Abstract
Individuals infected with HIV show moderate deficits in decision-making, but the ecological relevance of such deficits on everyday functioning has not previously been described. This study sought to examine the magnitude, cognitive correlates, and everyday functioning impact of risky decision-making impairment in HIV-associated neurocognitive disorders (HAND). Participants included 68 HIV+ individuals with HAND, 78 HIV+ individuals without HAND, and 51 HIV- comparison participants, who were administered the Iowa Gambling Task (IGT) alongside a comprehensive neuropsychological test battery and self-report measures assessing aspects of everyday functioning. HIV+ individuals with HAND performed more poorly on the IGT relative to the other two groups, most notably during the last three trial blocks. Within the HIV+ group, IGT performance during the last three trial blocks was most strongly associated with cognitive flexibility, but was not significantly related to declines in instrumental activities of daily living (IADLs), unemployment, or medication non-adherence. While overall IGT performance across the last three trial blocks may be helpful diagnostically in identifying decision-making impairment in HAND, examination of alternate, more specific metrics (e.g., individual deck selections across trial blocks) may be more useful in delineating the role of poor decision-making in HIV-related disability, and should be examined in future research.
Keywords: HIV, cognitive impairment, decision-making, everyday functioning, cognitive flexibility
HIV-associated neurocognitive disorders (HAND) have persisted in the era of combination antiretroviral therapy (cART; Heaton et al., 2010). Recent estimates suggest that nearly 50% of HIV-infected individuals experience HAND (Heaton et al., 2010), which is thought to primarily reflect underlying frontostriatal neuropathology (Ellis, Langford, & Masliah, 2007), and is commonly expressed in areas of episodic memory, executive functions, attention and working memory, and psychomotor skills (Reger, Welsh, Razani, Martin, & Boone, 2002). Nearly half of individuals with HAND experience significant difficulties with their everyday activities (e.g., Blackstone et al., 2012; Heaton et al., 2004a), including suboptimal medication adherence (e.g., Hinkin et al., 2004) and vocational difficulties (e.g., van Gorp et al., 2007). Despite the reliable link between neurocognitive impairment and functional declines in HIV infection, much of this research has relied on standard clinical neuropsychological tests, which may be limited in their ecological relevance due to a number of factors (e.g., testing environment, inability to use compensatory strategies; Long & Kibby, 1995). For example, some individuals with HIV show considerable functional impairment in their day-to-day life despite normal performance on traditional laboratory-based measures (e.g., Heaton et al., 2004a). One explanation for the discordance between performance on standard neuropsychological tests and real-life functioning is that traditional assessment measures may not fully capture the cognitive demands required for successful everyday functioning. Therefore, examination of more recently adapted experimental measures developed out of strong neurocognitive theory and designed to more closely simulate real-life situations may provide more ecologically valid measurements of the cognitive abilities required for successful everyday functioning in HIV (for a review, see Woods, Moore, Weber, & Grant, 2009).
Decision-making is one cognitive construct not traditionally assessed in the evaluation of HAND that may play a unique role in the adverse functional and health-related consequences of HIV infection. As defined by Bechara, Damasio, and Damasio (2000), decision-making refers to complex cognitive and affective processes involved in the “ability to select the most advantageous response from an array of possible behavioral choices.” One of the most widely used laboratory-based measures of decision-making is the Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994). The IGT was designed to simulate real-life decision-making by incorporating factors such as rewards and punishments, uncertainty, implicit rule learning, and response to feedback (for a review, see Bechara et al., 2000). In brief, the IGT requires participants to make selections one at a time from one of four card decks, whereby each selection results in winning a specified amount of money, though simultaneously, some selections result in penalties that vary in amount and frequency across decks. The goal of the task is to maximize profit across 100 trials. In order to do so, participants must learn over the course of the task that two of the decks are disadvantageous (i.e., “risky”) decks, as they involve higher immediate gains after each selection but also occasional higher penalties, while the other two decks are considered advantageous (i.e., “safe”) decks, and involve low immediate gains after each selection and occasional low penalties. Decision-making impairment (e.g., making disproportionately more selections from the disadvantageous decks across the course of the task, relative to healthy participants), has been observed in a wide array of clinical conditions (e.g., frontal lobe lesions, substance use disorders; Bechara et al., 1994; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2006) and found to be associated with underlying neural damage in prefrontal regions, as well as within limbic structures typically associated with emotional or somatic states (Bechara, Damasio, & Damasio, 2003; Bechara, Damasio, Tranel, & Damasio, 1999; Bechara & Martin, 2004; Brand, Recknor, Grabenhorst, & Bechara, 2007; Ernst et al., 2002; Lawrence, Clark, Labuzetta, Sahakian & Vyakarnum, 2008; Li, Lu, D'Argembeau, Ng, & Bechara, 2010). Moreover, impaired decision-making on the IGT has been linked to adverse real-life functional consequences in substance dependent individuals, including medical (e.g., hospitalizations, chronic medical conditions) and legal problems (Verdejo-Garcia et al., 2006), as well as unemployment (Bechara et al., 2001).
Consistent with the imaging and lesion research demonstrating an association between impaired IGT performance and frontal systems dysfunction, IGT deficits have been observed in HIV-infected individuals, both with (e.g., Martin et al., 2004) and without (e.g., Hardy, Hinkin, Levine, Castellon, & Lam, 2006) substance use disorders. While HIV-infected individuals perform comparably to seronegative comparisons during the initial portions of the task, they make disproportionately more selections from risky decks as the task progresses (e.g., Hardy et al., 2006; Martin et al., 2004). HIV-associated deficits on the IGT may be particularly driven by Deck B (Hardy et al., 2006), which is considered to be the “riskiest” deck given its consistently higher rewards in light of its infrequent but larger penalties relative to each of the other decks. It has been posited that HIV-infected individuals may have difficulties inhibiting risky selections due to poorer inhibition to the lure of high rewards and/or problems remembering the infrequent but large penalties that ensued from these selections (Hardy et al., 2006). However, other studies have found that overall IGT performance was not strongly related to attention, working memory, or other aspects of executive functions in HIV (e.g., Gonzalez et al., 2005; Hardy et al., 2006; Martin et al., 2004; Wardle, Gonzalez, Bechara, & Martin-Thormeyer, 2010), which may suggest that underlying cognitive processes involved in decision-making are somewhat dissociable from those measured by traditional neuropsychological measures.
Although decision-making impairment has been observed in HIV infection, it has not been specifically examined in the context of HAND, which is the neurobehavioral hallmark of neuroAIDS. Moreover, no studies have assessed the direct everyday functioning correlates of HIV-associated decision-making impairment. Recent research has examined the role of decision-making processes in the relationship between positive (i.e., sensation-seeking; Gonzalez et al., 2005) and negative (i.e., emotional distress; Wardle et al., 2010) emotional states and engagement in risky sexual practices. Specifically, they found that increased sensation-seeking and greater emotional distress were predictive of riskier sexual practices, though only in HIV+ individuals with intact IGT performance. The authors interpret this finding to suggest that there may in fact be a significant association between decision-making processes and functional outcomes in HIV, though it may only be observed when the neural circuitry underlying affective decision-making processes is intact. However, no studies have examined the value of decision-making in predicting important everyday outcomes common to HIV infection, including declines in instrumental activities of daily living (IADLs), unemployment, and cART nonadherence. It stands to reason that poorer decision-making amongst HIV-infected individuals could increase the risk of numerous adverse long-term health-related consequences (e.g., poorer immune function and health due to medication nonadherence). For example, HIV-infected individuals with decision-making impairment may not be able to fully evaluate the consequences of poor adherence and the adverse implications that such choices may have on their health and subsequently choose to not take their medications at times and/or alter their doses. Given such potential implications, this study sought to investigate the profile, cognitive correlates, and everyday functioning consequences of risky decision-making abilities as measured by the IGT in individuals with HAND.
Method
Participants
This study included 146 HIV-infected participants and 51 HIV seronegative comparison subjects enrolled in NIH-funded studies conducted within the San Diego HIV Neurobehavioral Research Program (HNRP). HIV infection was indicated by enzyme linked immunosorbent assays (ELISA) and a Western Blot confirmatory test, and Hepatitis C Virus (HCV) diagnoses were confirmed through detection of HCV IgG antibody in plasma by ELISA. Individuals were excluded if they met Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV; American Psychiatric Association, 1994) criteria for current substance dependence (i.e., within one month of evaluation) as determined by the Composite International Diagnostic Interview (CIDI; Version 2.1; World Health Organization, 1998), or if they tested positive for illicit drugs (other than marijuana) on a urine toxicology screen or for alcohol on a breathalyzer test on the day of evaluation. Participants with histories of severe psychiatric (e.g., schizophrenia) or neurological (e.g., active CNS opportunistic infections, closed head injuries) illness, or an estimated verbal IQ score of less than 70 on the Wide Range Achievement Test, Revision 3 (WRAT-3 Revised; Wilkinson, 1993) were also excluded. Diagnoses for psychiatric disorders common in HIV-infected and substance using populations, including Major Depressive Disorder (MDD) and Antisocial Personality Disorder (ASPD) were established using the CIDI and the Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1996), respectively. Attention–Deficit/Hyperactivity Disorder (ADHD) was assessed using the Diagnostic Interview Schedule (DIS-IV; Robins, Helzer, Croughan, & Ratcliff, 1981).
Procedure
The procedures involved in this study were approved by the human subjects institutional review board at the University of California, San Diego. Each participant provided written, informed consent, and was administered a computerized version of the Iowa Gambling Task (IGT; Bechara et al., 2000) alongside a comprehensive medical, psychiatric, and neuropsychological evaluation. A detailed description of the original gambling task may be found in Bechara et al. (1994). Briefly, the IGT is a 100-item measure designed to assess real-world decision-making in a laboratory setting, whereby participants are given a hypothetical loan of $2000 and asked to choose cards one at a time from one of 4 decks (i.e., A, B, C, or D) that involve monetary gains and losses. Each card selection (i.e., each individual trial) results in winning a specified amount of money, although some selections simultaneously result in a penalty, varying in amount and frequency across decks. The goal of the task is to maximize profit across 100 trials. In order to maximize profit, participants must learn over time that two of the decks (i.e., A and B) are disadvantageous (i.e., “risky”) decks, as they involve higher immediate gains after each selection (i.e., $100) but also occasional higher penalties (e.g., 20 more frequent but relatively smaller penalties in Deck A ranging from $150-300 and four infrequent but larger penalties of $1250 in Deck B). Choosing primarily from the “risky” decks will result in a net monetary loss. Decks C and D are classified as advantageous (i.e., “safe”) decks, and involve low immediate gains (i.e., $50) after each selection and occasional low penalties (i.e., 20 more frequent but relatively smaller penalties ranging from $25-75 in Deck C, and four infrequent but larger penalties of $250 in Deck D), resulting in an overall net gain. IGT performance was indexed by the total number of choices from the advantageous decks (i.e., total number of selections from Deck C + total number of selections from Deck D) minus the total number of choices from the disadvantageous decks (i.e., total number of selections from Deck A + total number of selections from Deck B) across five 20-trial blocks (i.e., Trials 1-20, 21-40, 41-60, 61-80, 81-100) where lower values (i.e., scores below zero) indicated more disadvantageous (i.e., “risky”) decision-making and higher values (i.e., scores above zero) denoted more advantageous, or “safe” decision-making.
The remaining neuropsychological test battery included an estimate of pre-morbid verbal IQ (i.e., the reading subtest of the WRAT-3; Wilkinson, 1993), and measures designed to assess neurocognitive domains relevant to HAND in accordance with Frascati research criteria (Antinori et al., 2007). Measures are listed below by domain:
(1) Speed of Information Processing: Trail Making Test Part A (Army Individual Test Battery, 1944; Heaton, Miller, Taylor & Grant, 2004), Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol and Symbol Search (Heaton, Taylor, & Manly, 2001; The Psychological Corporation, 1997); (2) Verbal Fluency: Controlled Oral Word Association Test (COWAT-FAS; Benton, Hamsher, & Sivan; 1983; Gladsjo et al., 1999), and a semantic verbal fluency task (i.e., animal fluency; Benton, Hamsher, & Sivan, 1983); (3) Attention/Working Memory: Paced Auditory Serial Addition Test (PASAT; Heaton et al., 2004b; Gronwall, 1977; Gronwall & Sampson, 1974), Wechsler Memory Scale-3rd Edition (WMS-III) Spatial Span (The Psychological Corporation, 1997); (4) Executive Functions: Wisconsin Card Sorting Test-64 Card Version (WCST-64) Perseverative Responses (Kongs, Thompson, Iverson, & Heaton, 2000), Trail Making Test Part B-A (TMT B-A; Army Individual Test Battery, 1944; Heaton et al., 2004b); (5) Learning: Hopkins Verbal Learning Test-Revised (HVLT-R) Total Trial 1-3 Recall (Benedict, Schretlen, Groninger, & Brandt, 1998), Brief Visuospatial Memory Test-Revised (BVMT-R) Total Trial 1-3 Recall (Benedict, 1997); (6) Memory: HVLT-R Delayed Recall (Benedict, Schretlen, Groninger, & Brandt, 1998), BVMT-R Delayed Recall (Benedict, 1997); (7) Motor: Grooved Pegboard Dominant and Non-dominant hand (Heaton et al., 2004b; Klove, 1963).
Raw scores from the individual neuropsychological tests listed were converted to demographically adjusted T-scores and used to derive clinical ratings for each of the aforementioned individual cognitive domains as well as a global clinical rating. Ratings ranged from 0 (above average, T-score ≥ 55) to nine (severely impaired, T-score < 20) and were used to determine HAND diagnoses (i.e., a global clinical rating of ≥ 5 was considered impaired; see Woods et al., 2004 for further detail). Based on this classification, 47% (n = 68) of the HIV+ sample met criteria for HAND (i.e., “HAND+”). Individuals with HAND were further classified into HAND diagnoses using revised Antinori et al. (2007) research criteria. Of the 68 HIV+ individuals who met criteria for HAND, 74% (n=50) were further classified as having Asymptomatic Neurocognitive Impairment (ANI), and 26% (n = 18) as having Mild Neurocognitive Disorder (MND). No participant met diagnostic criteria for HIV-associated Dementia (HAD). In order to explore the cognitive correlates of IGT performance within the HIV+ group, raw scores from the test battery were converted to population-based z-scores for standardization and comparison purposes, and averaged to create domain-based z-scores that were used for analyses. The population-based z-scores were derived using raw score distributions from the clinical sample (i.e., HIV+ only), which was based on a standard published approach used in our previous studies (e.g., Zogg et al., 2011).
Demographic characteristics for the two HIV-infected samples (i.e., HAND- and HAND+) relative to the HIV- comparison subjects are presented in Table 1. The three groups did not differ in terms of most demographic factors (i.e., age, education, gender; ps > 0.10), though both HIV+ groups had slightly greater proportions of Caucasian individuals relative to the HIV- cohort (ps < 0.05). The HAND+ group had lower estimated verbal IQ scores and higher clinical ratings (indicating greater impairment) relative to both the HAND- and HIV- groups (ps < 0.05). With regard to psychiatric characteristics, more individuals within the HAND+ group met criteria for lifetime MDD relative to both the HIV- and HAND- groups (ps < 0.05). Both HIV+ groups had greater proportions of individuals with lifetime substance dependence diagnoses relative to the HIV- group (ps < 0.05), though they did not differ in proportions relative to each other (p > 0.10). There were no individuals in the HIV- group who met criteria for ADHD or ASPD, though rates of both conditions were comparable across the HIV+ groups (ps > 0.10). A greater proportion of the HAND+ group was co-infected with HCV relative to the HAND- group (p < 0.05), though there were no individuals in the seronegative comparison group with HCV. Thus, analyses with regard to confounding effects of HCV were restricted to the two HIV+ groups. Lastly, the two HIV+ groups did not differ in terms of HIV-disease characteristics (i.e., duration of HIV disease, nadir and current CD4 counts, plasma viral load concentrations, proportion of individuals diagnosed with AIDS), nor did they differ in proportions of individuals who were currently prescribed antiretroviral (ARV) medications (ps > 0.10).
Table 1.
Demographic, Psychiatric, Substance Use, and Medical Characteristics of the Study Participants
| Variable | HIV-(n = 51) | HIV+ (n = 146) | p-value | Group | |
|---|---|---|---|---|---|
|
| |||||
| HAND-(n = 78) | HAND+ (n = 68) | ||||
| Demographic Characteristics | |||||
| Age (years) | 40.8 (12.8) | 42.9 (7.6) | 43.8 (8.4) | 0.234 | ----- |
| Education (years) | 13.2 (2.2) | 13.2 (2.0) | 13.0 (2.1) | 0.852 | ----- |
| Sex (% male) | 86.3 | 89.7 | 91.2 | 0.693 | ----- |
| Ethnicity (% Caucasian) | 49.0 | 68.0 | 67.7 | 0.061 | ----- |
| Estimated Premorbid Verbal IQa | 101.8 (12.4) | 103.0 (8.7) | 95.4 (11.4) | <0.001 | HAND- = HIV- > HAND+ |
| Global Clinical Rating | 4.0 (1.3) | 3.3 (0.9) | 5.6 (0.8) | <0.001 | HAND+ > HIV- > HAND- |
| Psychiatric Characteristics | |||||
| MDDb (% LT) | 29.4 | 42.3 | 66.2 | <0.001 | HAND+ > HAND- = HIV- |
| Substance Dependenceb (% LT) | 17.7 | 62.8 | 73.5 | <0.001 | HAND+ = HAND- > HIV- |
| ADHDb (% LT) | ----- | 14.7 | 19.4 | 0.453 | ----- |
| ASPDb (% LT) | ----- | 10.7 | 13.4 | 0.613 | ----- |
| Medical Characteristics | |||||
| Hepatitis C Virus (%) | ----- | 25.6 | 42.7 | 0.030 | HAND+ > HAND- |
| HIV Disease Characteristics | |||||
| Duration of Infection (years) | ----- | 8.6 (4.0, 17.5) | 7.5 (3.0, 14.9) | 0.639 | ----- |
| Nadir CD4c count (cells/μl) | ----- | 200.0 (45.0, 340.0) | 160.0 (48.2, 322.5) | 0.612 | ----- |
| Current CD4c count (cells/μl) | ----- | 462.0 (320.5, 641.5) | 435.5 (310.5, 619.5) | 0.764 | ----- |
| Plasma viral load (% detectable) | ----- | 41.1 | 46.8 | 0.508 | ----- |
| Proportion with AIDS (%) | ----- | 52.6 | 54.6 | 0.820 | ----- |
| Proportion on ARVs (%) | ----- | 78.4 | 80.0 | 0.814 | ----- |
Note. MDD = Major Depressive Disorder; LT = Lifetime; ADHD = Attention Deficit Hyperactivity Disorder; ASPD = Antisocial Personality Disorder; CD4 = Cluster of differentiation 4. ARV = antiretroviral therapy.
Based on the reading subtest of the Wide Range Achievement Test (WRAT-3).
Denotes any lifetime diagnosis.
Median (interquartile range).
Several additional self-report measures included in the comprehensive evaluation were also used to examine associations between IGT performance and functional outcomes. Analyses with regard to functional outcomes were conducted in the HIV-infected group only (n = 146) in order to reduce type I error risk.
Evaluation of Daily Functioning
Each participant completed a modified version of the Lawton and Brody (1969) Activities of Daily Living (ADL) Scale, which assesses the degree to which an individual can function independently in their daily activities, and has been used extensively in the evaluation of daily functioning abilities in the HIV literature (e.g., Heaton et al., 2004a; Woods et al., 2006). Briefly, participants are asked to rate both their current and best (i.e., highest previous) levels of functioning with regard to ten instrumental activities of daily living (IADLs), including managing finances, medication adherence, laundry, transportation, grocery shopping, housekeeping (cleaning), shopping, telephone use, cooking, and home repairs. Participants were classified as IADL dependent if they reported decline (i.e., current rating of functioning is lower than their best level of functioning) in two or more IADLs (consistent with Heaton et al., 2004). Using this system, 83% (n = 115) of the HIV-infected cohort with IADL data were classified as “IADL Independent”, and 17% (n = 24) were considered “IADL Dependent”. The IADL Dependent and Independent HIV-infected samples were comparable with respect to most demographic (e.g., age, education, ethnicity, gender), medical (i.e., HCV), psychiatric (i.e., ADHD, ASPD, and lifetime substance use disorders) and HIV disease characteristics (e.g., AIDS status, nadir and current CD4 count, plasma viral load concentration) that may influence an individual's everyday functioning abilities (all ps > 0.10). However, the IADL Dependent group had higher rates of both HAND and lifetime MDD diagnoses, and had a greater proportion of individuals currently taking antiretroviral medications relative to the IADL Independent group (ps < 0.05).
Employment Status
A single item derived from the Patient's Assessment of Own Functioning Inventory (PAOFI; Chelune, Heaton, & Lehman, 1986), a self-report questionnaire in which an individual rates their current level of functioning in day-to-day life, was used to classify individuals as either employed (i.e., full-time only; n = 18) or unemployed (n = 104). The employed group did not differ from the unemployed group in terms of most demographic (i.e., age, education, ethnicity, sex), medical (i.e., HCV infection), psychiatric (i.e., lifetime MDD, ASPD, ADHD, and substance dependence diagnoses), and HIV disease (i.e., plasma viral load, proportion on ARVs, nadir and current CD4 counts, or duration of infection) characteristics (all ps > 0.10). A greater proportion of the unemployed group met criteria for HAND and had AIDS diagnoses (ps < 0.05) relative to the employed group.
Medication Adherence
Each HIV-infected participant was administered the ACTG Adherence to Anti-HIV Medications questionnaire, which is a self-report measure designed to assess medication adherence (e.g., how many pills missed and why) over the four days prior to their assessment. Analyses exploring the potential relationship between risky decision-making and poor medication adherence were restricted to those in the HIV+ group who were taking anti-HIV medications at the time of their assessment (n = 105). The primary variable of interest from the ACTG was an item indicating whether the participant missed any of their HIV medications in the last four days (yes/no). Of the 105 HIV-infected participants, only 12.4% (n = 13) reported missing a dose within the last four days and were classified as poor adherers. Those reporting poor adherence did not differ from those reporting good adherence (i.e., did not miss a dose over the four days prior to their assessment; n = 92) with regards to demographic characteristics (i.e., age, education, ethnicity, and gender), HAND diagnoses, HCV status, and lifetime ASPD and substance dependence diagnoses (ps > 0.10), though they had higher rates of lifetime ADHD and LT MDD diagnoses (ps < 0.05). The two HIV+ adherence groups did not differ on any HIV disease characteristics (e.g., proportion of individuals diagnosed with AIDS and on ARVs, plasma viral load concentrations, current and nadir CD4 counts, and duration of infection; ps > 0.10).
Results
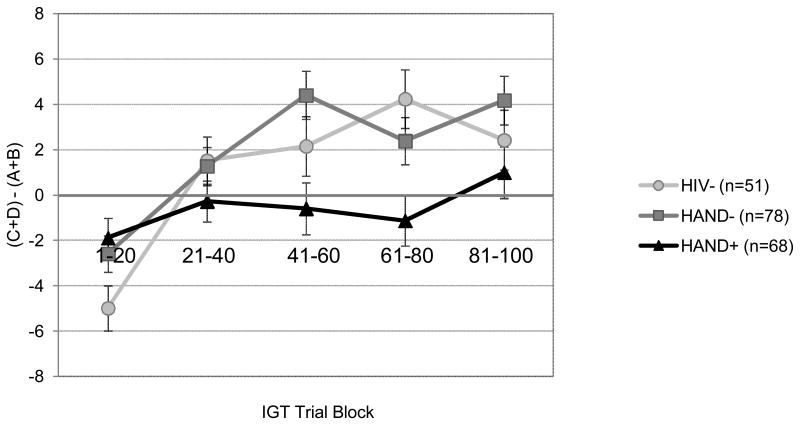
Although a few variables were non-normally distributed, the results did not differ when nonparametric statistics were used; as such, we adopted a parametric approach throughout the study. Hedge's g was used as an index of effect size, and the critical alpha was set to 0.05 for all analyses. First, a mixed design analysis of variance (ANOVA) was conducted in order to explore the effects of HAND on decision-making abilities on the IGT with trial block (i.e., Trials 1-20, 21-40, 41-60, 61-80, and 81-100) as the within-group variable and study group (i.e., HIV, HAND-, HAND+) as the between-subjects variable. The primary outcome measure was the total number of advantageous selections (i.e., C+D) minus the total number of selections from the disadvantageous decks (i.e., A+B), which was calculated separately for each of the five IGT trial blocks. Results revealed a significant group by trial block interaction [F(8, 382) = 3.65; p < 0.001; see Figure 1], as well as significant main effects for both study group [F(2, 194) = 3.40; p = 0.035] and trial block [F(4, 191) = 13.7; p < 0.001]. This interaction remained significant even when accounting for potentially confounding variables that differed between the study groups, including lifetime MDD and substance use diagnoses; p < 0.01). Moreover, none of these diagnoses were independently associated with IGT performance in this model (ps > 0.10). No significant associations were observed between HCV co-infection and IGT performance within the entire HIV+ group (ps > 0.10) or within the HAND+ group alone (ps > 0.10).
Figure 1.
Performance on the IGT task (i.e., total number of cards selected from the advantageous minus the disadvantageous decks) across five 20-trial blocks for the three study groups.
Follow-up analyses using independent samples t-tests were then conducted to further explore the significant study group by IGT trial block interaction. Relative to the HAND- group, the HAND+ group performed comparably during the first two trial blocks (i.e., Trials 1-20, 21-40; Hedge's g = 0.08 and -0.17 respectively; ps > 0.10), though made significantly more disadvantageous selections from the third trial block throughout the remainder of the task (i.e., Trials 41-60, 61-80, 81-100; Hedge's g = -0.45, -0.32 and -0.28, respectively; all ps < 0.05; See Figure 1). Aside from the initial trial block (i.e., Trials 1-20), the HAND group made more disadvantageous selections across the remainder of the Trial Blocks (i.e., Trials 21-40, 41-60, 61-80, 81-100) relative to the HIV- group, with a significant difference emerging for Trials 61-80 (Hedges g = -0.49; p = 0.002). The HIV- and HAND- groups performed comparably on each of the five 20-trial blocks (Hedge's g range -0.17 – 0.34 all ps > 0.05).
Next, planned correlational analyses were conducted between IGT task performance and population-based cognitive domain z-scores within the HIV+ group only (n = 146). The IGT variable chosen for the correlational analyses was the sum of each individual's overall performance (i.e., [C+D]-[A+B]) across the last three trial blocks (i.e., Trials 41-100). Previous research has suggested that performance across the last three trial blocks may be a more reliable estimate of decision-making, as these trials are considered to represent decision-making under risk rather than ambiguous conditions, as by this point, participants are more likely to have learned the rules of the task and developed a decision-making strategy (Monterosso, Ehrman, Napier, O'Brien, & Childress, 2001).
Raw scores for the individual tests were converted to population-based z-scores within the HIV+ group only (i.e., both HAND+ and HAND- groups) and averaged to create domain z-scores for use in the analyses. No significant correlations were observed between any of the cognitive domains (i.e., verbal fluency, executive functions, attention/working memory, information processing speed, learning, memory, and motor) and performance during the first two blocks of the IGT (i.e., Trials 1-40; Pearson's r correlation coefficient range = -0.11 to 0.04; ps > 0.10). However, IGT performance during the last three trial blocks (i.e., Trials 41-100) correlated significantly with executive functions (r = 0.25, p = 0.002), learning (r = 0.18; p = 0.016) and memory (r = 0.21; p = 0.010). Smaller correlations were also found between performance on Trials 41-100 and working memory (r = 0.15; p = 0.079) and motor skills (r = -0.14; p = 0.087), but not with verbal fluency or speed of information processing (rs = 0.11 and 0.06; ps > 0.10). When the significant cognitive domains (i.e., executive functions, learning, and memory) were entered into a regression predicting performance during the last three trial blocks of the IGT, the overall model was significant [F(3, 142) = 3.95; p = 0.010; adjusted R2 = 0.06], with executive functions emerging as the only significant independent predictor (β = 0.21; p = 0.028).
Finally, logistic regression analyses were conducted within the HIV-infected group only (n = 146) in order to examine “risky” decision-making (i.e., [C+D]-[A+B] across IGT Trials 41-100) as a potential independent predictor of adverse functional outcomes (i.e., dependence in activities of daily living, unemployment, and poor medication adherence). As both lifetime MDD and HAND diagnoses have been previously established as significant independent predictors of self-reported functional outcomes in HIV infection (e.g., Heaton et al., 2004a; Hinkin et al., 2002; van Gorp, Baerwald, Ferrando, McElhiney & Rabkin, 1999) they were included a priori in the regression models as covariates. Also included in the individual prediction models were any potentially confounding variables that differed between the functional outcome groups (e.g., AIDS status for the employed versus unemployed HIV-infected individuals). As shown in Table 2, results revealed that while the each of the overall models were significant, IGT Trials 41-100 did not emerge as a significant independent predictor of any of the aforementioned functional outcomes examined in this study (i.e., IADL Dependence, Employment Status, and Medication Adherence; ps > 0.10). Moreover, no significant associations were observed at the univariate level between the functional outcomes and IGT Trials 41-100 (ps > 0.10). Of note, these null findings regarding IGT effects did not differ when examining other commonly used IGT outcome variables (e.g., total net score of disadvantageous minus advantageous deck selections, total number of individual deck selections) as predictors of adverse functional outcomes (ps > 0.10). In the regression models, significant predictors of IADL dependence were HAND diagnoses (p = 0.014) and ARV status at a trend level (p = 0.074). Employment status was also associated with HAND diagnoses, as well as AIDS (ps < 0.05). The only significant predictor of medication adherence was lifetime ADHD diagnoses (p = 0.032). Lifetime MDD was not a significant predictor of any of the functional outcomes when included in the overall models (ps > 0.10).
Table 2.
Logistic regression analyses examining poorer (i.e., “risky”) decision-making as a predictor of adverse functional outcomes in the HIV-infected group (i.e., IADL dependence, unemployment, and poor medication adherence).
| Variable | B | SE | CI | Odds Ratio | X2 | p-value |
|---|---|---|---|---|---|---|
| IADL Dependence [Dependent] (df=4) | 18.25 | 0.001 | ||||
| IGT Trials 41-100 | 0.01 | 0.01 | [-0.01, 0.03] | 1.01 | 1.85 | 0.174 |
| HAND Diagnosis [HAND] | 0.68 | 0.28 | [0.16, 1.25] | 3.87 | 6.06 | 0.014 |
| Lifetime MDD [yes] | 0.47 | 0.29 | [-0.06, 1.08] | 2.57 | 2.74 | 0.100 |
| ARV Status [on] | 0.96 | 0.54 | [0.10, 2.42] | 6.78 | 3.20 | 0.074 |
| Employment Status [Unemployed] (df=4) | 11.52 | 0.021 | ||||
| IGT Trials 41-100 | 0.01 | 0.01 | [-0.01, 0.03] | 1.01 | 0.24 | 0.625 |
| HAND Diagnosis [HAND] | 0.79 | 0.33 | [0.18, 1.50] | 4.83 | 5.64 | 0.018 |
| Lifetime MDD [yes] | -0.46 | 0.30 | [-1.08, 0.11] | 0.40 | 2.40 | 0.122 |
| AIDS Status [yes] | 0.63 | 0.29 | [0.09, 1.24] | 3.55 | 4.75 | 0.029 |
| Medication Adherence [Poor Adherence] (df=4) | 11.90 | 0.018 | ||||
| IGT Trials 41-100 | 0.03 | 0.02 | [-0.01, 0.06] | 1.03 | 2.53 | 0.112 |
| HAND Diagnosis [HAND] | -0.22 | 0.33 | [-0.89, 0.42] | 0.64 | 0.45 | 0.502 |
| Lifetime MDD [yes] | 0.68 | 0.42 | [-0.06, 1.65] | 3.90 | 2.67 | 0.102 |
| Lifetime ADHD [yes] | 0.82 | 0.38 | [0.07, 1.60] | 5.16 | 4.58 | 0.032 |
Note. IADL = Instrumental activities of daily living. IGT = Iowa Gambling Task. HAND = HIV-associated neurocognitive disorder. MDD = Major Depressive Disorder. ARV = antiretroviral therapy. ADHD = Attention Deficit/Hyperactivity Disorder.
Discussion
This study extends the prior literature on decision-making in HIV by examining the profile, cognitive correlates, and everyday functioning outcomes of IGT performance specifically in persons with HAND. Findings provide further support for poorer (i.e., “riskier”) decision-making in HAND, which is consistent with the previous literature on decision-making as assessed by the IGT in HIV (e.g., Hardy et al., 2006; Martin et al., 2004) as well as in conditions with similar underlying frontostriatal systems dysfunction (e.g., Parkinson's disease; Perretta, Pari, & Beninger, 2005). Specifically, while all three study groups made more selections from the disadvantageous decks initially (i.e., during the first block, which some have referred to as “exploratory behavior” rather than explicit decision-making style; e.g., Dunn et al., 2006), only the HIV- and neurocognitively unimpaired HIV-infected individuals adopted a more effective strategy shortly thereafter (i.e., selecting more from advantageous versus disadvantageous decks). However, the HAND group continued to primarily choose from the disadvantageous decks throughout the course of the task. It is unlikely that this pattern of decision-making impairment in HAND is better explained by potentially confounding factors, as the two HIV+ groups (i.e., HAND+ and HAND-) were well matched demographically (e.g., education) and on other psychiatric (e.g., ADHD, ASPD), substance use (i.e., lifetime substance dependence), and HIV disease characteristics (e.g., nadir CD4 count, plasma viral load) that may impact cognitive performance. While there were greater proportions of individuals within the HAND+ group with MDD and HCV relative to the HAND- group, including these variables in the analyses did not modify the effect of HAND on decision-making.
Within the entire HIV+ group, IGT performance during the initial portion of the task (i.e., Trials 1-40) was not significantly associated with any of the cognitive domains assessed in this study, including executive functions, attention/working memory, information processing speed, learning and memory, and motor skills. Conversely, the majority of cognitive domains assessed were significantly associated with IGT performance during the latter three trial blocks (i.e., Trials 41-100), including executive functions, working memory, and learning and memory. Executive functions, as indexed by putative measures of cognitive flexibility (i.e., Trails B-A and WCST perseverative responses), emerged as the strongest cognitive predictor of successful task performance during Trials 41-100. This is consistent with research specifically examining the convergent and divergent validity of the IGT, which demonstrated that executive functions play a unique role in the latter three trial blocks only (i.e., Trials 41-100; Gansler, Jerram, Vannorsdall & Schretlen, 2011), which may also help explain their greater diagnostic utility (Roca et al., 2008). This evidence is critical to the interpretation of IGT data and highlights the importance of examining alternate metrics, which may be more informative and have greater construct and criterion validity relative to the conventional IGT measure (i.e., CD-AB for Trials 1-100). Nonetheless, given the multifactorial nature of HAND and the wide range of cognitive profiles (Dawes et al., 2008), it is important to examine which specific cognitive aspects of HAND might be driving an omnibus effect on the IGT. While there is evidence to suggest specific cognitive abilities associated with the IGT (e.g., executive functions; Hardy et al., 2006), some have also suggested that the cognitive demands of the IGT may be somewhat dissociable from those assessed by the measures included in our cognitive battery used to classify HAND. Thus, a thorough examination of the cognitive correlates of the IGT across HAND groups is necessary in order to fully understand the underlying cognitive mechanisms of decision-making ability in HIV.
Relatedly, the existing literature regarding the cognitive correlates of decision-making impairment in HIV and in the IGT literature in general has been largely inconsistent, this may be due, in part, to the different IGT outcome variables examined across studies. For example, some studies have used the overall index of performance collapsed across the entire task (i.e., the conventional metric CD-AB for Trials 1-100), which is arguably an inaccurate representation of decision-making abilities given the potential neural and cognitive dissociations between the earlier and later portions of the task (e.g., Monterosso et al., 2001). Along these lines, our findings are consistent with recent evidence suggesting that the latter portion of the task may be a more accurate representation of decision-making processes under specified risk conditions (e.g., Monterosso et al., 2001) and as mentioned before, may rely more heavily on specific executive functions (e.g., set shifting; Brand et al., 2007; Gansler et al., 2011). Our findings are also consistent with recent imaging studies suggesting that frontostriatal regions (e.g., dorsolateral prefrontal cortex; Fellows & Farah, 2005) may be more prominently involved in decision-making under specified risk and less so when the conditions are ambiguous (for a review, see Dunn, Dalgleish, & Lawrence, 2006). Future research is needed to further delineate the cognitive correlates of decision-making performance across the IGT, and may benefit from particular attention towards specific executive aspects such as cognitive flexibility. To the extent that poor decision-making could be attributed to cognitive inflexibility (e.g., an inability to recognize alternate approaches to a problem), this research could be highly informative with regard to specifically tailored cognitive rehabilitation interventions aimed at improving decision-making abilities in HIV. For example, an individual with lower cognitive flexibility who turns to drugs as a means of problem solving may not be able to recognize alternate strategies to problems and continue using drugs or adopt other approaches that have proven unsuccessful in the past in order to resolve discomfort. Interventions specifically targeted at improving cognitive flexibility may enhance this individual's ability to think through alternate approaches and provide the cognitive resources needed to choose a more advantageous strategy.
The most surprising outcome of this study was that risky decision-making was not associated with HIV-related functional disability. It was anticipated that poor decision-making would negatively affect functional outcomes (e.g., medication adherence) given the strong and reliable associations between executive dysfunction and disability in HIV (e.g., poor medication adherence, IADL declines, unemployment; Hinkin et al., 2002; Hinkin et al., 2004). Impaired performance on the IGT in substance using populations has also been associated with real-life functional consequences such as unemployment (Bechara et al., 2001) and medical and legal problems (e.g., Verdejo-Garcia et al., 2006). However, the IGT did not predict any of the functional outcomes examined in this study, including poor medication adherence, unemployment, or dependence in instrumental activities of daily living (IADLs). Also somewhat surprising was that lifetime MDD was also not associated with any of the functional outcomes when included in our overall models, nor was it associated with IGT performance within the HAND group, though this latter effect is consistent with research that has failed to find additive effects of depression and HIV infection on cognition (e.g., Cysique et al., 2007). One possible explanation of these null findings particularly with regard to our functional outcome analyses is the small sample sizes of some of the functional outcome groups. For example, only 18 out of 122 HIV-infected individuals (i.e., HAND and noHAND) were employed full-time, and only 13 out of the 105 HIV+ individuals prescribed cART were classified as suboptimal adherers. Analyses of lifetime MDD and HAND within the HIV+ groups decreased the sample sizes even further (e.g., only four individuals in the HAND group with lifetime MDD reported poor medication adherence). Future studies with larger samples sizes are needed in order to fully delineate the relationships between decision-making and these functional outcomes, as performance on the IGT has been linked to adverse medical problems (e.g., hospitalizations, chronic medical conditions; Verdejo-Garcia et al., 2006), as well as unemployment (Bechara et al., 2001). Of note, despite the small sample sizes in these analyses, we nevertheless observed significant relationships between other variables expected to relate to the functional outcomes (e.g., HAND), and MDD was associated with IADL dependence at the univariate level.
Another possible explanation for the unexpected null findings is that there may be important emotional (e.g., apathy) or personality (e.g., sensation-seeking) complexities that mediate/moderate the relationship between risky decision-making and adverse functional consequences in HIV infection. For example, two studies in HIV have demonstrated a potentially mediating role of IGT performance in the relationship between dispositional characteristics (e.g., sensation-seeking) and adverse functional outcomes (Gonzalez et al., 2005; Wardle et al., 2011). Specifically, while there were no direct associations between poor decision-making and increased sexual risk in HIV-infected individuals, both sensation-seeking (Gonzalez et al., 2005) and emotional distress (Wardle et al., 2010) were correlated with increased risky sexual practices, though only among individuals with normal IGT performance. Moreover, imaging studies have found associations between impaired decision-making and neural damage in limbic structures typically associated with emotional or somatic states (e.g., Bechara et al., 2003). Future research should strongly consider the potential influence of dispositional personality traits and emotional factors when examining the relationship between IGT performance (as indexed by various different IGT metrics, including individual decks, as well as overall disadvantageous minus advantageous selections over trial blocks) and adverse functional outcomes (e.g., poor medication adherence).
The lack of association between decision-making and functional outcomes may also be attributed to particular characteristics of the decision-making task employed in this study. First, some have raised concern regarding the potential to run out of cards in one deck and essentially be “forced” to choose from other decks towards the end of the task after exhausting another. While this is certainly of concern when examining IGT data, it did not appear to confound our results. Upon specific examination of our data, only four individuals in our sample reached any deck limit by the end of the fourth block (i.e., Trial 80) and only two additional individuals reached any deck limit per group by the end of the task. Analyses revealed nearly identical results even if we excluded these individuals. Another task-related limitation is that this study examined decision-making only in the context of performance on the IGT, which is a complex, multi-factorial task, with a number of different outcome metrics available that have been used as indicators of “poor decision-making,” though with varying degrees of construct and criterion validity. As the primary aim of this study was to identify the pattern of IGT impairment in HAND, we were primarily interested in performance across the different trial blocks as this metric has demonstrated diagnostic significance (e.g., Roca et al., 2008). However, as mentioned above, previous research has demonstrated the utility of examining alternative IGT metrics in HIV-infected individual (e.g., total number of selections from Deck B; Hardy et al., 2006). While beyond the scope of this study, post hoc examination of our data did not reveal any significant differences between the groups for overall selections from Deck B, nor did the number of selections from Deck B predict the functional outcomes within the HIV+ group (ps > 0.10). It is possible that other factors (e.g., personality features, depression, working memory) may influence the relationship between Deck B selections and functional outcomes, and this may be an interesting future direction, alongside examining alternate metrics (e.g., trial by trial performance, and individual deck patterns of performance over time). There are also a number of alternate experimental performance-based measures of risky decision-making (e.g., the Balloon Analogue Risk Test) that show good convergent validity with self-report measures of risk-related constructs (e.g., addictive behaviors; Lejuez et al., 2002), and may be better indicators of real-world risk taking behaviors and stronger predictors of functional outcomes in HIV-infected individuals. Future studies should include multiple measures addressing varying aspects of decision-making in their neurocognitive batteries in order to fully evaluate and capture decision-making ability in HAND and its relationship with functional outcomes.
Finally, the functional outcomes assessed in this current study were largely based on self-report and are by no means exhaustive. It is nevertheless worth noting that our measurement of functional outcomes reported was multimodal, including both objective (i.e., employment) and self-report (e.g., IADL declines) measures. There are a number of significant real-world consequences not examined in this study that could result from poor decision-making and could be examined through laboratory-based functional measures or real world outcomes. For example, it is possible that decision-making ability may play a critical role in automobile driving (e.g., traffic violations, accidents), as has been shown with other aspects of executive dysfunction (e.g., Marcotte et al., 2004). Prior research in substance dependent individuals suggests that objective measures of real-life functioning may be better predictors of decision-making on the IGT relative to self-report measures, which could be confounded by poor insight into problems (e.g., Verdejo-Garcia et al., 2006). Thus, the sensitivity of future research on this topic may benefit from the inclusion of performance-based and/or behavioral measures of medication management (e.g., MMT-R; Heaton et al., 2004a), adherence (e.g., medication event monitoring system), vocational functioning (e.g., Valpar; Heaton et al., 2004a), employment (e.g., frequency of mistakes at the workplace), and various other ADLs (e.g., shopping and financial management; Heaton et al., 2004a).
Acknowledgments
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert Heaton, Ph.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H., Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Steven Paul Woods, Psy.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.
This research was supported by National Institutes of Health grants P30-MH62512, P01-DA12065, T32-DA31098, and L30-DA034362. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in Neurology. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd D, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington, D.C.: War Department, Adjutant General's Office; 1944. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision-making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/S0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision-making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test – Revised. Odessa, Florida: Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, Grant I for the CHARTER Group. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Networks. 2006;19:1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsychology. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients' complaints of disability. In: Tarter RE, Goldstein G, editors. Advances in Clinical Neuropsychology. Vol. 3. New York: Plenum Press; 1986. pp. 95–126. [Google Scholar]
- Cysique LA, Deutsch R, Atkindon JH, Young C, Marcotte TD, Dawson L The HNRC Group. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society. 2007;13:1–11. doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- Dawes DD, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre SL, Heaton RK the HNRC Group. Variable patterns of neuropsychological performance in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2008;30:613–626. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eaton LA, Kalichman SC. Changes in transmission risk behaviors across stages of HIV disease among people living with HIV. Journal of the Association of Nurses in AIDS Care. 2009;20:39–49. doi: 10.1016/j.jana.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nature Reviews Neuroscience. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Gansler DA, Jerram MW, Vannorsdall TD, Schretlen DJ. Does the Iowa Gambling Task measure executive function? Archives of Clinical Neuropsychology. 2011;26:706–717. doi: 10.1093/archlin/acr082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/S1355617705050186. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–375. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gronwall DM, Sampson H. The psychological effects of concussion. Auckland, New Zealand: Auckland University Press; 1974. [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Grant I for the CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H The HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004a;10:317–331. doi: 10.1017/S1355617704102130. doi: 10.10170S13556177041022130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Odessa, FL: Psychological Assessment Resources, Inc.; 2004b. [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky DS, Heaton RK, Chelune G, Ivnik R, Bornstein RA, Prifitera A, Ledbetter M, editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Stefaniak M. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.WNL.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefanaik M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kløve H. Grooved Pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test – 64 Card Computerized Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Lawrence A, Clark L, Labuzetta JN, Sahakian B, Vyakarnum S. The innovative brain. Nature. 2008;456:168–169. doi: 10.1038/456168a. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037/1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Kibby MY. Ecological validity of neuropsychological tests: A look at neuropsychology's past and the impact that ecological issues may have on its future. Advances in Medical Psychotherapy. 1995;8:59–78. [Google Scholar]
- Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ the HIV Neurobehavioral Research Center (HNRC) Group. A multimodal assessment of driving performance in HIV infection. Neurology. 2004;63:1417–1422. doi: 10.1212/01.WNL.0000141920.33580.5D. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/S1355617704107054. [DOI] [PubMed] [Google Scholar]
- Mazas C, Finn P, Steinmetz J. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1036–1040. doi: 10.1111/j.1530-0277.2000.tb04647.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O'Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Patton JM, Stanford MS, Barratt ES. Factor Structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768∷AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Perretta J, Pari G, Beninger RJ. Effects of Parkinson's Disease on two putative nondeclarative memory tasks: probabilistic classification and gambling. Cognitive and Behavioural Neurology. 2005;18:185–92. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/S1355617702813212. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule - Its History, Characteristics, and Validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Roca M, Torralva T, Meli F, Fiol M, Calcagno ML, Carpintiero S, Correale J. Cognitive deficits in multiple sclerosis correlate with changes in fronto-subcortical tracts. Multiple Sclerosis. 2008;14:364–369. doi: 10.1177/1352458507084270. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Toplak ME, Sorge GB, Benoit A, West RF, Stanovich KE. Decision-making and cognitive abilities: A review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clinical Psychology Review. 2010;30:562–581. doi: 10.1016/j.cpr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Baerwald JP, Ferrando SJ, McElhiney MC, Rabkin JG. The relationship between employment and neuropsychological impairment in HIV infection. Journal of the International Neuropsychological Society. 1999;5:534–539. doi: 10.1017/s1355617799566071. [DOI] [PubMed] [Google Scholar]
- van Gorp W, Rabkin J, Ferrando S, Mintz J, Ryan E, Borkowski T, McElhiney Neuropsychiatric predictors of return to work in HIV/AIDS. Journal of the International Neuropsychological Society. 2007;13:80–89. doi: 10.1017/S1355617707070117. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/S1355617706060486. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Gonzalez R, Bechara A, Martin-Thormeyer EM. Iowa Gambling Task performance and emotional distress interact to predict risky sexual behavior in individuals with dual substance and HIV diagnoses. Journal of Clinical and Experimental Neuropsychology. 2010;32:1110–1121. doi: 10.1080/13803391003757833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test Administration Manual. 3rd. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive Neuropsychology of HIV-Associated Neurocognitive Disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Scott JC, Grant I The HNRC Group. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. Journal of Clinical and Experimental Neuropsychology. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, Version 2.1) Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- Zogg J, Woods SP, Weber E, Doyle K, Grant I the HIV Neurobehavioral Research Programs (HNRP) Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]