SUMMARY
Elevated levels of circulating tissue factor-bearing microparticles (TFMP) have been associated with an increased risk of developing venous thromboembolism (VTE) in cancer patients. We performed a randomized phase II study to evaluate the cumulative incidence of VTE in advanced cancer patients with lower levels of TFMP not receiving thromboprophylaxis and those with higher levels of circulating TFMP randomized to enoxaparin or observation. The cumulative incidence of VTE at 2 months in the higher TFMP group randomized to enoxaparin (N=23) was 5.6% while the higher TFMP group observation arm (N=11) was 27.3% (Gray test P=0.06). The cumulative incidence of VTE in the low TFMP was 7.2% (N=32). No major haemorrhages were observed in the enoxaparin arm. The median survival for patients with higher levels of TFMP followed by observation was 11.8 months compared with 17.8 months on enoxaparin (P=0.58). In a prospective randomized trial, increased numbers of circulating TFMP detected by impedance flow cytometry identified cancer patients with a high incidence of VTE. Enoxaparin demonstrated a clear trend towards reducing the rate of VTE in patients with elevated levels of TFMP, with an overall rate of VTE similar in magnitude to the lower TFMP group.
Keywords: microparticles, venous thromboembolic events, cancer associated thrombosis, low molecular weight heparin
INTRODUCTION
Low molecular weight heparin (LMWH) has established efficacy in the treatment of venous thromboembolism (VTE) in cancer, but its role preventing thrombosis in cancer outpatients is a matter of debate. Large randomized clinical trials have demonstrated about a 50% relative risk reduction in thrombotic events in cancer cohorts treated with prophylactic LMWH (Agnelli, et al 2012, Agnelli, et al 2009). However, with an overall VTE rate of only 3–5%, oncologists have resisted prescribing LMWH to unselected asymptomatic outpatients with advanced cancer. A targeted approach to preventing VTE in cancer outpatients who are at a high risk of VTE is therefore warranted.
Pathological changes in microparticle populations contribute to the hypercoagulability associated with various disease states, including malignancy. Microparticles are vesicular structures measuring less than 1 μm in size and are derived from a number of cells within the vascular compartment including leucocytes, platelets, red blood cells, and endothelial cells (Zwicker, et al 2011). Some microparticles are considered procoagulant due to the exposure of negatively charged phospholipids on the external membrane leaflet for the support of thrombin generation (Chang, et al 1993, Satta, et al 1994). Subpopulations of microparticles also express tissue factor, the in vivo initiator of blood coagulation (Falati, et al 2003, Giesen, et al 1999). Circulating tissue factor-bearing microparticles augment thrombus formation in animal models (Chou, et al 2004) and tumor-derived microparticles accumulate at the site of vascular injury (Thomas, et al 2009).
We previously evaluated the hypothesis that tumour-derived circulating microparticles expressing tissue factor are associated with an increased risk of thrombosis in cancer patients (Zwicker, et al 2009). In a case control study, elevations in plasma tissue factor-bearing microparticles measured by impedance-based flow cytometry were associated with a 4-fold increased risk of VTE in cancer outpatients. We now report the results of a randomized prospective trial of LMWH (enoxaparin) versus observation in preventing VTE in cancer patients with higher levels of plasma tissue factor-bearing microparticles. We also assessed the rates of VTE in patients with lower levels of circulating tissue factor-bearing microparticles who did not receive thromboprophylaxis. This study demonstrated that higher levels of tissue factor-bearing microparticles identify a cancer cohort at substantially higher risk of developing VTE and that enoxaparin demonstrated a clear trend towards reducing the rate of VTE in such patients.
METHODS
Eligibility
Participants were required to have histologically confirmed malignancy for which standard curative therapies do not exist. Eligible malignancies included: adenocarcinoma of the pancreas (locally advanced or metastatic), colorectal (stage IV), non-small cell lung cancer (stage III or IV), relapsed or stage IV ovarian, or surgically unresectable or metastatic gastric adenocarcinoma. All patients were required to be within four weeks of first or second line therapy for the malignancy, a life expectancy estimated to be greater than six months, and an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2. The following clinical laboratory values were required: absolute neutrophil count ≥ 1.0 × 109/l, platelet count ≥ 100 × 109/l, aspartate transaminase or alanine transaminase ≤3.0-fold upper limit of normal, creatinine clearance ≥ 40 ml/min. Patients were excluded if they had known brain metastases, history of VTE within the previous five years, or any history of significant haemorrhage requiring transfusion or hospitalization within the last 5 years (outside of a surgical setting). Additional exclusion criteria included: history of allergy to heparin compounds, history of heparin-induced thrombocytopenia, prothrombin time or partial thromboplastin time >1.2-fold upper limits of normal, a familial bleeding diathesis, disseminated intravascular coagulation, or requirement for anticoagulation or an antiplatelet agent (>81 mg aspirin daily). All patients voluntarily gave written informed consent prior to initiation of study procedures. The protocol was approved by the institutional review boards of the 10 participating medical centres and centrally by the Dana Farber/Harvard Cancer Center. Study coordination, randomization, and monitoring were performed by the Quality Assurance Office for Clinical Trials (QACT) at Dana Farber/Harvard Cancer Center.
Study Design
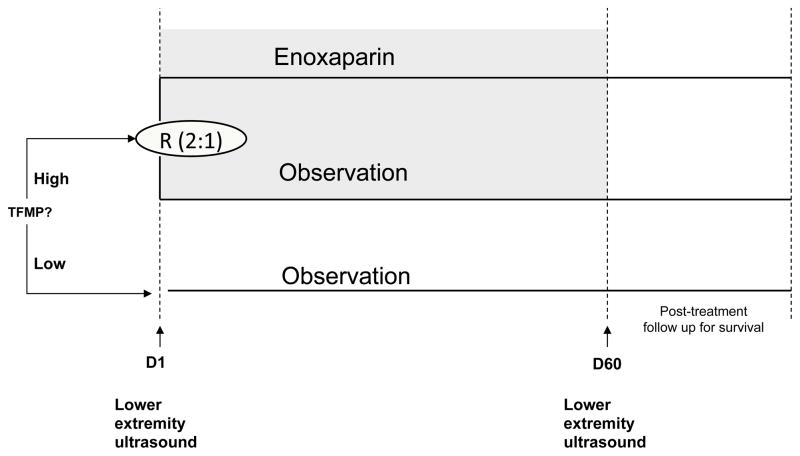
The study was a randomized phase II trial to evaluate the cumulative incidence of VTE in cancer outpatients (Figure 1). Following measurement of tissue factor-bearing microparticle in plasma, those with higher levels were randomized (2:1) to enoxaparin 40 mg subcutaneously once daily or observation. Randomization was stratified based on cancer diagnosis. Participants with lower levels of tissue factor-bearing microparticles were observed without anticoagulation. Both the treating physicians and patients were blinded to microparticle status in the observation arms.
Figure 1. MicroTEC trial schema.
The plasma concentration of tissue factor-bearing microparticles (TFMP) was measured following enrollment. Individuals with higher concentrations of tissue factor-bearing microparticles were randomized 2:1 to enoxaparin 40 mg daily versus observation. Individuals with low tissue factor-bearing microparticles were followed without anticoagulation. Study subjects were scheduled to undergo lower extremity ultrasound on Day 1 and Day 60 to evaluate for an incidental proximal deep vein thrombosis.
Baseline and follow-up VTE assessment
Bilateral lower extremity ultrasound evaluations were performed following randomization and at day 60 (± 7 days) in order to assess for the presence of an incidental proximal deep vein thrombosis. The cumulative incidence of venous thromboembolic events at 2 months was the primary endpoint. The composite VTE endpoint included any symptomatic proximal or distal lower extremity deep vein thrombosis, symptomatic pulmonary embolism or fatal pulmonary embolism diagnosed by autopsy, or asymptomatic proximal deep vein thrombosis diagnosed by screening compression ultrasound. Criteria for diagnosis of qualifying VTE included: new non-compressible lower extremity deep venous segment by compression ultrasound (below the popliteal vein thrombus qualified only if symptomatic); intraluminal defects in two or more views on pulmonary angiography; sudden contrast cut-off of one or more vessels greater than 2.5 mm in diameter on a pulmonary angiogram; a high probability ventilation/perfusion (VQ) lung scan showing one or more segmental perfusion defects with corresponding normal ventilation (mismatch defect); abnormal spiral computed tomography (CT) showing thrombus in pulmonary vessels (subsegmental or larger). Major haemorrhage was defined according to International Society on Thrombosis and Haemostasis guidelines (Schulman and Kearon 2005). Toxicity and endpoint monitoring were performed every 30 days. Patients were followed for survival every 3 months upon completion of the 60-day active study period.
Measurement of tissue factor-bearing microparticles and D-dimer
Blood was drawn by peripheral venepuncture into 3.2% citrate. Plasma was separated at 2100 g for 20 min within 1 h of specimen collection. A second centrifugation was performed at 2100 g for 20 min to generate platelet-free plasma and stored in aliquots at −80°C until analysis. Measurement of tissue factor-bearing microparticles was performed by impedance-based flow cytometry using a Beckman Coulter SC Quanta flow cytometer modified specifically for microparticle analysis (Beckman Coulter, Miami, FL) (Zwicker, et al 2009). Humanized monoclonal antibodies cH36 against human tissue factor (generously provided by Altor Bioscience, Miramar, FL) and purified human IgG control antibodies (Sigma Aldrich, St Louis, MO) were labelled with Alexa 488 (Invitrogen, Grand Island, NY). The concentration of tissue factor-bearing microparticles for each plasma sample was measured in triplicate along with internal positive and negative controls as previously described (Zwicker, et al 2009). Determination of higher tissue factor-bearing microparticle concentration was performed using a reference repository of plasmas from sixty cancer patients. The top tercile of tissue factor-bearing microparticle concentrations from the reference specimens (3.5 × 104 microparticles/μl) was considered the cut-off point for “high” and corresponds with previously described “detectable” levels. D-dimer was measured in plasma thawed at 37°C using a commercial assay (Roche Diagnostics, Indianapolis, IN).
Statistical analysis
The aim of this phase II trial was to estimate the cumulative incidence of symptomatic or proximal venous thromboembolic events (VTE) at two months in subjects with locally advanced or metastatic cancer, based on higher or lower levels of tissue factor-bearing microparticles. The study was originally initiated as a phase III trial but in response to external constraints was re-configured as a randomized phase II trial with the primary objective of prospectively determining the cumulative incidence of VTE in the three arms. Patients were analysed on an intention-to-treat basis following randomization. Based on our previous observation regarding the cumulative incidence of VTE in patients with high levels of tissue factor-bearing microparticles not treated with primary thromboprophylaxis, we anticipated a cumulative rate in this group of approximately 40% as compared to 5% in the group treated with enoxaparin (Zwicker, et al 2009). A true cumulative incidence rate of 5% would be considered therapeutically promising in a high tissue factor-bearing microparticle arm receiving enoxaparin. Based on 90% exact binomial confidence intervals, we estimated that with 70 enrolled patients that there would be a 90% chance of concluding that the treatment is effective when the true cumulative incidence rate was 5%, and less than a 10% chance of concluding the treatment was effective when the true cumulative incidence rate was 40% or greater.
The cumulative incidence of VTE was assessed by a competing risk analysis; the Fine and Gray regression model was used to estimate the hazard ratio for the primary comparison and differences in the incidence rates of the high tissue factor-bearing microparticle arms were evaluated by the Gray test (Campigotto, et al 2012, Fine and Gray 1999, Gray 1988). Statistical significance was defined as P value <0.05. Differences between the baseline characteristics for categorical variables (sex, diagnosis, stage, ECOG performance status, and number of prior lines of therapy) were analysed by 2-sided Fisher exact test. Median values were calculated for continuous variables including age, months from cancer diagnosis to registration, haemoglobin, white blood cell count, platelet count, and body-mass index; differences between the three groups were assessed by 2-sided Kruskal Wallis test. Long-term survival analysis according to higher or lower tissue factor-bearing microparticle concentrations was performed by Kaplan-Meier method and statistical differences assessed by the log-rank test. The Spearman’s rank correlation coefficient was used to assess the linear dependence between concentration of tissue factor-bearing microparticles at baseline and other baseline quantitative measurements.
RESULTS
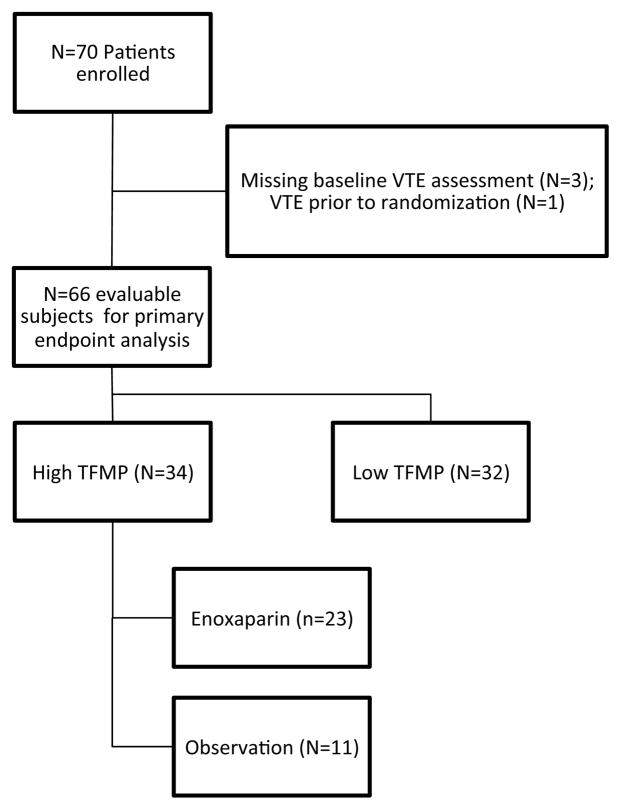
Seventy patients were enrolled in the study across 10 study sites. A total of 66 patients were evaluable for primary endpoint analysis (Figure 2). Four patients were excluded from analysis due to the absence of assessment for deep vein thrombosis in three and diagnosis of VTE prior to randomization in one. Among the cancer patients with higher circulating tissue factor-bearing microparticles, 23 were randomized to enoxaparin and 11 were followed without thromboprophylaxis. There were 32 cancer patients monitored on the observation arm with lower levels of tissue factor-bearing microparticles. The baseline characteristics for the three arms are shown in Table I. The predominant cancer diagnosis was pancreatic cancer (30 of 66), followed by non-small lung cancer (21 of 66), and colorectal cancer (15 of 66). The majority of patients had metastatic disease (N=52, 78.8%), ECOG performance status of 0 or 1 (N=61, 92.4%), and were receiving initial chemotherapy for their malignancy (N=51, 77.3%). The median time from cancer diagnosis to registration was 1.5 months and did not differ between three groups (2-sided Kruskal-Wallis test P=0.20). There were no statistically significant differences measured between the three groups in terms of baseline characteristics including age, gender, body-mass index, diagnosis, stage, number of prior chemotherapy regimens, or performance status. The median baseline white blood cell count (6.95 × 109/l), haemoglobin (125 g/l), and platelet count (246.5 × 109/l) were similar across the groups (P > 0.05).
Figure 2. Flow diagram of MicroTEC enrollment.
A total of 70 patients were enrolled in the trial with data available for primary endpoint venous thromboembolism (VTE) analysis in 66 patients (34 with high levels of tissue factor-bearing microparticles (TFMP) and 32 with lower levels of tissue factor-bearing microparticles).
TABLE I.
Baseline Characteristics
| Characteristic | High TFMP (Enoxaparin) N=23* | High TFMP (Observation) N=11* | Low TFMP (Observation) N=32* |
|---|---|---|---|
| Median Age, years (range) | 68.1 (46.6–80.1) | 67.5 (28.8–78.7) | 62.8 (42.7–83.8) |
| Male gender, n (%) | 14 (61%) | 5 (46%) | 19 (59%) |
| Median BMI, kg/m2 (range) | 23.8 (16.6–31.6) | 23.8 (20.0–34.4) | 26.3 (19.0–48.7) |
| Diagnosis, n (%) | |||
| Pancreatic Cancer | 11 (48) | 6 (54) | 13 (41) |
| Non-small cell lung | 9 (39) | 3 (27) | 9 (28) |
| Colorectal | 3 (13) | 2 (18) | 10 (31) |
| Stage, n (%) | |||
| III | 5 (22) | 2 (18) | 7 (22) |
| IV | 18 (78) | 9 (82) | 52 (78) |
| ECOG Status, n (%) | |||
| 0 | 7 (30.5) | 5 (45) | 14 (44) |
| 1 | 15 (65) | 3 (27.5) | 17 (53) |
| 2 | 1 (4.5) | 3 (27.5) | 1 (3) |
| Number of prior lines of therapy, n (%) | |||
| 0 | 18 (78.3) | 9 (81.8) | 24 (75) |
| 1 | 5 (21.7) | 2 (18.2) | 8 (25) |
No statistically significant differences (P>0.05) in baseline characteristics were detected for the three arms (analysis by 2-sided Fisher exact or Kruskal Wallis tests).
TFMP, tissue factor-bearing microparticles; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Cumulative incidence of VTE
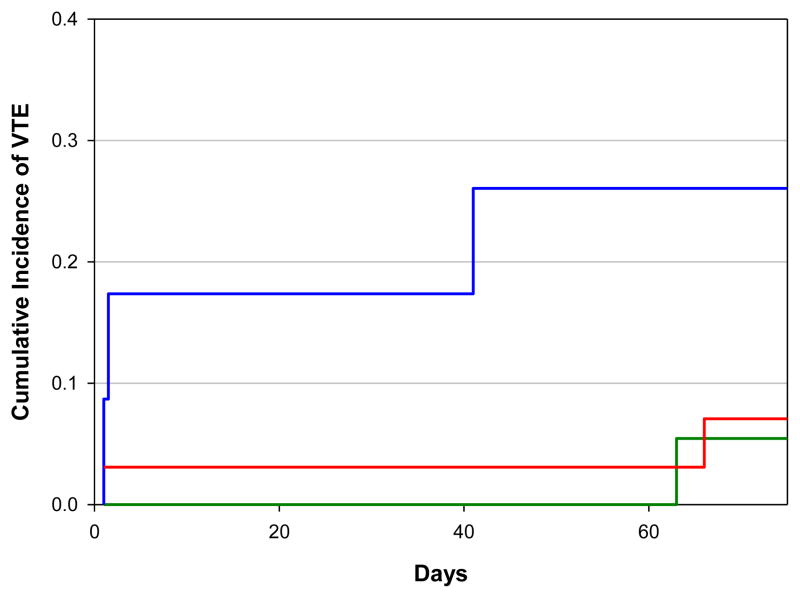
The cumulative incidence of VTE at 2 months in cancer patients with higher levels of tissue factor-bearing microparticles receiving enoxaparin was 5.6% (95% confidence interval [CI], 0 to 16.6%) versus 27.2% (95% CI, 0 to 55.1%) in the group not receiving primary thromboprophylaxis (Figure 3). Subjects with higher tissue factor-bearing microparticles randomized to observation were 7 times more likely to develop a VTE than those receiving enoxaparin (hazard ratio of 6.70, 95% CI 1.03 to 43.17, Gray’s test P value=0.06). The overall rate of VTE at 2 months in those with lower levels of tissue factor-bearing microparticles followed without primary thromboprophylaxis was 7.2% (95% CI, 0 to 17.1%). There were a total of 6 venous thromboembolic complications diagnosed among the 3 groups. Five of the deep vein thrombi were incidental and diagnosed by protocol-mandated lower extremity ultrasound evaluation. The deep vein thrombi were either bilateral (N=2) or included the superficial femoral and popliteal veins (N=3). One patient developed shortness of breath and was diagnosed with a pulmonary embolism in the right middle lobe pulmonary artery by CT angiogram. One sudden death occurred during the observation period in a patient who acutely developed respiratory distress and died prior to the arrival of emergency medical services. Autopsy was declined and the event was not considered a primary endpoint (study subject was randomized to the higher microparticle observation arm).
Figure 3. Cumulative incidence of venous thromboembolism with higher or lower concentration of tissue factor-bearing microparticles.
The cumulative incidence of venous thromboembolism (VTE) at 2 months in the group of patients with higher levels of tissue factor-bearing microparticles randomized to observation was 27.2% (blue) compared with 5.6% (green) in the group treated with enoxaparin (Gray test P value =0.06). The cumulative incidence of VTE at 2-months for the cohort with lower levels of tissue factor-bearing microparticles was 7.2% (red).
Safety and overall survival
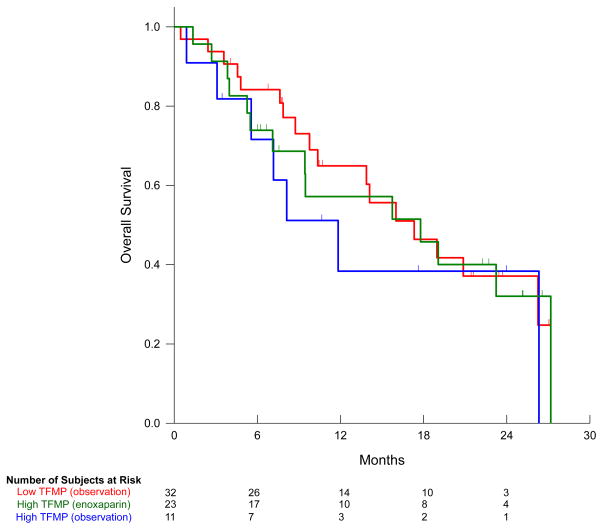
Overall, there were no major haemorrhages or serious adverse events attributed to the use of enoxaparin. One study subject with pancreatic cancer on the observation arm died following an upper gastrointestinal bleed. The median survival for patients with high levels of tissue factor-bearing microparticles on observation was 11.8 months (95%CI, 5.4 to 18.2 months) compared with 17.8 (95% CI, 5.2 to 30 months) for those receiving enoxaparin (Log-rank test P=0.58). In the arm with lower levels of tissue factor-bearing microparticles the median survival was 17.3 months (95% CI, 10.3 to 24.3 months) as shown in Figure 4.
Figure 4. Overall survival based on tissue factor-bearing microparticle status.
Median survival for study subjects with higher concentration of tissue factor-bearing microparticles (TFMP) on observation (blue) was 11.8 months compared with 17.8 months on enoxaparin (green, Log-rank test P-value=0.58). The median survival for lower tissue factor-bearing microparticle arm was 17.3 months (red).
Correlation between tissue factor-bearing microparticles and D-dimer
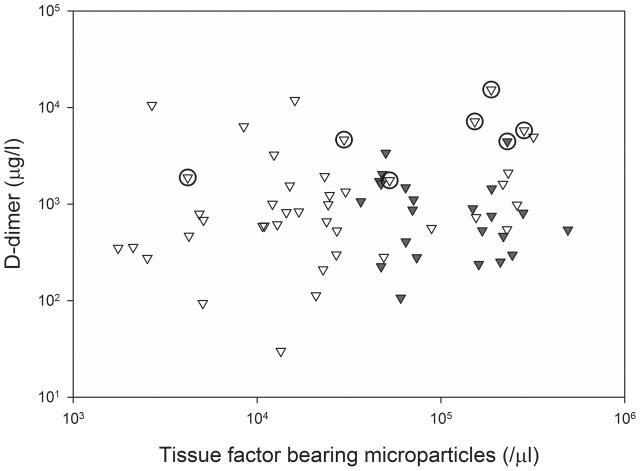
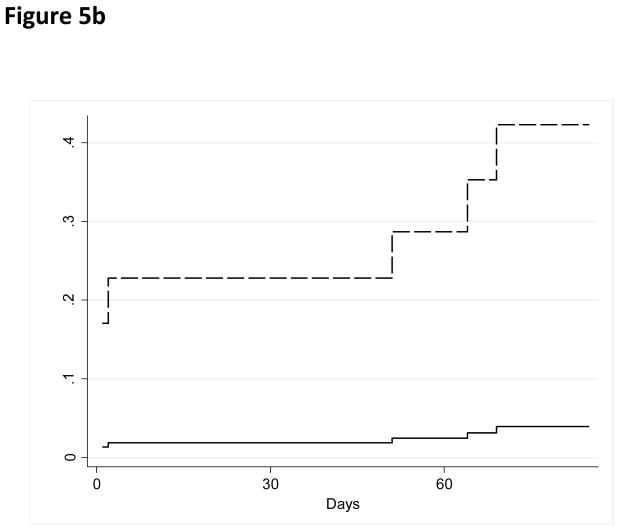
The baseline values of tissue factor-bearing microparticles did not correlate with D-dimer values (Spearman’s coefficient 0.10, P=0.41). The median D-dimer value for all patients was 815 μg/l (range 30–15,331 μg/l) and did not differ significantly between the three arms (two-sided Kruskal Wallis test P=0.21). Similarly, there was no correlation between the concentrations of tissue factor-bearing microparticles and white blood cell count, haemoglobin concentration, platelet count, ECOG performance status, number of prior chemotherapy regimens, or stage by univariate analysis. The majority of VTE were diagnosed in study subjects both with very elevated D-dimer and higher levels of tissue factor-bearing microparticles (Figure 5A). In an exploratory analysis (inclusive of the study subject diagnosed with VTE prior to randomization), the cumulative incidence of VTE was 43% (95% CI, 12.2 to 73.9%) at 2 months in the group with very elevated D-dimer values (>1500 μg/l) and higher levels of tissue factor-bearing microparticles (N=12). By comparison, the cumulative incidence of VTE was 4.2% (95% CI, 0% to 10.1%) for all other study subjects (N=55, Gray’s test P value=0.0001, Figure 5B).
Figure 5. Correlation between concentration of tissue factor-bearing microparticles and D-dimer with VTE.
a) Baseline concentration of tissue factor-bearing microparticles did not correlate with D-dimer values (Spearman coefficient 0.10). Dark triangles represent subjects randomized to enoxaparin and open triangles represent subjects followed without anticoagulation. The open circles represent subjects diagnosed with a VTE primary endpoint (including a study subject diagnosed with VTE prior to randomization). b) Cumulative incidence of VTE for subjects with very elevated D-dimer values (>1500 μg/l) and higher tissue factor-bearing microparticles (N=12, dashed line) compared with all other study subjects (N=55, Gray’s test P value=0.0001, solid line).
DISCUSSION
The primary objective of this prospective, phase II randomized trial was to establish the cumulative incidence rates in three groups of cancer patients: those with higher levels of tissue factor-bearing microparticles, those with higher levels of tissue factor-bearing microparticles treated with enoxaparin thromboprophylaxis, and those with lower levels of tissue factor-bearing microparticles. We observed a 27% cumulative incidence of VTE in cancer patients with higher levels of tissue factor-bearing microparticles. By comparison, only 7% of cancer patients with lower levels of tissue factor-bearing microparticles developed VTE in the absence of prophylactic anticoagulation. These data parallel our previous retrospective data describing the association between elevations of tissue factor-bearing microparticles and the development of thromboembolic complications (Zwicker, et al 2009). Although the study was not formally powered to compare the cumulative incidence of patients with higher levels of tissue factor-bearing microparticles randomized to enoxaparin or observation, the use of enoxaparin resulted in an 80% risk reduction compared to observation and provides justification for the conduct of a larger phase III trial.
A number of different methodologies have been evaluated for the measurement of microparticle populations including flow cytometry (Lacroix, et al 2010), dynamic light scatter (Gyorgy, et al 2011), particle tracking (Aleman, et al 2011), enzyme-linked immunosorbent assay (Lee, et al 2012), atomic force microscopy (Gyorgy, et al 2011, Yuana, et al 2010) and microparticle-based functional assays (Manly, et al 2010, Tesselaar, et al 2007, Thaler, et al 2012). Without an efficient and accurate standard for the measurement of tissue factor-bearing microparticle concentrations, the optimal methodology for microparticle size measurement, characterization, and enumeration is not known. Consequently, validation of an assay’s utility is dependent upon establishing an association with a clinical phenotype. Acknowledging the limitations of light scatter flow cytometry for microparticle sizing and detection (Shapiro 2003, van der Pol, et al 2012), we previously described an impedance-based flow cytometer optimized for microparticle measurement (Zwicker, et al 2009). Using this instrumentation, the current clinical study confirms that higher levels of circulating tissue factor-bearing microparticles are associated with an increased risk of thrombosis. Other groups have evaluated plasma tissue factor activity in cancer patients and observed an association between increased tissue factor activity and thrombosis (Khorana, et al 2008a, Manly, et al 2010, Tesselaar, et al 2009, Tesselaar, et al 2007). However, a recent analysis of banked plasma specimens from the Vienna Cancer and Thrombosis Study failed to identify a significant association between high plasma tissue factor activity and the development of VTE (Thaler, et al 2012). In a non-malignant cohort, we did not find a correlation between the number of tissue factor-bearing microparticles and tissue factor activity (Weitz, et al 2012). Lack of correlation between assays that measure tissue factor antigen or activity may, in part, be due to the complex mechanisms thought to regulate tissue factor activity in vivo such as decreased exposure to tissue factor pathway inhibitor, phosphatidylserine exposure, or tissue factor encryption (Bach and Rifkin 1990, Chen, et al 2006, Dietzen, et al 2004, Girard, et al 1989).
The prognostic utility of D-dimer measurements in cancer patients has been limited by lack of specificity (Ay, et al 2009). Microparticle-associated tissue factor activity was not previously shown to correlate with plasma D-dimer concentrations (Thaler, et al 2012, Weitz, et al 2012). We similarly did not observe a relationship between D-dimer values and concentrations of tissue factor-bearing microparticles, although there was an increased rate of VTE in patients with higher levels of tissue factor-bearing microparticles and very elevated D-dimer levels. The cumulative incidence of VTE at two months was 43% in this high risk population identified by elevated tissue factor-bearing microparticles and D-dimer (>1500 μg/l) and potentially represents a useful set of biomarkers to identify individuals with circulating tissue factor that is functionally active in vivo. Prospective studies are necessary to validate the potential prognostic utility of this approach.
While several randomized trials demonstrated the efficacy of LMWH in preventing VTE in cancer outpatients, the absolute benefit is modest. A notable finding of the current study is that deep vein thrombosis is underdiagnosed. All except one of the qualifying thrombotic events in this trial were identified by a protocol-mandated bilateral lower extremity ultrasound. The thrombi identified by screening ultrasound were typically extensive, involving both lower extremities or multiple proximal veins within the deep venous system. Implementation of routine ultrasound testing in future clinical trial design would probably increase the number of venous thrombotic events diagnosed and thus improve the likelihood of observing a greater absolute risk reduction with primary thromboprophylaxis.
The Food and Drug Administration (FDA) Oncologic Drug Advisory Committee recently voted against the approval of semuloparin, an ultra-LMWH for primary thromboprophylaxis in cancer patients, and emphasized the need to establish a risk-adapted approach to primary thromboprophylaxis (FDA 2012). Another randomized study is currently ongoing to assess the efficacy of dalteparin prophylaxis in high-risk patients based on a VTE-risk model that incorporates histological diagnosis, body mass index, and blood counts (ClinicalTrials.gov identifier NCT00876915) (Khorana, et al 2008b). Within this context, we report the results of the first randomized clinical trial evaluating a biomarker-driven anticoagulation strategy in cancer patients and confirm the potential prognostic utility of tissue factor-bearing microparticles detected by impedance-based flow cytometry. Enoxaparin, at prophylactic dosing, appears safe and effective in preventing thrombosis in cancer patients with elevated tissue factor-bearing microparticles. Based on these findings, we are initiating a placebo controlled, randomized, multi-centre trial supported by the National Heart, Lung, and Blood Institute to confirm the role of primary thromboprophylaxis in cancer patients with elevated tissue factor-bearing microparticles.
Acknowledgments
This work was supported by grants from the National Institutes of Health, K23 HL84052 (JIZ) and R01 HL095084 (BF), as well as a research grant from Sanofi (JIZ). We thank Anita Rodrigues, Marie Mahony RN, Laurie Hornor RN, Lisa Fabry RN, and Kelly Tammaro PharmD for their efforts in clinical research coordination; Gus Alban and Leo Puljanowski and Beckman Coulter for cytometry technical support; Dr. Gary Horowitz for technical assistance with D-dimer analysis; Dr. Hing Wong at Altor Biosciences for the gift of humanized tissue factor antibody. We are indebted to the patients for participating in this trial and acknowledge the critical roles played by all the participating investigators and coordinators.
Footnotes
Contribution: Clinical trial was developed by JIZ, BF, HAL, KAB, DN, and FC. Research conducted by JIZ, HAL, KAB, TC, RR, SM, CMK, JE, VR, HJL, AB, BB and statistical analysis performed by FC, DN, and JIZ. Manuscript written by JIZ and BF and reviewed by all authors.
Conflict-of-interest disclosure: HAL has served on steering committees for Sanofi; CMK has received research funds and served on advisory boards for Sanofi and Esai. No other authors report relevant conflicts-of-interest.
References
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte-and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–2261. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124–4129. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campigotto F, Neuberg D, Zwicker JI. Biased estimation of thrombosis rates in cancer studies using the method of Kaplan and Meier. J Thromb Haemost. 2012;10:1449–1451. doi: 10.1111/j.1538-7836.2012.04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Zhao J, Wiedmer T, Sims PJ. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J Biol Chem. 1993;268:7171–7178. [PubMed] [Google Scholar]
- Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. FDA Briefing Document. Oncologic Drugs Advisory Committee Meeting; June 20,2012; 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM308561.pdf. [Google Scholar]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999:496–509. [Google Scholar]
- Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:11141–11154. [Google Scholar]
- Gyorgy B, Modos K, Pallinger E, Paloczi K, Pasztoi M, Misjak P, Deli MA, Sipos A, Szalai A, Voszka I, Polgar A, Toth K, Csete M, Nagy G, Gay S, Falus A, Kittel A, Buzas EI. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008a;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008b;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- Shapiro HM. Practical Flow Cytometry. Wiley-Liss; New York: 2003. [Google Scholar]
- Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1364–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost. 2012;10:919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- Weitz IC, Razavi P, Rochanda L, Zwicker J, Furie B, Manly D, Mackman N, Green R, Liebman HA. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res. 2012;130:361–368. doi: 10.1016/j.thromres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–323. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JI, Trenor CC, 3rd, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31:728–733. doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]