Abstract
Background
Previous literature suggests that cell death pathways activated after cerebral ischemia differ between the sexes. While caspase-dependent mechanisms predominate in the female brain, caspase-independent cell death induced by activation of Poly (ADP-ribose) polymerase (PARP) predominates in the male brain. PARP-1 gene deletion decreases infarction volume in the male brain, but paradoxically increases damage in PARP-1 knockout females.
Purpose
This study examined stroke induced changes in NAD+, a key energy molecule involved in PARP-1 activation in both sexes.
Methods
Mice were subjected to Middle Cerebral Artery Occlusion and NAD+ levels were assessed. Caspase-3 activity and nuclear translocation was assessed 6 hours after ischemia. In additional cohorts, Nicotinamide (500mg/kg i.p.) a precursor of NAD+ or vehicle was administered and infarction volume was measured 24 hours after ischemia.
Results
Males have higher baseline NAD+ levels than females. Significant stroke-induced NAD+ depletion occurred in males and ovariectomized females but not in intact females. PARP-1 deletion prevented the stroke induced loss in NAD+ in males, but worsened NAD+ loss in PARP-1 deficient females. Preventing NAD+ loss with nicotinamide reduced infarct in wild-type males and PARP-1 knockout mice of both sexes, with no effect in WT females. Caspase-3 activity was significantly increased in PARP-1 knockout females compared to males and wild-type females, this was reversed with nicotinamide.
Conclusions
Sex differences exist in baseline and stroke-induced NAD+ levels. Nicotinamide protected males and PARP knockout mice, but had minimal effects in the wild-type female brain. This may be secondary to differences in energy metabolism between the sexes.
Keywords: NAD+, Nicotinamide, PARP-1, Caspase, Sex Differences
1.1 INTRODUCTION
Studies have shown that sexual dimorphism exists in cell death pathways activated by cerebral ischemia (Siegel et al., 2010). In experimental stroke models cell death in females is triggered primarily by caspase-dependent mechanisms (Renolleau et al., 2007, Liu et al., 2009b). This differs from the apparent caspase independent mode of cell death in males, characterized by peroxynitrite (OONO−) formation, poly ADP-ribose polymerase (PARP-1) activation, and apoptosis inducing factor (AIF) translocation (Du et al., 2004, Hagberg et al., 2004, McCullough et al., 2005, Renolleau et al., 2007, Li and McCullough, 2009, Liu et al., 2009b, Yuan et al., 2009, Siegel and McCullough, 2010, Siegel et al., 2010). nNOS and PARP-1 deletion and pharmacological inhibition reduced stroke in male mice, but exacerbated injury in female mice (McCullough et al., 2005). PARP-1 gene deletion was also protective in neonatal hypoxia-ischemia (HI) in males but had no effect in female pups (Hagberg et al., 2004). Neonatal HI activated PARP-1 signaling in post-natal day 9 (P9) males, while P9 females exhibited increased caspase-3 activity (Zhu et al., 2006). These data suggest that PARP-1 plays an important role in ischemic cell death in the male brain.
PARP-1 is a nuclear enzyme activated by DNA damage that when activated can lead to cell death (Jagtap and Szabo, 2005). PARP-1 generates toxic poly ADP-ribose (PAR) polymers leading to the nuclear translocation of AIF (Andrabi et al., 2006, Yu et al., 2006), a process that utilizes nicotinamide adenine dinucleotide (NAD+) (Dawson and Dawson, 2004). In stroke, over activation of PARP-1 leads to increased PAR polymer formation, NAD+ depletion, subsequent adenine triphosphate (ATP) loss, and ultimately energy failure and cell death (Jagtap and Szabo, 2005). NAD+ depletion is a necessary step for PARP-1-mediated cell death in astrocytes (Alano et al., 2004), and is upstream of mitochondrial AIF release (Alano et al., 2010). Exogenous administration of nicotinamide, a precursor to NAD+, preserved NAD+ levels, reduced PAR polymer formation, and was protective in cortical neurons in vitro (Liu et al., 2009a). Similarly, direct NAD+ repletion has been shown to prevent PARP-1-mediated cell death in vivo (Ying et al., 2003).
Although PARP-1-mediated cell death plays an important role in the ischemic male brain, sex differences in NAD+ have not been previously evaluated. Considering the key role played by NAD+ in PARP-1-mediated cell death, we hypothesized sex differences may exist in response to NAD+ depletion during ischemia. This was evaluated in both wild type (WT) and PARP-1 knockout (KO) mice after reversible middle cerebral artery occlusion (MCAO). The effects of NAD+ repletion on ischemic outcome were also evaluated.
2.1 MATERIALS AND METHODS
2.1.1 Experimental Animals
PARP-1 KO and WT littermate mice on a SV129 background (Jackson Labs, Bar Harbor, ME) were utilized in this study. Animals were either male, intact female, or ovariectomized (ovx) females housed in cages with a 12-hour light/dark schedule, and provided with food and water ad libitum (6–8 weeks of age). Ovariectomy was performed under Isoflurane anesthesia 14 days prior to stroke, as described previously (McCullough et al., 2003). Removal of ovarian hormones was confirmed by analysis of serum estrogen levels (IBL, Hamburg, Germany) and uterine weights (Li et al., 2010). Nicotinamide (Sigma, St. Louis, MO) or vehicle was administered (500mg/kg i.p.) immediately before stroke.
2.1.2 Ischemic Model
Animals were randomized into stroke and sham groups. Focal cerebral ischemia was induced by 90 minutes of reversible MCAO as described previously (McCullough et al., 2004). Briefly, under Isoflurane anesthesia, a 6.0 silicone coated suture (Doccol Corporation, Redlands, CA) was inserted through an external carotid artery stump, advanced through the internal carotid artery, and occluded the right middle cerebral artery. Temperature was maintained at 37°C throughout the surgical procedure. Sham animals underwent the same surgery, but the suture was not inserted into the internal carotid artery. Animals were sacrificed 60 minutes after induction of ischemia (“intra-ischemic cohort”), 30 minutes after reperfusion, 6 hours (for Western and activity assays) or 24 hours after ischemia for determination of infarction volume. Nicotinamide was shown to have no effect on physiological parameters or temperature (Mokudai et al., 2000). Neurological deficit was scored as follows: 0, no deficit; 1, forelimb weakness and torso turning to ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; 4, no spontaneous locomotor activity or barrel rolling.
2.1.3 NAD+ Assay
NAD+ levels were assessed using the EnzyChrom NAD+/NADH Assay Kit (BioAssay Systems, Hayward, CA). Briefly, 20mg of tissue localized to the core/peri-infarct region was isolated from both sham and stroke WT and PARP-1 KO mice. Tissue was isolated either after 60 minutes of ischemia or 30 minutes after reperfusion. Tissue was immediately placed into extraction buffer to limit NAD+ degradation. The tissue was lysed and optical density was measured at 565nm. NAD+ levels were quantified by comparison to NAD+ standards.
2.1.4 Terminal Histopathology
Brains were harvested 24 hours after ischemia, sliced into five 2mm sections, and stained with 1.5% triphenyltetrazolium chloride (Sigma, St. Louis, MO) at 37°C for 7 minutes. Infarction volume was measured by a blinded investigator with Sigma Scan and expressed as a percentage of the contralateral structure corrected for edema (McCullough et al., 2004).
2.1.5 Subcellular Fractionation
Protein was extracted 6 hours after 90 minute MCAO and separated into cytosolic and nuclear fractions as described (Yuan et al., 2009). Protein concentrations were determined by BCA protein assay (Pierce, Rockford, IL).
2.1.6 Western Blot Analysis
Equal amounts of protein were loaded per lane of a 4–15% SDS poly-acrylamide gel (Bio-Rad Laboratories, Hercules, CA), transferred to a PVDF membrane, blocked with 5% milk and probed with antibodies for αII Spectrin (1:500 Millipore, Billerica, MA), Caspase-3 (1:500 Santa Cruz Biotechnology, Santa Cruz, CA), MIF (1:1000 Santa Cruz Biotechnology, Santz Cruz, CA), and Histone (1:1000 Abcam, Cambridge, MA) in 4% BSA at 4°C overnight followed by incubation with horseradish peroxidase secondary antibody (GE Healthcare, Piscataway, NJ). Blots were incubated with signal detection reagent (Pierce, Rockville, IL), and developed on film (Denville, Metuchen, NJ).
2.1.7 Statistics
Data from individual experiments were presented as mean±SEM. ANOVA (with Tukey post hoc correction, when appropriate) was used for the comparison of the means between the experimental groups (P<0.05 was considered statistically significant). The neurological deficit scores were assessed with Mann Whitney. All infarct analysis was performed by a blinded investigator.
3.1 RESULTS
3.1.1 Sex Differences in NAD+ Levels
Biologic sex played a significant role in the ability to maintain NAD+ levels either during or after ischemia. Although WT female mice have less NAD+ than males at baseline (Figure 1A bars 1 vs. 3), a significant stroke-induced decrease in NAD+ was seen selectively in males during the intra-ischemic period (Figure 1A bars 1 vs. 2). Ovx females exhibited a pattern of NAD+ loss similar to that of males, with a significant drop in stroke-induced intra-ischemic NAD+ levels (Figure 1A bars 5 vs. 6). Interestingly, PARP-1 gene deletion ameliorated the stroke-induced NAD+ loss seen in males and ovx females (Figure 1B bars 1 vs. 2 and 5 vs. 6), but paradoxically led to increased ischemic NAD+ loss in KO females (Figure 1B bars 3 vs. 4). The intra-ischemic NAD+ loss seen in WT males, persisted 120 minutes after ischemia into reperfusion (Figure 1C bars 1 vs. 2). The NAD+ levels in WT and ovx females were not significantly different in the reperfusion period although the drop in ovx females was still present (Figure 1C bars 3 vs. 4 and 5 vs. 6). PARP-deletion ameliorated the stroke-induced decrease in NAD+ seen in males during reperfusion (Figure 1D bars 1 vs. 2) but did not lead changes in NAD levels in gonadally intact females (Figure 1D bars 3 vs. 4) or ovx females (Figure 1D bars 5 vs. 6) 120 minutes after ischemia. This demonstrates that unlike males, intact females can maintain NAD+ levels during ischemia, while PARP-1 gene deletion causes NAD+ depletion in intact females and maintained NAD+ levels in males.
Figure 1. Sex Differences in NAD+ Levels.
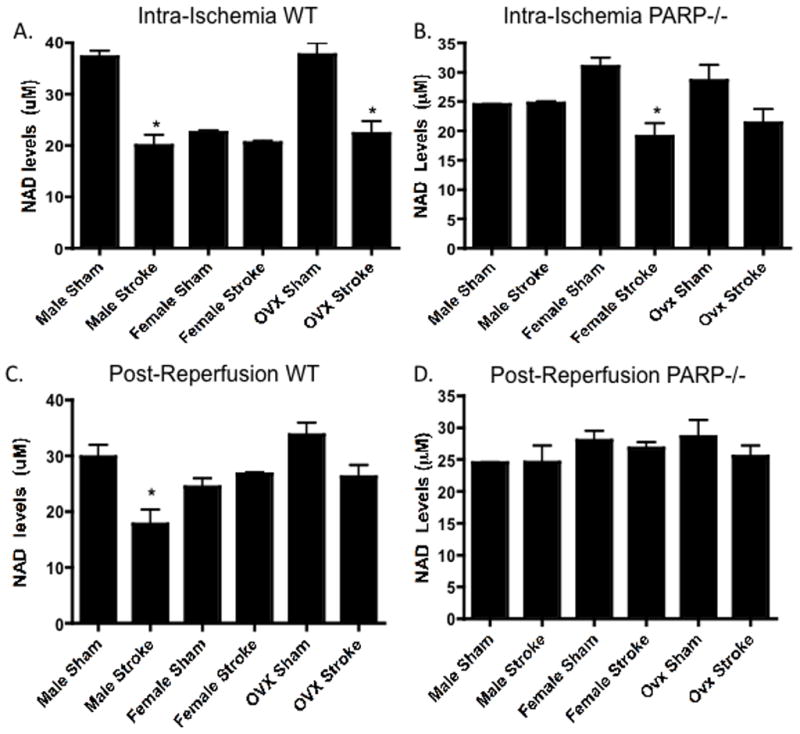
NAD+ levels were analyzed 60 minutes after ischemic onset in male, female, and ovx female WT (A) and PARP-1−/− (B) mice (n=8/group: p<0.05). Stroke-induced NAD+ depletion was seen in WT males, and WT Ovx females. PARP-1−/− males maintained their NAD+ levels, while PARP-1−/− females experienced a loss of NAD+ after stroke. NAD+ levels were analyzed 30 minutes after reperfusion in male, female, and Ovx female WT (C) and PARP-1−/− (D) mice (n=8/group: p<0.05). Stroke-induced NAD+ depletion was seen in WT males. PARP-1−/− males maintained their NAD+ levels in all sexes after stroke.
3.1.2 Nicotinamide’s Effect on Stroke Outcome
In order to directly assess the effect of NAD+ repletion on stroke outcome nicotinamide was administered to both WT and PARP-1 KO male, female, and ovx female mice. No differences in physiological parameters were seen in any of the groups. Consistent with previous studies, deletion of PARP-1 reduced infarction volume in males (Figure 2 bar 1 vs. 7: 46.4%±5.6 vs. 25.5%±6.7 n=10), whereas the loss of PARP-1 led to an exacerbation of injury in gonadally intact females (Figure 2 bar 3 vs. 9: 31.8%±3.9 vs. 52.7%±5.4 n=10). Nicotinamide treatment decreased stroke size in the WT male brain (Figure 2 bar 1 vs. 2: 46.4%±5.6 vs. 30.8%±5.2 n=10), and further reduced infarction volume in PARP-1 KO males (Figure 2 bar 7 vs. 8: 30.8%±5.2 vs. 8.9%±3.9 n=10). Infarct size in WT females was unaffected by nicotinamide treatment (Figure 2 bar 3 vs. 4: 31.8%±3.9 vs. 32.1%±4.9 n=10), but infarct was smallest in this group, due to the protective effect of endogenous estrogen. However, ovariectomized WT females also did not show a significant benefit suggesting this effect is independent of activational hormone effects. Interestingly, although the PARP-1 KO female brain was extremely vulnerable to ischemic insult, nicotinamide treatment drastically reduced infarction volume compared to vehicle (Figure 2 bar 9 vs. 10: 52.7%±5.4 vs. 21.5%±4.8 n=10). These data suggested that NAD+ repletion protects male, PARP-1 KO male, and PARP-1 KO female brains.
Figure 2. Sex Differences in Response to Nicotinamide Treatment.
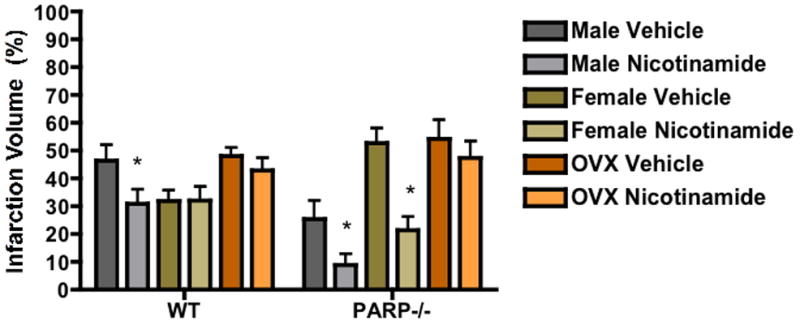
Nicotinamide was administered i.p. (500mg/kg) 20 minutes before MCAO to male, female, and Ovx female WT and PARP-1−/− mice (n=10/group: p<0.05). Infarction volume was measured 24 hours after ischemia. Nicotinamide was protective in WT males, PARP-1−/− males, and PARP-1−/− females.
3.1.3 Sex Differences in Caspase Activity in WT and PARP-1−/−
As caspase-mediated cell death is an energy dependent process and plays an integral role in ischemic cell death in the female brain (Siegel et al., 2010), we examined the effect of nicotinamide treatment on caspase activity. Caspase activity was determined by measuring Caspase-3 cleavage by a specific target of caspases, αII-Spectrin (Williams et al., 2003). No spectrin cleavage was seen in sham brains of either sex or either genotype (Figure 3). Stroke induced spectrin cleavage in both males and females. WT females demonstrated a significant stroke-induced increase in both the activity (Figure 3A lanes 2 vs. 4 p<0.05) and nuclear translocation (Figure 3B lanes 2 vs. 4 p<0.05) of Caspase-3 as compared to males. PARP-1 KO females exhibited the same stroke-induced increase in activity (Figure 3C lanes 2 vs. 4 p<0.05) and nuclear translocation (Figure 3D lanes 2 vs. 4 p<0.05) of Caspase-3 as did males. Next, the effect of nicotinamide treatment was examined in relation to activity and nuclear translocation of Caspase-3.
Figure 3. Sex Differences in Caspase-3 Activity.
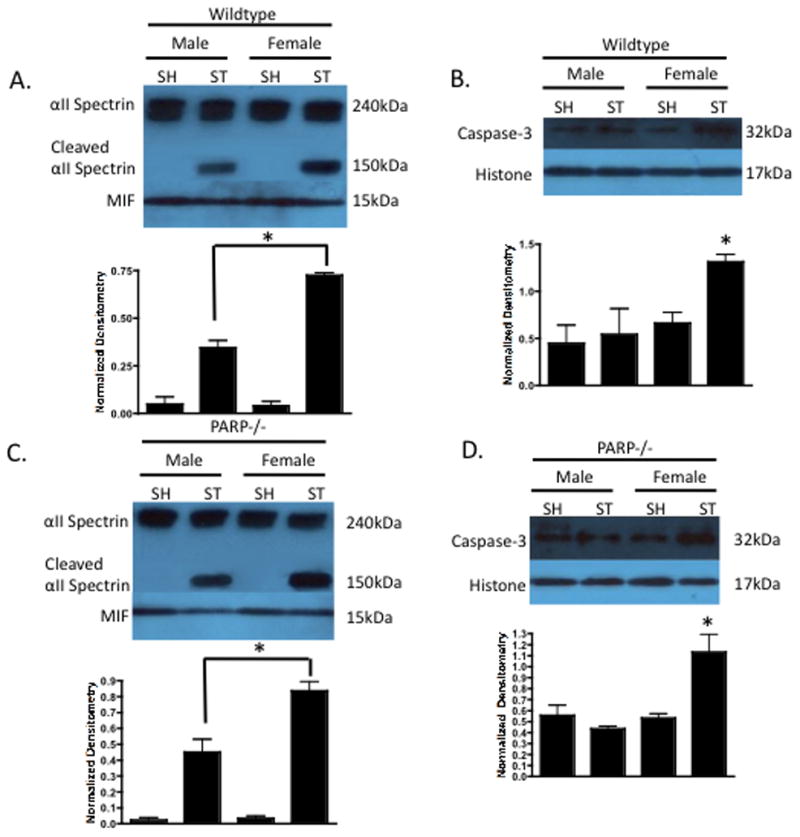
(A) Wildtype male and female cytosolic fractions 6 hours after 90 minute MCAO were probed for caspase activity using αII-Spectrin cleavage (150kDa). MIF was utilized a cytosolic purity and loading control. Females exhibited increased caspase activity compared to males after stroke. (B) Wildtype nuclear fractions 6 hours after 90 minute MCAO were probed for caspase-3 with Histone as a nuclear purity and loading control. Females exhibited a stroke-induced increase in nuclear caspase-3 compared to males. (C) PARP-1−/− females had increased caspase activity and nuclear caspase-3 (D) as compared to the ischemic male brain.
3.1.4 The Effect of Nicotinamide on Caspase Activity in the Ischemic Female Brain
Interestingly, vehicle treated PARP-1 KO females had a significant increase in caspase activity (Figure 4A lanes 2 vs. 4 p<0.05) and nuclear translocation of caspase-3 (Figure 4B lanes 2 vs. 4 p<0.05) compared to WT females, suggesting that this pathway is up-regulated in PARP-1 KO females. Nicotinamide treatment completely ameliorated the stroke-induced caspase activity (Figure 4C lanes 2 vs. 4 p<0.05) and translocation (Figure 4D lanes 2 vs. 4 p<0.05) of Caspase-3 in both WT females and PARP-1 KO females.
Figure 4. Nicotinamide Alters Caspase Activity in Females.
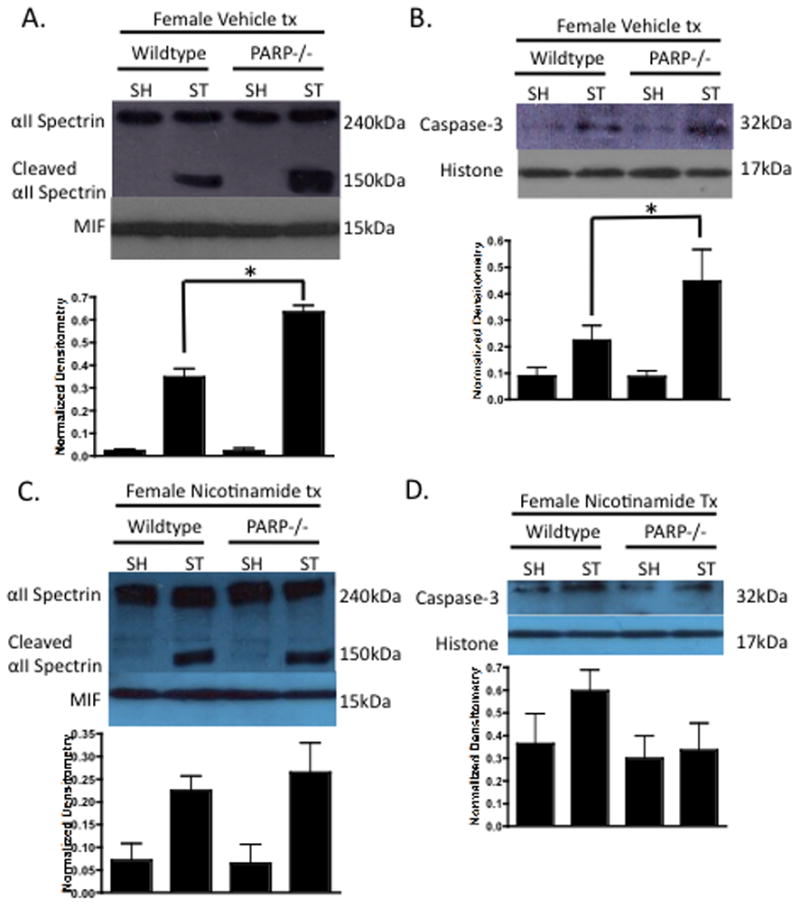
(A) Vehicle treated female WT and PARP-1−/− cytosolic fractions 6 hours after 90 minute MCAO were probed for caspase activity using αII-Spectrin cleavage (150kDa). MIF was utilized a cytosolic purity and loading control. PARP-1−/− females exhibited increased caspase activity compared to WT females after stroke. (B) Vehicle treated female nuclear fractions 6 hours after 90 minute MCAO were probed for caspase-3 with Histone as a purity and loading control. PARP-1−/− females exhibited a stroke-induced increase in nuclear caspase-3 compared to WT. (C) Nicotinamide treated WT and PARP-1−/− females illustrated equivalent caspase activity and nuclear caspase-3 (D).
3.1.5 The Effect of PARP-1 deletion in Males and PARP-1 Gene Deletion Combined with Nicotinamide Treatment in Females
The deletion of PARP-1 led to no significant changes in caspase activity (Figure 5A lanes 2 vs. 4 p<0.05) or nuclear translocation (Figure 5B lanes 2 vs. 4 p<0.05) in the ischemic male brain. Caspase-3 activity (Figure 5C lanes 2 vs. 4 p<0.05) and nuclear translocation (Figure 5D lanes 2 vs. 4 p<0.05) were drastically reduced in nicotinamide treated PARP-1 KO females as compared to vehicle treated PARP-1 KO females. These data suggest that nicotinamide may be able to overcome the detrimental effects of PARP-1 gene deletion in the female brain, as the PARP-1 KO female is more vulnerable to ischemic insult due to increased caspase activity.
Figure 5. Sex Differences in Caspase-3 Activity in PARP-1−/−.
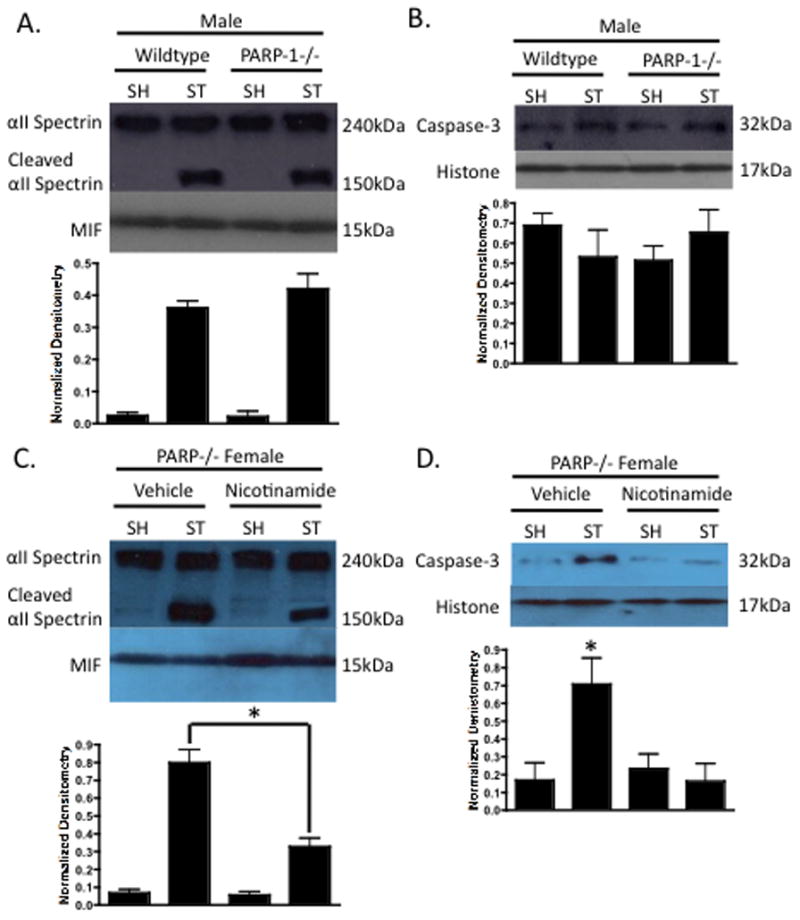
(A) Male WT and PARP-1−/− cytosolic fractions 6 hours after 90 minute MCAO were probed for caspase activity using αII-Spectrin cleavage (150kDa). MIF was utilized a cytosolic purity and loading control. No difference in caspase activity was found between male WT and PARP-1−/− animals. (B) Male WT and PARP-1−/− nuclear fractions 6 hours after 90 minute MCAO were probed for caspase-3 with Histone as a purity and loading control. Equivalent nuclear caspase-3 was observed in both groups. (C) Nicotinamide treated PARP-1−/− females exhibited a stroke-induced decrease in caspase activity, and nuclear caspase-3 (D) as compared to vehicle treated PARP-1−/− females.
4.1 DISCUSSION
The activation of PARP-1 has been shown to be a major inducer of cell death in the ischemic male brain (Siegel and McCullough, 2010). Although, gene deletion or pharmacological inhibition of PARP-1 is neuroprotective in males, it paradoxically exacerbates ischemic injury in females (McCullough et al., 2005), which was the focus of this study. The main substrate for PARP-1 activity is NAD+ (Chiarugi, 2002) and neuronal death arises in part due to excessive activation of PARP-1, subsequent NAD+ depletion and consequent energy failure. This study has discovered several important findings regarding sex differences in NAD+ and its role in caspase activation. WT males experienced a significant loss of NAD+ both during and after ischemia, while loss of PARP-1 eliminated the stroke-induced loss of NAD+ (Figure 1). This is consistent with previous work showing that PARP-1 induces cell death by depleting intracellular NAD+ (Ying et al., 2007) and this is substantiated by the amelioration of ischemia-induced drop in NAD+ in PARP knockout males. WT females had lower baseline NAD+ levels compared to males, but did not show significant NAD+ loss either during stroke or in early reperfusion. Importantly, PARP-1 KO females had a significant intra-ischemic loss of NAD+ (Figure 1); similar to what was seen in WT males, although NAD+ levels recovered 30 minutes after reperfusion. This is the first report of sex specific NAD+ changes in WT or PARP-1 KO mice and definitively ties energy depletion (NAD+ loss) to PARP-1 activation in males. Surprisingly, ovary-intact females had minimal NAD+ loss. It is possible that this simply reflects the improved energy dynamics secondary to the significantly smaller infarct seen in intact females; however NAD+ levels also differed at baseline between the sexes (Figure 1). Caspase activity and translocation were also increased in the ischemic female brain as compared to males, despite the smaller infarcts (Figure 3). Ovx females showed a more “male-like” pattern of NAD+ loss (Figure 1). Importantly, in PARP-1 knockout animals, this pattern was reversed; the NAD+ depletion seen in WT males was ameliorated in PARP-1 KO males, whereas the loss of PARP-1 exacerbated energy failure and NAD+ loss in females.
Previous studies have shown that PARP-1 deletion leads to a loss of PAR polymer formation, a measure of PARP-1 activation in both male and female PARP-1 KO mice, but despite an almost complete lack of PAR and subsequent AIF translocation, neuroprotection is only seen in the male brain (Yuan et al., 2009). This suggests that there is some alternative mechanism that leads to the exacerbation of injury seen in PARP deficient females. The precursor of NAD+, nicotinamide, has been shown to enhance NAD+ levels (Canto et al., 2012). NAD+ repletion by nicotinamide has been shown to be protective in the males both in vitro and in vivo (Klaidman et al., 2003, Sadanaga-Akiyoshi et al., 2003, Chong et al., 2005, Liu et al., 2009a, Suzuki et al., 2010) via preservation of ATP and improved energy dynamics. NAD+ is a substrate and energy source for activity of the PARP-1 enzyme (Belenky et al., 2007). In this study, Nicotinamide was protective in WT males, PARP-1 KO males, and PARP-1 KO females (Figure 2) but not in WT females. This may also be confounded by the smaller infarct seen in intact females (a possible floor effect), but nicotinamide did not statistically reduce infarct in ovx females despite their large infarcts. In addition, nicotinamide further reduced infarct in PARP-1 KO males despite the fact that this cohort had the smallest infarcts, making a floor effect less likely. This also suggests that nicotinamide also protects by mechanisms other than reducing PARP activation likely via preservation of mitochondrial energy dynamics, as has been suggested by others (Klaidman et al., 2003).
Caspase-mediated cell death has been shown to play an integral role in the ischemic female brain (Lang and McCullough, 2008). Caspase activation is ATP dependent process (Delivoria-Papadopoulos et al., 2007), and ATP levels are significantly decreased during stroke (Sims and Anderson, 2002). Studies also suggest that NAD+ and ATP levels are tightly correlated in ischemic brain (Klaidman et al., 2003). NAD+ depletion has been linked to mitochondrial membrane depolarization and mitochondrial permeability transition pore (MPT) formation (Chong et al., 2004). Disruption of the mitochondrial membrane leads to cytochrome-c release and activation of the caspase cascade (Renolleau et al., 2008, Broughton et al., 2009). Caspase-3 activity was measured by cleaved αII-Spectrin and nuclear translocation of Capase-3. As expected, females had more caspase activity and nuclear translocation as compared to males (Figure 3). PARP-1 KO females, which have exacerbated infarction volumes (Figure 2), also experienced increased caspase activity that and this was ameliorated with nicotinamide treatment (Figure 4). Previous work also suggests PARP-1 KO shifts the cell death program from caspase-independent to caspase-dependent mechanisms in XX neuronal cultures (Sharma et al., 2011). Similarly, nicotinamide treatment decreased caspase activation in PARP-1 KO females to levels seen in WT females (Figure 4,5). These data suggest nicotinamide treatment is protective in the PARP-1 KO female brain as it decreases caspase activation.
These data are also in agreement with a previous study, which illustrated caspase inhibition was neuroprotective in PARP-1−/− females (Liu et al., 2011). Caspases have been shown to interact and cleave PARP-1 (Ferrer and Planas, 2003). The data presented in this study suggest that the loss of PARP-1 is detrimental in the ischemic female brain due to associated increase in caspase activity and the inherent sensitivity of females to caspase-induced cell death. Interestingly, due to the predominance of PARP-mediated cell death in the ischemic male brain, males are less affected by caspase activation (Siegel and McCullough, 2010, Siegel et al., 2010), and show less benefit than females from caspase inhibition.
It has been well established that estrogen is neuroprotective in experimental stroke models (Hurn and Brass, 2003). In this study only WT ovx females experienced a stroke-induced loss of NAD+ 60 minutes into ischemia (Figure 1). NAD+ levels were unchanged in PARP-1−/− ovx female mice, and nicotinamide treatment did not protect the ovx female brain. It has been suggested that estrogen and the estrogen receptor may play a role in the actions PARP-1. The estrogen receptor has been shown to complex with PARP-1, which is enhanced by estrogen, and inhibits the ability of PARP-1 to recognize DNA strand breaks (Szabo et al., 2006). This could account in part for the ability of the female brain to withstand PARP-1 mediated cell death as compared to males. The loss of estrogen would enhance PARP-1 mediated cell death making the ischemic ovx female brain more similar to the ischemic male brain.
This study suggested that treatment with an NAD+ precursor, nicotinamide (Maiese et al., 2009), led to decreased ischemic damage in males, PARP-1 KO males, and PARP-1 KO females (Figure 2). Nicotinamide was utilized as a tool to further understand the mechanism by which NAD+ loss occurs in stroke, and therefore was given at the time of stroke. The clinical potential of nicotinamide has yet to be elucidated, especially in females. A previous study found treatment with nicotinamide was neuroprotective when administered 2 hours after stroke (Mokudai et al., 2000). Further studies are necessary to determine whether nicotinamide is an appropriate therapeutic target as the AIM HIGH trial found a surprising increase in stroke incidence when extended-release high dose Niacin (which is converted to nicotinamide in vivo, but may not have the same pharmacological effects) was combined with statins but sex specific outcomes were not reported (Boden et al., 2011).
Nicotinamide has been shown to be involved in multiple pathways including oxidative stress, cell survival, and death (Belenky et al., 2007, Maiese et al., 2009) as well as energy failure; therefore alternative pathways may also play a role. We did not directly measure ATP levels in this study as the focus was on the interaction between PARP and NAD in the different sexes. Several other studies have shown that levels of ATP and NAD+ are highly correlated and that nicotinamide supplementation prevents both the depletion of ATP and NAD at 6 and 24 h of reperfusion in males (Yang et al., 2002, Klaidman et al., 2003).
Energy dynamics in stroke, especially in combination with studies of sex differences, remain understudied. The data presented in this study raises many questions regarding the possible upstream contribution to the sex differences seen in NAD+ and PARP-1 activity. While these experiments focused on the PARP-1-mediated NAD+ depletion, studying the sub-cellular distribution of NAD+ is an important future direction of study. At this point it is not known if sex differences exist in either the synthesis or mitochondrial utilization of NAD+. We attempted to determine both cytosolic and mitochondrial NAD+ levels initially but this presented significant technical challenges. The fractionation techniques take considerable time and this led to rapid NAD+ degradation. We therefore were unable to fractionate our samples as the method for assessing NAD+ levels required the tissue to be immediately placed into extraction buffer to preserve NAD+, which lysed all cell membranes and is a recognized limitation of these studies.
Another family of proteins that has garnered much recent attention are Sirtuins. These proteins are members of the protein deacetylase family, which are the only histone deactyalses (HDACs) that require NAD for their enzymatic activity (Imai et al., 2000). Sirtuins play a role in both energy metabolism and longevity. Previous studies have shown that SIRT-1 plays a role in cerebral ischemia and other neurodegenerative diseases (Sun et al., 2010), but sex differences in the expression or function of this protein family have not yet been evaluated. SIRT-6 has been implicated in increasing lifespan in male mice, which is one of the first reports of sex differences in any member of the Sirtuin family (Kanfi et al., 2012).
The lack of neuroprotection in females was somewhat surprising, and requires further study and confirmation in other models and by other groups. Interestingly, PARP-1 deletion clearly exacerbated energy failure in females and nicotinamide treatment ameliorated this effect (Figure 2) due to its effect on Caspase-3 activity. If estrogen normally restrains energy failure or PARP activation, nicotimamide may still have considerable clinical utility as the vast majority of women with stroke are well past menopause. However the lack of effect in acutely ovx females suggests that further studies are needed before considering translation to the clinic. Utilizing aged animals rather than young ovx animals is likely to be a better “translational” approach as seen with recent studies examining the PARP inhibitor Minocycline. Sex differences in the neuroprotective response were seen in young animals in a reperfusion model, but not in an embolic clot model or in aged mice (Hoda et al., 2011). Overall, this study suggests that sex differences may be present in cellular energy dynamics and apoptosis. Potential sex differences in mitochondrial function, longevity and aging (Tower, 2006, Hughes and Hekimi, 2011) have only recently been identified and remain to be explored in stroke.
Highlights.
Cell death pathways differ between the sexes.
Poly (ADP-ribose) polymerase (PARP) gene deletion decreased stroke damage in males and increased damage in females.
Stroke induced changes in NAD+ differed in the male and female brain.
Nicotinamide reduced infarct in wild-type males and PARP-1 knockout mice of both sexes but not in wild-type females.
Energy metabolism may differ between the sexes.
Acknowledgments
This work was supported by the NIH (pre-doctoral NRSA to CS and RO1 NS055215-06 to LDM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A. Poly(ADP-ribose) polymerase: killer or conspirator? The ‘suicide hypothesis’ revisited. Trends Pharmacol Sci. 2002;23:122–129. doi: 10.1016/S0165-6147(00)01902-7. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M, Gorn M, Ashraf QM, Mishra OP. ATP and cytochrome c-dependent activation of caspase-9 during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett. 2007;429:115–119. doi: 10.1016/j.neulet.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34:338–341. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Benashski SE, Siegel C, Liu F, McCullough LD. Adenosine monophosphate activated protein kinase inhibition is protective in both sexes after experimental stroke. Neurosci Lett. 2010;482:62–65. doi: 10.1016/j.neulet.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009a;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke. 2011;42:1090–1096. doi: 10.1161/STROKEAHA.110.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009b;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin B(3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke. 2000;31:1679–1685. doi: 10.1161/01.str.31.7.1679. [DOI] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14:46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- Sadanaga-Akiyoshi F, Yao H, Tanuma S, Nakahara T, Hong JS, Ibayashi S, Uchimura H, Fujishima M. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res. 2003;28:1227–1234. doi: 10.1023/a:1024236614015. [DOI] [PubMed] [Google Scholar]
- Sharma J, Nelluru G, Wilson MA, Johnston MV, Hossain MA. Sex-specific activation of cell death signalling pathways in cerebellar granule neurons exposed to oxygen glucose deprivation followed by reoxygenation. ASN Neuro. 2011;3 doi: 10.1042/AN20100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, McCullough LD. NAD+ depletion or PAR polymer formation: which plays the role of executioner in ischaemic cell death? Acta Physiol (Oxf) 2010 doi: 10.1111/j.1748-1716.2010.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: possible molecular mechanisms. J Neurosci Res. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Okuda H, Nishida K, Fujimoto S, Nagasawa K. Protective effect of nicotinamide against poly(ADP-ribose) polymerase-1-mediated astrocyte death depends on its transporter-mediated uptake. Life Sci. 2010;86:676–682. doi: 10.1016/j.lfs.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Szabo C, Pacher P, Swanson RA. Novel modulators of poly(ADP-ribose) polymerase. Trends Pharmacol Sci. 2006;27:626–630. doi: 10.1016/j.tips.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ST, Smith AN, Cianci CD, Morrow JS, Brown TL. Identification of the primary caspase 3 cleavage site in alpha II-spectrin during apoptosis. Apoptosis. 2003;8:353–361. doi: 10.1023/a:1024168901003. [DOI] [PubMed] [Google Scholar]
- Ying W, Garnier P, Swanson RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]


