Abstract
Recognition memory judgments can be based on recollection of qualitative information about an earlier study event or on assessments of stimulus familiarity. Schizophrenia is associated with pronounced deficits in overall recognition memory and these deficits are highly predictive of global functioning. However, the extent to which these deficits reflect impairments in recollection or familiarity is less well understood. In the current paper, we reviewed studies that utilized remember-know-new (RKN), process dissociation (PD), and receiver operating characteristic (ROC) procedures to investigate recollection and familiarity in schizophrenia. We also performed a quantitative reanalysis of these study results to obtain recollection and familiarity estimates that account for methodological differences between studies. Contrary to previous conclusions that recollection is selectively impaired in schizophrenia, we found evidence for both familiarity and recollection deficits across studies, suggesting multi-focal medial temporal lobe and/or prefrontal cortex dysfunction. The familiarity deficits were more variable with frequent small to medium rather than medium to large effect sizes, suggesting that familiarity could be potentially utilized as a compensatory ability while recollection is conceptualized as a therapeutic target for new treatment development.
Keywords: Schizophrenia, Episodic Memory, Memory Retrieval, Medial Temporal Lobe, Recollection, Familiarity
INTRODUCTION
Episodic long-term memory is severely impaired in patients with schizophrenia (1, 2). This deficit is a core feature of the illness (3) and highly predictive of functional outcome (4, 5). However, not all aspects of episodic memory are equally affected. For instance, memory performance is disproportionally impaired when patients must organize information during encoding (6), when memory for relationships between items rather than for individual item features is required (7, 8), and when retrieval is tested using recall versus recognition tasks (1). To the extent that successful recall performance requires recollection of contextual aspects of the encoding event, whereas recognition memory can be based on the assessment of item familiarity (9), these results suggest that patients may suffer from a selective deficit in recollection, while familiarity may be preserved. Many recent studies directly examining recollection and familiarity in schizophrenia have supported this hypothesis, suggesting a substantial recollection impairment and preservation of, or increased reliance on, familiarity processes (10-21), but other studies do not support this conclusion (22-28). Moreover, as described in more detail below a number of methodological issues complicate interpretation of and integration across extant findings. The goal of the current paper is to review and reanalyze published studies of recollection and familiarity in order to establish more definitively the relative impact of schizophrenia on these two retrieval processes.
Episodic memories can be retrieved on the basis of recollection or familiarity (for a review, see (29)). Recollection reflects a retrieval process whereby qualitative details of an event or episode are accessed, such as meeting someone and remembering who they are and that you saw them at the gym last week. Familiarity, in contrast, reflects a signal detection retrieval process, or an assessment of stimulus recency in the absence of recollection, such as recognizing another shopper at the grocery store, but being unable to retrieve their name or remember where you saw them last. Recollection and familiarity are functionally separable (for reviews, see (29, 30)) and rely on dissociable brain networks (31, 32). For example, within the medial temporal lobe (MTL), recollection is supported by the hippocampus and parahippocampal cortex (PHC), whereas the perirhinal cortex (PRC) may be sufficient to support familiarity-based recognition (for reviews, see (32-34)). Establishing the differential impact of schizophrenia on these two retrieval processes may, therefore, inform pathophysiological models of memory dysfunction and prove useful in developing future pharmacological or behavioral treatments.
Multiple experimental and computational procedures have been used to dissociate the relative contributions of recollection and familiarity to recognition memory performance in schizophrenia (see Table 1). The majority of studies have employed the remember-know-new (RKN) procedure (35), in which subjects respond “remember” when they can recollect qualitative information about the study event, and respond “know” when an item is recognized on the basis of familiarity in the absence of recollection. More recently, studies have utilized the receiver operating characteristic (ROC) method in which participants rate their subjective confidence (high, medium, low) that an item is either old or new. These confidence ratings are used to plot ROC curves that can be analyzed to estimate the contributions of recollection and familiarity (36, 37). One study also examined recognition memory with the process dissociation (PD) procedure (38), which measures recollection as the ability to accurately retrieve source information and familiarity as the ability to recognize items that are not recollected.
Table 1.
Summary of remember-know-new (RKN), process dissociation procedure (PD), and receiver operating characteristic (ROC) studies included in the review. For RKN studies that calculated recollection and familiarity estimates, recollection and familiarity results are reported in addition to response proportion results. Inclusion criteria for RKN studies in the reanalysis were reported mean response proportions for each group for “remember,” “know,” and “new” responses. HV = healthy volunteers; PT = patients; * = effect of schizophrenia originally reported as statistically significant (p < 0.05).
| Study | Study Type |
N=HV/PT | Experimental Materials and Procedures |
Remember Deficit | Know Deficit | Know Benefit | Recollection Deficit | Familiarity Deficit | Included in Reanalysis |
|---|---|---|---|---|---|---|---|---|---|
| Bonner- Jackson et al., 2008 (14) |
RKN | N=15/18 | fMRI scanning during incidental or intentional encoding of words |
* | X | ||||
| Danion et al., 1999 (10) |
RKN | N=25/25 | Physical objects paired by self or experimenter at encoding |
* | * | X | |||
| Danion et al., 2003 (23) |
RKN | N=24/24 | Emotional and neutral words | * | * | * | * | X | |
| Drakeford et al., 2006 (13) |
RKN | N=16/14 | Auditory sentences | * | * | X | |||
| Edelstyn et al., 2003 (12) |
RKN | N=16/10 | Faces and words; Two schizophrenia patients with delusional misidentification not included in this reanalysis |
* | * | X | |||
| Grillon et al., 2005 (24) |
RKN | N=24/24 | Words that are read, repeated, or refreshed at encoding |
* | X | ||||
| Grillon et al., 2010 (16) |
RKN | N=25/25 | Words that are read, repeated, or refreshed at encoding |
* | X | ||||
| Guillaume et al., 2007 (28) |
PD | N=20/20 | Emotional faces paired with background scenes at encoding; Inclusion phase: “Old” to any face seen previously; Exclusion phase: “Old” only to faces with identical expression and background to encoding |
* | X | ||||
| Huron et al., 1995 (17) |
RKN | N=30/30 | High- and low-frequency words | * | |||||
| Huron et al., 2003 (21) |
RKN | N=24/24 | Words and pictures | * | X | ||||
| Huron and Danion, 2002 (11) |
RKN | N=30/30 | Semantically-related words (e.g. Deese, 1959; Roediger & McDermott, 1995) |
* | X | ||||
| Martin et al., 2011 (22) |
RKN | N=24/25 | Faces and words; Same, similar, or new stimuli at retrieval |
* | * | X | |||
| Neumann et al., 2007 (18) |
RKN | N=20/20 | Positive and negative emotional pictures |
* | * | ||||
| Ragland et al., 2012 (25) |
ROC | N=19/20 | fMRI scanning during words presented three at a time with, item-specific (rehearse) or relational (reorder) encoding |
* | * | X | |||
| Ragland et al., 2012 (26), Experiment 1 |
ROC | N=74/104 | Pictures encoded either alone (living/nonliving judgment) or in pairs (size judgment) |
* | * | X | |||
| Ragland et al., 2012 (26), Experiment 2 |
ROC | N=64/49 | Words encoded either alone (living/nonliving judgment) or in pairs (size judgment) |
* | * | X | |||
| Sonntag et al., 2003 (20) |
RKN | N=21/21 | Words, instructed to either remember or forget at encoding |
X | |||||
| Tendolkar et al., 2002 (19) |
RKN | N=14/14 | ERP recording during retrieval of words |
* | * | ||||
| Thoma et al., 2006 (27) |
ROC | N=(11/11)/ 11 |
Words; Patient group divided into high and low negative symptoms groups |
* | * | X | |||
| van Erp et al., 2008 (15) |
RKN | N=35/35 | Word-picture pairs | * | * | * | X |
Across methods, results from these studies have consistently suggested that schizophrenia patients exhibit deficits in recollection, but the fate of familiarity has been less clear (see Table 1). For example, 16 of 19 studies reported that recollection was reduced in schizophrenia patients relative to demographically matched controls (10-19, 21-23, 25-27), whereas only three (1 PD and 2 RKN) found no significant group differences (20, 24, 28). In contrast, when familiarity was examined, seven studies (1 PD, 3 ROC, 3 RKN) indicated that familiarity was reduced (22-28), seven (all RKN) that familiarity was unaffected (11, 14-17, 20, 21), and five (all RKN) that familiarity was increased, rather than decreased (10, 12, 13, 18, 19), in patients with schizophrenia. These mixed conclusions regarding familiarity may reflect the fact that there is more heterogeneity in the effects of schizophrenia on familiarity versus recollection. Alternatively, these mixed results may arise due to differences in the manner in which the familiarity estimates have been derived across these different studies.
Methodological variability in familiarity estimation has been particularly marked in studies using the RKN procedure. Many studies of schizophrenia that used the RKN procedure have estimated effects on familiarity by simply comparing the proportion of “know” responses between patient and control groups. One problem with this approach is that the proportion of “know” responses does not take into account that “remember” responses can also be associated with some degree of familiarity (39). Another issue is that a “know” response can only be given to an item that has not received a “remember” response, so a reduction in “remember” responses could spuriously inflate the proportion of “know” responses. Thus, it is possible that previous studies of schizophrenia using the RKN procedure either under- or over-estimated familiarity-based recognition in schizophrenia. In addition, some, but not all, studies have accounted for response bias using signal detection-based models. Response bias incorporation is critical because response bias often differs between healthy individuals and patients with schizophrenia (40), and could, therefore, spuriously impact group differences in memory parameter estimates.
In the current paper, we attempted to obtain a more definitive understanding of the relative impact of schizophrenia on recollection and familiarity by taking a consistent and systematic approach to characterizing recollection and familiarity across studies of recognition memory in schizophrenia. As in a prior study of amnestic patients (e.g., (41)), we performed a summary reanalysis of group means reported in the RKN, ROC, and PD literature to obtain comparable recollection and familiarity estimates across studies. We predicted that reanalysis of RKN findings will reveal both recollection and familiarity deficits in schizophrenia patients, consistent with the pattern seen in studies using ROC and PD methods.
METHODS AND MATERIALS
Literature Search
We reviewed 19 published journal articles examining recollection and familiarity in schizophrenia using any of three different recognition memory paradigms: RKN (N=15), PD (N=1), and ROC (N=3; Table 1). A PubMed search was conducted to identify studies using any of these paradigms to compare recognition memory between patients diagnosed with schizophrenia or schizoaffective disorder and matched healthy controls. Search terms were “schizophrenia” with any of the following: “recollection,” “familiarity,” “remember,” “know,” “process dissociation,” or “receiver operating characteristic.” Studies employing these paradigms to test cued recall or autobiographical memory were excluded. Reference lists of included studies were also reviewed for relevant studies not detected in the original database search. This search yielded twelve RKN studies that reported proportions of hits and false alarms for studied (old) and unstudied (new) items receiving “remember” and “know” responses and could, therefore, be included in the reanalysis, along with the PD and ROC studies (Table 1).
Quantification of recollection and familiarity effects across studies
For each of the RKN studies, summary estimates of recollection and familiarity (Table 1) were calculated for each group (schizophrenia patients and healthy controls). When multiple experimental conditions were tested within a study, behavioral measures were averaged across condition, resulting in one recollection and one familiarity estimate for each group per study. Reanalysis of RKN studies yielded recollection and familiarity estimates scaled as probabilities, which are comparable to estimates derived from PD calculations. However, for ROC studies, only recollection is scaled as a probability, while familiarity is estimated using signal detection measure d’. To eliminate scaling differences between studies and allow for direct comparison between recollection and familiarity, d’ estimates obtained from ROC studies were converted into probabilities as follows: the average false alarm rate (across groups) for a particular study was subtracted from the hit rate corresponding to each group’s d’ score (42, 43). It should be noted that the use of average false alarm rate was arbitrary and did not impact between-groups effects, as it was applied identically to each group. One ROC study (26) presented results from two independent samples, yielding two sets of recollection and familiarity estimates.
Reanalysis provided estimates of recollection and familiarity for each group, but no measures of within-group variance for each study, precluding the use of standard meta-analytic procedures to draw statistical inferences across studies. However, six studies (3 ROC, 1 PD, 2 RKN) reported group means and variability measures for recollection and familiarity estimates that were obtained according to the computational procedures below (15, 23, 25-28). Therefore, to estimate the effects of schizophrenia on each process, effect sizes (Cohen’s d) were calculated for each of these six studies, averaged across experimental condition, separately for recollection and familiarity.
Computational Procedures
RKN studies
Recollection is calculated based on accurate “remember” responses, correcting for “remember” false alarm rate, as follows:
where Rold is the proportion of “remember” responses to previously studied items and Rnew is the proportion of “remember” responses to nonstudied items.
To account for response non-independence, the proportion of “know” responses must be divided by the proportion of trials in which a “know” response could have been made (i.e., when a “remember” response was not made). Thus, familiarity is calculated for both old and new items as follows:
And overall familiarity, correcting for false alarms is estimated as:
PD studies
In the inclusion condition, an old item can be correctly recognized as old if it is recollected or if it is sufficiently familiar. Therefore, in the inclusion condition, the probability of responding “old” to an item that was previously seen can be represented by:
In the exclusion condition, however, only recollection is diagnostic of accurate “old” judgments to items from the target list, while items from the nontarget list could be incorrectly accepted if they are familiar but not recollected. Therefore, in the exclusion condition the probability of responding “old” to an item that was seen in only the indicated studied list can be represented by:
These equations were combined and reduced to solve for recollection and familiarity:
ROC studies
Identical to the inclusion condition analysis described above, an accurate “old” judgment can be made based on recollection or sufficient familiarity in the absence of recollection. False alarms, on the other hand, are driven by familiarity exceeding a response criterion. Therefore, the probability of responding “old” to previously seen and new objects at any one point i on the ROC can be represented by:
Familiarity is assumed to reflect a signal-detection process, whereby successful recognition on the basis of familiarity is a function of the strength of the familiarity of old items relative to new items. Therefore, the familiarity of old and new items can be represented as follows:
Sensitivity, or d’, is the distance between the means of the two Gaussian distributions of old and new item familiarity, Φ is there area under the cumulative normal distribution, and c is the response criterion, reflecting response bias (41).
Therefore, an ROC with 6 points will have a set of 12 equations. These computations assume that recollection and d’ remain constant across the ROC, but that ci varies. The solver function in Excel (see (44)) was used to find the best fitting Recollection and d’ parameters for these equations by minimizing the sum of squared errors between predicted and observed data.
According to signal detection theory, d’ can be represented by:
Therefore, to scale a d’ familiarity estimate derived from ROCs as a probability, this equation can be solved for Hit Rate by taking the standard normal cumulative distribution of d’ plus the inverse normal density of the false alarm rate.
RESULTS
Reanalysis of RKN studies
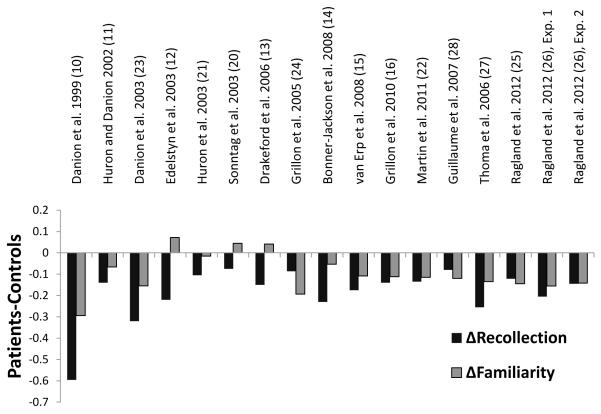
Figure 1 illustrates group differences in recollection and familiarity after reanalysis of the RKN data. Reanalysis of the familiarity estimates from the RKN studies had a dramatic effect on the pattern of group differences. Across studies, there was an inverse effect of reanalysis between groups, with the magnitude of the familiarity estimates decreasing in the patient sample and increasing in the control sample. After reanalysis, all but three of the RKN studies that reported patient benefits in familiarity based on “know” response proportions yielded reduced familiarity in patients compared to controls. Thus, reanalysis of the data revealed more consistent patient deficits in familiarity, in contrast to previous results that reported unimpaired or above-average patient familiarity.
Figure 1.
Recollection and familiarity deficits for RKN, ROC, and PD studies. Differences in reanalyzed recollection and familiarity estimates for each RKN study, combined with recollection and familiarity differences reported in all PD and ROC studies. Negative-going bars indicate patient deficits.
Recollection and Familiarity across Studies
To investigate the consistency of group differences in recollection and familiarity across studies, we examined difference scores for each test procedure. When group differences (patient – control) in recollection were calculated, the average difference was −0.20 across reanalyzed RKN studies, −0.18 across ROC studies, and −0.08 for the PD study. For familiarity, the average patient-control difference was −0.08 for reanalyzed RKN studies, −0.14 for ROC studies, and −0.12 for the PD study. Additionally, we calculated effect sizes (Cohen’s d) for each of the studies that estimated recollection and familiarity following recommended computational methods, as detailed in Computational Procedures. As can be seen in Table 2, effect sizes of schizophrenia on recollection were large (−0.9 to −1.86) in three studies (23, 26, 27) and medium (−0.59 to −0.72) in four studies (15, 25, 26, 28), whereas effect sizes on familiarity were medium to large (−0.79 to −0.92) in three studies (25, 27, 28), and small to medium (−0.20 to −0.55) in four studies (15, 23, 26).
Table 2.
Effect sizes of schizophrenia on recollection and familiarity. Cohen’s d was calculated on group means and standard deviations, averaged across conditions, reported in six studies that obtained recollection and familiarity estimates according to the computational procedures detailed in the Methods section. Negative effect sizes reflect patient deficits.
| Study | Study Type | Recollection | Familiarity |
|---|---|---|---|
| Danion et al., 2003 (23) | RKN | −1.86 | −0.48 |
| van Erp et al., 2008 (15) | RKN | −0.72 | −0.20 |
| Guillaume et al., 2007 (28) | PD | −0.59 | −0.91 |
| Thoma et al., 2006 (27) | ROC | −1.67 | −0.79 |
| Ragland et al., 2012 (25) | ROC | −0.63 | −0.92 |
| Ragland et al., 2012 (26), Experiment 1 | ROC | −0.90 | −0.55 |
| Ragland et al., 2012 (26), Experiment 2 | ROC | −0.60 | −0.43 |
DISCUSSION
The dominant hypothesis in the recognition memory literature on schizophrenia has been that recollection is selectively impaired relative to familiarity (10-19, 23). In the current paper, we reviewed nineteen studies of recollection and familiarity in schizophrenia, and found that this hypothesis has primarily been driven by RKN studies, whereas studies using PD and ROC procedures suggested deficits in both retrieval processes. To help resolve these discrepancies, we performed a summary reanalysis to obtain computationally comparable estimates of recollection and familiarity across different study procedures, while taking into account potential confounds such as response non-independence and response bias. With application of these methods, a more consistent pattern of results was observed. In agreement with previous ROC and PD studies, and contrary to studies utilizing RKN methods, reanalysis of the data revealed that schizophrenia affects both retrieval processes.
The Nature of Recognition Impairments in Schizophrenia
It is widely acknowledged that individuals with schizophrenia have difficulty recollecting contextual details of study events, as reflected by poor performance on source memory tasks (e.g., (45)) and a reduced proportion of “remember” responses during RKN testing (10-19, 23). In contrast, these studies have suggested that familiarity is either intact or at least relatively preserved, as patients typically report an equal or greater number of “know” responses compared to healthy controls. As noted earlier, however, the rate of “know” responses does not adequately measure familiarity, because “remember” and “know” responses are non-independent, and because familiarity would be expected to contribute to both “know” and “remember” responses. When these factors were accounted for (Figure 1), deficits in both familiarity and recollection were evident. This finding suggests a new interpretation of previous studies of schizophrenia that used the RKN procedure: because “remember” responses are often associated with recollection and a high degree of familiarity, previously documented reductions in “remember” responses in patients with schizophrenia are indicative of deficits in both recollection and familiarity (46).
Although our current findings emphasize that both recollection and familiarity are impacted by schizophrenia, results of the reanalysis also align with previous conclusions that recollection is strongly impacted. Across studies, recollection deficit effect sizes were large or medium, compared to mostly medium or small sized effects on familiarity, with the magnitude of effect sizes appearing larger for recollection than for familiarity in all but three of the examined studies. The familiarity impairment was also less consistent, with about a quarter of studies suggesting either a small familiarity impairment or a slight familiarity advantage in patients. In contrast, all studies revealed a recollection deficit (Figure 1).
Putative neural underpinnings of recollection and familiarity deficits in schizophrenia
Previous neuroimaging and focal lesion studies have demonstrated that regions within the MTL and PFC play a critical role in recollection and familiarity components of recognition memory performance. Within the MTL, functional imaging and lesion studies in humans have consistently demonstrated that the hippocampus is critical for the encoding and retrieval of the contextual details necessary for successful recollection (31-33, 42, 47, 48). This conclusion has been supported by animal lesion studies in rats and monkeys employing recollection-like memory paradigms (33, 49-52). In contrast, functional imaging and focal lesion studies have demonstrated that familiarity can be supported by the PRC without hippocampal involvement (33, 42, 48, 51, 52). Within the prefrontal cortex (PFC), it is unclear whether different subregions might differentially support recollection and familiarity, but available evidence suggests that the PFC is critical for both processes, particularly when control of strategic memory processes is important for task performance (43, 53-56). Based on the results of our review, we hypothesize that recollection and familiarity impairments in schizophrenia do not solely reflect disruption limited to the hippocampus, as selective hippocampal dysfunction would not yield familiarity deficits across studies. Instead, it is likely that dysfunction in other areas, including the PFC (57) and perirhinal cortex (58-60) additionally contribute to memory deficits in schizophrenia by affecting familiarity.
Limitations and Future Implications
Although RKN, PD, and ROC paradigms can yield quantitatively comparable recollection and familiarity effects, each of these procedures has different methodological limitations to consider for future studies of recognition memory in schizophrenia. A potential limitation of both PD and RKN methods is that they are procedurally complex and metacognitively demanding, raising concerns about their implementation in schizophrenia patients with known metacognitive deficits (40, 61). Although metacognition is also required for making the confidence judgments required by the ROC procedure, reliable confidence ratings can be obtained in children as young as age 6 (62), and our previous research has shown that patients with schizophrenia can generate appropriate response distributions to permit successful modeling of recollection and familiarity estimates (26).
While reanalysis of previous study data increased the consistency of group difference results, there was still marked variability in the pattern of effects across studies. Although too few studies of recollection and familiarity in schizophrenia currently exist to draw strong conclusions, it is likely that procedural differences, both at encoding and retrieval, contributed to discrepancies in effects across studies within each method. For instance, within the ROC studies, one study used intentional encoding and a list discrimination procedure at retrieval (27), while the other studies used incidental encoding and subjective recollection judgments at retrieval (25, 26). It is also possible that sample differences may account for some of the variability in results across studies. For example, several studies have shown that both recollection and familiarity may be affected similarly by cognitive factors such as IQ (27) and clinical factors such as the severity of negative, but not positive, symptoms (25, 27). Examination of individual difference factors should be an important component of any future large-scale study of recollection and familiarity in schizophrenia.
Characterizing recollection and familiarity deficits in schizophrenia may lead to better-targeted treatment for memory impairments in the disorder. For instance, as familiarity deficits appear to have small to medium rather than medium to large effect sizes, familiarity may be better treated as a compensatory process during cognitive training, in which patients learn strategies to increasingly rely on familiarity strength to improve overall memory performance. However, increased reliance on familiarity is not likely to fully overcome patients’ episodic retrieval deficits. Therefore, it will also be important to develop new pharmacological and cognitive training procedures aimed at improving recollection. These recollection training procedures are already being developed for healthy aging and mild cognitive impairment individuals, and hold promise for translation to individuals with schizophrenia (63, 64).
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants R01MH084895, R01MH59352, and RO1MH83734 and by the NSF GRFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 2.Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. J Abnorm Psychol. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- 3.Clare L, McKenna PJ, Mortimer AM, Baddeley AD. Memory in schizophrenia: what is impaired and what is preserved? Neuropsychologia. 1993;31:1225–1241. doi: 10.1016/0028-3932(93)90070-g. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 6.Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychol Med. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- 7.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis. Brain Cogn. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 8.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson JR, Bower GH. A propositional theory of recognition memory. Mem Cognit. 1974;2:406–412. doi: 10.3758/BF03196896. [DOI] [PubMed] [Google Scholar]
- 10.Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- 11.Huron C, Danion J. Impairment of constructive memory in schizophrenia. Int Clin Psychopharmacol. 2002;17:127–133. doi: 10.1097/00004850-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Edelstyn NM, Drakeford J, Oyebode F, Findlay C. Investigation of conscious recollection, false recognition and delusional misidentification in patients with schizophrenia. Psychopathology. 2003;36:312–319. doi: 10.1159/000075831. [DOI] [PubMed] [Google Scholar]
- 13.Drakeford JL, Edelstyn NM, Oyebode F, Srivastava S, Calthorpe WR, Mukherjee T. Auditory recognition memory, conscious recollection, and executive function in patients with schizophrenia. Psychopathology. 2006;39:199–208. doi: 10.1159/000093524. [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Jackson A, Yodkovik N, Csernansky JG, Barch DM. Episodic memory in schizophrenia: the influence of strategy use on behavior and brain activation. Psychiatry Res. 2008;164:1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Erp T, Lesh T, Knowlton B, Bearden C, Hardt M, Karlsgodt K, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillon ML, Krebs MO, Gourevitch R, Giersch A, Huron C. Episodic memory and impairment of an early encoding process in schizophrenia. Neuropsychology. 2010;24:101–108. doi: 10.1037/a0015544. [DOI] [PubMed] [Google Scholar]
- 17.Huron C, Danion J, Giacomoni F, Grangé D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry. 1995;152:1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- 18.Neumann A, Blairy S, Lecompte D, Philippot P. Specificity deficit in the recollection of emotional memories in schizophrenia. Conscious Cogn. 2007;16:469–484. doi: 10.1016/j.concog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus-Dumke A, van Eijndhoven P, et al. Contributions of the medial temporal lobe to declarative memory retrieval: manipulating the amount of contextual retrieval. Learn Mem. 2008;15:611–617. doi: 10.1101/lm.916708. [DOI] [PubMed] [Google Scholar]
- 20.Sonntag P, Gokalsing E, Olivier C, Robert P, Burglen F, Kauffmann-Muller F, et al. Impaired strategic regulation of contents of conscious awareness in schizophrenia. Conscious Cogn. 2003;12:190–200. doi: 10.1016/s1053-8100(03)00016-3. [DOI] [PubMed] [Google Scholar]
- 21.Huron C, Danion J, Rizzo L, Killofer V, Damiens A. Subjective qualities of memories associated with the picture superiority effect in schizophrenia. J Abnorm Psychol. 2003;112:152–158. [PubMed] [Google Scholar]
- 22.Martin CD, Baudouin JY, Franck N, Guillaume F, Guillem F, Tiberghien G, et al. Impairment not only in remembering but also in knowing previously seen faces and words in schizophrenia. Psychiatry Res. 2011 doi: 10.1016/j.psychres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Danion J, Kazes M, Huron C, Karchouni N. Do patients with schizophrenia consciously recollect emotional events better than neutral events? Am J Psychiatry. 2003;160:1879–1881. doi: 10.1176/appi.ajp.160.10.1879. [DOI] [PubMed] [Google Scholar]
- 24.Grillon M, Johnson M, Danion J, Rizzo L, Verdet C, Huron C. Assessing a minimal executive operation in schizophrenia. Psychiatry Res. 2005;137:37–48. doi: 10.1016/j.psychres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Ragland JD, Blumenfeld RS, Ramsay IS, Yonelinas A, Yoon J, Solomon M, et al. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. Neuroimage. 2012;59:1719–1726. doi: 10.1016/j.neuroimage.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragland JD, Ranganath C, Barch DM, Gold JM, Haley B, Macdonald AW, et al. Relational and Item-Specific Encoding (RISE): Task Development and Psychometric Characteristics. Schizophr Bull. 2012;38:114–124. doi: 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoma P, Zoppelt D, Wiebel B, Daum I. Recollection and familiarity in negative schizophrenia. Neuropsychologia. 2006;44:430–435. doi: 10.1016/j.neuropsychologia.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Guillaume F, Guillem F, Tiberghien G, Martin F, Ganeva E, Germain M, et al. Use of the process dissociation procedure to study the contextual effects on face recognition in schizophrenia: familiarity, associative recollection and discriminative recollection. Psychiatry Res. 2007;149:105–119. doi: 10.1016/j.psychres.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diana RA, Reder LM, Arndt J, Park H. Models of recognition: a review of arguments in favor of a dual-process account. Psychon Bull Rev. 2006;13:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 35.Tulving E. Memory and consciousness. Canadian Psychology/Psychologie canadienne. 1985;26:1–12. [Google Scholar]
- 36.Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- 37.Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: a formal dual-process model and an analysis of receiver operating characteristics. J Exp Psychol Learn Mem Cogn. 1999;25:1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- 38.Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- 39.Yonelinas AP, Jacoby LL. The Relation between Remembering and Knowing as Bases for Recognition: Effects of Size Congruency. Journal of Memory and Language. 1995;34:622–643. [Google Scholar]
- 40.Moritz S, Woodward TS, Jelinek L, Klinge R. Memory and metamemory in schizophrenia: a liberal acceptance account of psychosis. Psychol Med. 2008;38:825–832. doi: 10.1017/S0033291707002553. [DOI] [PubMed] [Google Scholar]
- 41.Yonelinas A, Kroll N, Dobbins I, Lazzara M, Knight R. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- 42.Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 43.Aly M, Yonelinas AP, Kishiyama MM, Knight RT. Damage to the lateral prefrontal cortex impairs familiarity but not recollection. Behav Brain Res. 2011;225:297–304. doi: 10.1016/j.bbr.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- 45.Waters FA, Maybery MT, Badcock JC, Michie PT. Context memory and binding in schizophrenia. Schizophr Res. 2004;68:119–125. doi: 10.1016/S0920-9964(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 46.Yonelinas AP, Jacoby LL. Dissociating automatic and controlled processes in a memory-search task: beyond implicit memory. Psychol Res. 1995;57:156–165. doi: 10.1007/BF00431277. [DOI] [PubMed] [Google Scholar]
- 47.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Ranganath C, Yonelinas A, Cohen M, Dy C, Tom S, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q J Exp Psychol B. 2005;58:202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- 50.Eichenbaum H, Fortin N, Sauvage M, Robitsek RJ, Farovik A. An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory. Neuropsychologia. 2010;48:2281–2289. doi: 10.1016/j.neuropsychologia.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- 53.Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39:827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]
- 55.Turriziani P, Smirni D, Oliveri M, Semenza C, Cipolotti L. The role of the prefrontal cortex in familiarity and recollection processes during verbal and non-verbal recognition memory: an rTMS study. Neuroimage. 2010;52:348–357. doi: 10.1016/j.neuroimage.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 57.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim K, DeWitt I, Ditman T, Zalesak M, Greenhouse I, Goff D, et al. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull. 2006;32:332–340. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karnik-Henry MS, Wang L, Barch DM, Harms MP, Campanella C, Csernansky JG. Medial temporal lobe structure and cognition in individuals with schizophrenia and in their non-psychotic siblings. Schizophr Res. 2012;138:128–135. doi: 10.1016/j.schres.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- 61.Bacon E, Huet N, Danion JM. Metamemory knowledge and beliefs in patients with schizophrenia and how these relate to objective cognitive abilities. Conscious Cogn. 2011;20:1315–1326. doi: 10.1016/j.concog.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 62.Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- 63.Jennings JM, Webster LM, Kleykamp BA, Dagenbach D. Recollection training and transfer effects in older adults: Successful use of a repetition-lag procedure. Aging, Neuropsychology, and Cognition. 2005;12:278–298. doi: 10.1080/138255890968312. [DOI] [PubMed] [Google Scholar]
- 64.Jennings JM, Jacoby LL. Improving memory in older adults: Training recollection. Neuropsychological Rehabilitation. 2003;13:417–440. [Google Scholar]