Abstract
The causes of major depression remain unknown. Antidepressants elevate monoamine concentrations, particularly serotonin, but it remains uncertain which downstream events are critical to their therapeutic effects. We report that endogenous serotonin selectively potentiated excitatory synapses formed by the temporoammonic (TA) pathway with CA1 pyramidal cells via activation of 5-HT1BRs, without affecting nearby Schaffer collateral synapses. This potentiation was expressed postsynaptically by AMPA-type glutamate receptors and required calmodulin-dependent protein kinase-mediated phosphorylation of GluA1 subunits. Because they share common expression mechanisms, long-term potentiation and serotonin-induced potentiation occluded each other. Long-term consolidation of spatial learning, a function of TA-CA1 synapses, was enhanced by 5-HT1BR antagonists. Serotonin-induced potentiation was quantitatively and qualitatively altered in a rat model of depression, restored by chronic antidepressants, and required for the ability of chronic antidepressants to reverse stress-induced anhedonia. Changes in serotonin-mediated potentiation, and its recovery by antidepressants, implicate excitatory synapses as a locus of plasticity in depression.
Depression is a leading cause of mortality and morbidity worldwide1 and probably results from a combination of genetic and environmental risk factors. Although the genes responsible for depression remain difficult to identify, a common risk factor is stress2. Current treatments for depression remain unsatisfactory, partly because the underlying biological changes in brain function in depression remain poorly understood. The discovery that changes in monoamine levels alter the affective state of patients led to the hypothesis that a dysfunction of monoamine signaling, particularly serotonin (5-HT), causes depression3. Elevation of serotonin levels with conventional antidepressants, such as selective serotonin-reuptake inhibitors (SSRIs) and tricyclic antidepressants, may modulate neuronal excitability and plasticity by altering the transcription, translation, and phosphorylation state of target proteins1,3. Neither the principal neural circuits nor the key proteins that are modulated by serotonin are known, however. Serotonin is capable of regulating the glutamatergic system, and evidence of changes in excitatory synaptic transmission in models of depression is accumulating4,5,6, but it remains unclear how these two neurotransmitter systems interact.
Depression is likely caused by dysfunction in a variety of brain regions and cell types, including the hippocampus7,8. Altered hippocampal function may also influence adversely the activity of the cortex, amygdala, and other structures associated with reward, motivation, and emotionality. For example, the hippocampal formation provides a major source of excitatory input to the nucleus accumbens (NAc)9, where chronic stress weakens AMPAR-mediated excitatory synaptic transmission10.
The highest concentration of serotoninergic fibers in the forebrain is in stratum lacunosum-moleculare (SLM) of hippocampal areas CA1 and CA311, where the axons of layer III neurons in the entorhinal cortex form excitatory synapses with the distal apical dendrites of pyramidal cells. This temporoammonic (TA) pathway is required for some spatial recognition tasks and for long-term consolidation of spatial memory12. In order to understand the function of serotonin better, we studied its actions at TA-CA1 synapses, and observed that serotonin potentiates these synapses postsynaptically and that this potentiation is altered in a rat model of depression.
RESULTS
Potentiation of TA-CA1 synapses by serotonin
Field excitatory postsynaptic potentials (fEPSPs) were recorded in SLM of area CA1 of acutely prepared rat hippocampal brain slices in response to stimulation of the TA pathway (Supplementary Fig. 1a). Bath application of the tricyclic antidepressant imipramine (2 μM) or the SSRIs fluoxetine (20 μM) or citalopram (10 μM), in order to acutely elevate extracellular serotonin, all increased the slope of TA-CA1 fEPSPs (Fig. 1a,b, Supplementary Fig. 1b,c). Potentiation of synaptic transmission by serotonin is surprising because most neuromodulators inhibit synaptic transmission in the mammalian CNS, although there is evidence that serotonin can increase glutamate release in some systems13,14.
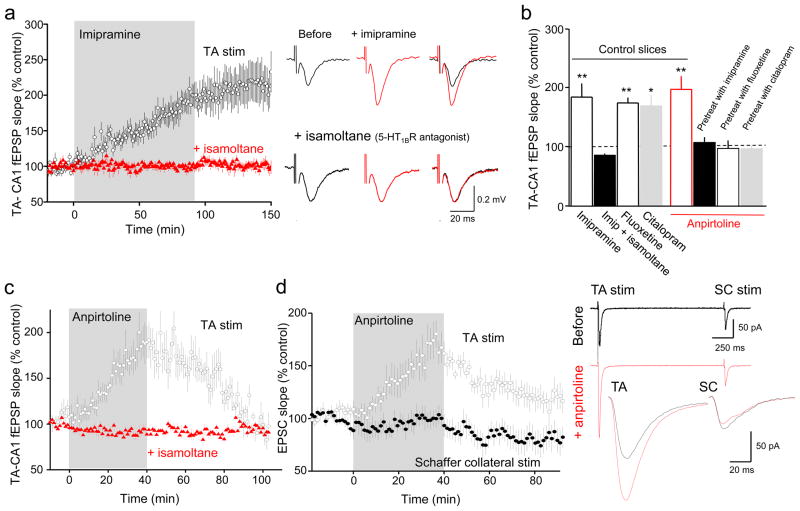
Figure 1. 5-HT1BR activation selectively potentiates TA-CA1 cell excitatory synapses.
(a) Promoting accumulation of endogenous serotonin by bath application of the tricyclic antidepressant imipramine (2 μM) potentiated TA-CA1 fEPSPs in SLM of area CA1 of acutely prepared hippocampal slices (n=8 slices) in control saline (black), but not in the slices pretreated with 5-HT1BR antagonist isamoltane (10 μM) (red; n=9 slices). Sample traces before (black) and 60 min after imipramine application (red) in control saline (upper row) or in the presence of isamoltane (lower row) are shown at right. (b) Group data showing the effect of antidepressants on TA-CA1 fEPSPs in control slices (fluoxetine, n=4 slices; citalopram, n=3 slices) and the effect of anpirtoline (50 μM) on TA-CA1 fEPSPs in control slices (red, n=11 slices; ANOVA F(3,20)=9.751, p<0.001) or slices pretreated for 60 min with antidepressants (n=5 slices pretreated with imipramine; n=5 slices with fluoxetine; n=3 slices with citalopram; F(3,20)=7.32, p=0.002). Bonferroni post-hoc tests revealed that isamoltane prevented the increase in fEPSP slope observed with antidepressants alone (p<0.05 vs. Imipramine, Fluoxetine, or Citalopram), and that anpirtoline treatment differed from each of these pretreatment+anpirtoline conditions (p<0.05). (c) The selective 5HT1BR agonist, anpirtoline, reversibly increased the slope of TA-CA1 fEPSPs in SLM of area CA1 (black circles; n=11 slices). Isamoltane (10 μM) blocked the effect of anpirtoline on TA-CA1 fEPSPs (red triangles; n=5 slices). (d) Anpirtoline selectively enhanced TA-CA1 EPSCs recorded in whole-cell voltage-clamp mode, but not simultaneously evoked SC-CA1 EPSCs in a two-pathway experimental design (n=9 cells). Sample traces are shown at right. The selective increase in TA-CA1 fEPSPs by serotonin, but not SC-CA1 fEPSPs, is consistent with the selective localization of 5-HT1BRs in SLM. *p<0.05, **p<0.01 compared with before anpirtoline or antidepressant; paired t-test.
5-HT1B receptor (R) mRNA is present in CA1 cells at high levels and 5-HT1BR binding sites are concentrated in SLM15,16. We therefore asked whether 5-HT1BRs mediated the effects of acute elevation of serotonin on TA-CA1 synaptic transmission. Imipramine application did not potentiate TA-CA1 fEPSP slope in slices pretreated with the antagonist isamoltane (10 μM) (Fig. 1a, b), nor did imipramine or fluoxetine affect TA-CA1 fEPSPs in slices of hippocampus taken from mice lacking 5-HT1BRs17 (Supplementary Fig. 2a, b). In contrast, the 5-HT1AR antagonist, NAN-190 (10 μM) had no effect on potentiation of TA-CA1 fEPSPs by imipramine (Supplementary Fig. 2c). Consistent with these findings, a selective agonist for 5-HT1BRs, anpirtoline (50μM)18, enhanced the slope of TA-CA1 fEPSPs (Fig. 1c). Unlike the acute potentiating effects of the antidepressants, the effects of anpirtoline on fEPSP slope were fully reversed after 60 min of wash-out. Application of isamoltane during washout of imipramine decreased the potentiation (Supplementary Fig. 1d), suggesting that the maintained potentiation induced by antidepressants was due to a persistent increase in serotonin levels. Anpirtoline also produced a doubling of TA-CA1 excitatory postsynaptic potential (EPSP) slope in whole-cell voltage- (Fig. 1d) and current-clamp (Supplementary Fig. 2d) recordings. Anpirtoline had no significant effect on fEPSPs in slices pretreated with isamoltane (Fig. 1c). Another 5-HT1BR selective agonist, CP94253 (5μM), also potentiated TA-CA1 fEPSP slope (Supplementary Fig. 2e). Anpirtoline antagonizes 5-HT3 receptors at higher concentrations19, however the 5-HT3 receptor antagonist Y-25130 (5μM) had no effect on TA-CA1 fEPSP slope (Supplementary Fig. 2e), indicating that anpirtoline’s effect was mediated by 5-HT1BRs. Anpirtoline-induced potentiation was unaffected by depletion of serotonin with p-chlorophenylalanine20 (Supplementary Fig. 1e), indicating that anpirtoline’s effect was mediated by postsynaptic heteroreceptors and not from an action on presynaptic inhibitory autoreceptors on serotonergic nerve terminals21. Finally, application of anpirtoline had no significant effect on TA-CA1 fEPSP slope in slices pretreated for 60 min with antidepressants (Fig. 1b), presumably because the endogenous serotonin had already maximally activated 5-HT1BRs, occluding the potentiation.
Serotonin-mediated potentiation was specific for the TA-CA1 excitatory synapses in SLM, where 5-HT1BRs are concentrated. Neither anpirtoline (Fig. 1d, Supplementary Fig. 2d) nor fluoxetine (Supplementary Fig. 2f) had any significant effect on Schaffer collateral (SC)-CA1 cell responses to stimuli delivered in stratum radiatum (SR). Activation of 5-HT1BRs by serotonin thus produces a highly localized potentiation of excitatory synapses.
TA inputs are relatively ineffective in inducing action potential firing in CA1 pyramidal cells, although modest SC activation facilitates this process22. Anpirtoline enhanced the supralinear summation of TA-SC EPSPs and promoted TA-SC -induced action potential firing (Supplementary Fig. 3a, b). Activation of 5-HT1BRs by endogenously released serotonin and subsequent potentiation of the TA-CA1 pathway thus promotes action potential output from area CA1.
Molecular basis of 5-HT -mediated potentiation
Is 5-HT1BR-mediated potentiation of TA-CA1 fEPSPs mediated pre- or postsynaptically? Neither the amplitude of the TA fiber volley (106±3% of control, n=7, p>0.05) nor the paired-pulse ratio of TA-CA1 fEPSPs (1.39±1.3 before, 1.34±1.0 after anpirtoline, n=11, p>0.05; elicited with pairs of TA stimuli separated by 50ms) were changed significantly by anpirtoline (Fig. 2a). Furthermore, anpirtoline potentiated the AMPAR-mediated component of the EPSC, recorded under voltage-clamp at –70 mV, significantly (161±5% of control, n=7, t(6)=4.808, p=0.003) whereas the NMDAR-mediated component of the EPSC at +40 mV was unaffected (83±13% of control, n=5, t(4)=1.838, p=0.14; Fig. 2b). A similar selective potentiation of pharmacologically isolated AMPA receptor-mediated fEPSPs was also observed in extracellular recordings (Supplementary Fig. 3c). 5-HT1BR-induced potentiation is thus inconsistent with classical presynaptic expression mechanisms.
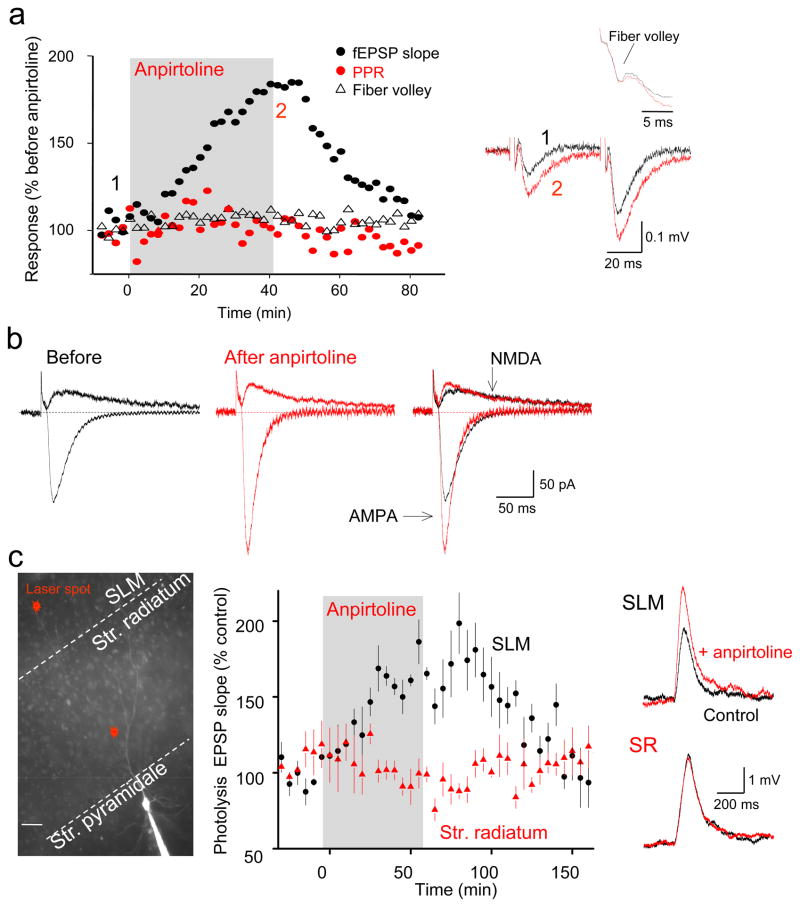
Figure 2. Expression of 5-HT1BR-induced potentiation of TA-CA1 synapses is mediated postsynaptically.
(a) Time course of changes of TA-CA1 fEPSP slope (black circles), fiber volley (black triangles), and paired-pulse ratio (PPR, red circles) before and after anpirtoline application in a typical experiment. Representative traces shown at right, with the fiber volley at higher resolution above. Neither fiber volley (t(6)=0.039, p=0.97) nor PPR (t(10)=1.17, p=0.27) changed with anpirtoline treatment. (b) Whole-cell voltage-clamp recordings of EPSCs at −70 and +40 mV in the same cell before (black) and 40 min after application of anpirtoline (red). (c) Left: Image of a CA1 cell filled with Alexa 594 from a somatic whole-cell pipette indicating sites of microphotolysis of caged glutamate on distal apical dendrites in SLM or oblique dendrites in SR of CA1 pyramidal cells. Scale bar = 20 μm. Right: bath application of anpirtoline increased photolysis-evoked EPSPs elicited from dendrites in SLM in acutely prepared hippocampal brain slices (n=5 cells) but not from dendrites in SR (n=6 cells in separate experiments), indicating that 5-HT1BR activation selectively enhanced postsynaptic glutamate responses at sites of TA synapse formation. Sample EPSP-like photolysis responses elicited from dendrites in SLM and SR before (black) and after anpirtoline application (red) are shown at right.
Selective 5-HT1BR-induced enhancement of AMPA receptor-mediated responses at TA synapses may result from a postsynaptic increase in AMPAR function. This hypothesis was tested using microphotolysis of caged glutamate23. With photostimuli delivered in SLM, anpirtoline application produced a near doubling of the slope of AMPAR-mediated photolysis-evoked EPSPs (phEPSPs)(Fig. 2c). Consistent with its lack of effect on SC-CA1 EPSPs, anpirtoline did not increase the slope of AMPAR-mediated phEPSPs elicited with microphotolysis of caged glutamate onto proximal oblique dendrites in SR (Fig. 2c). Potentiation of phEPSPs was not accompanied by changes in either holding current or input resistance (not shown). Activation of 5-HT1BRs thus specifically and selectively increases the number or efficacy of postsynaptic AMPA receptors in the distal apical dendrites of CA1 pyramidal neurons in a highly localized manner.
Stimulation of 5-HT1BRs can activate several protein kinases24,25, including calcium/calmodulin-dependent protein kinase (CaMK). We first analyzed threonine 286 on CaMKII, which is autophosphorylated when CaMK is activated26, in SLM tissue wedges subdissected from hippocampal slices. Anpirtoline increased CaMK T286 phosphorylation with a time-course comparable to the induction of the potentiation (Fig. 3a, b). Incubation of hippocampal slices with KN62 (5 μM), a specific CaMK inhibitor, blocked the effect of anpirtoline on TA-CA1 fEPSP slope (Fig. 3c). Similarly, intracellular dialysis with the selective CaMK inhibitor peptide, autocamtide-2-related inhibitory peptide (AIP, 2 μM)(Fig. 3c) blocked potentiation of TA-CA1 EPSP slope by anpirtoline. In contrast, anpirtoline-induced potentiation of fEPSPs was not affected by the protein kinase A (PKA) inhibitor, PKI 14-22 (1 μM)(Fig. 3c). We conclude that CaMK is activated rapidly in response to 5-HT1BR activation and that this activation is necessary for the potentiation of TA-CA1 transmission.
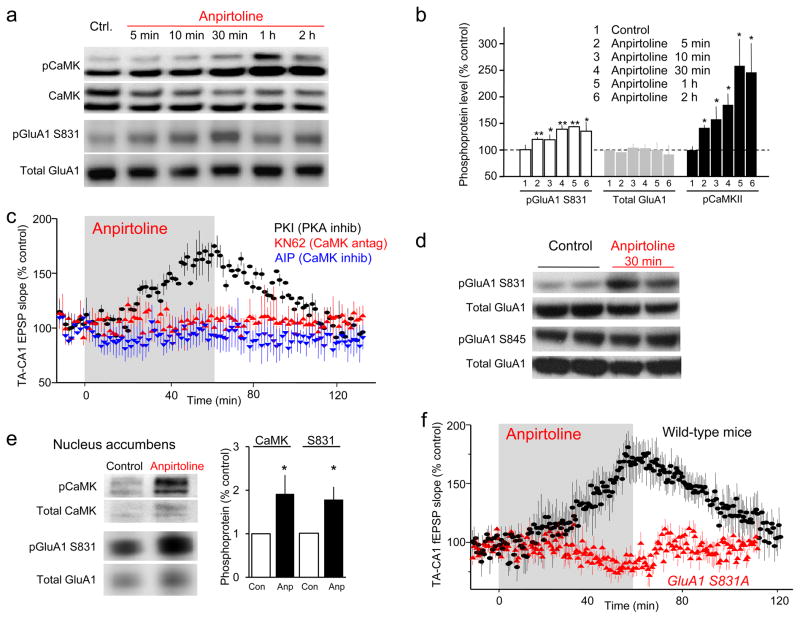
Figure 3. Anpirtoline activates CaMK and increases phosphorylation of GluA1 at serine 831 in SLM tissue.
(a,b) Representative Western blots and quantification showing that anpirtoline (50 μM, 2 hr) time dependently increased phosphorylation of alpha and beta CaMK at T286 (molecular weights 52 and 60 kD; F(5,18)=4.651, p=0.007) and GluA1 at S831 (F(5,24)=3.706, p=0.013), but did not affect total GluA1 expression (F(5,18)=0.863, p=0.524) in isolated CA1 SLM tissue sections, subdissected from whole hippocampal slices. (c) The effect of anpirtoline on TA-CA1 fEPSPs was blocked by bath application of the specific CaMK inhibitor, KN62 (5 μM)(n=6 slices), but not by bath application of the cell-permeable PKA inhibitor, PKI 14-22 (1 μM)(n=3 slices). Intracellular dialysis of the CaMK inhibitor, autocamtide-2-related inhibitory peptide (AIP, 2 μM) via patch-pipettes (n=5 cells) blocked potentiation of TA-CA1 EPSPs recorded in whole-cell mode. (d) Anpirtoline increased phosphorylation of GluA1 at S831 in isolated CA1 SLM tissue sections but not at S845. (e) Anpirtoline (50 μM for 60 min) increased phosphorylation of CaMK T286 (left, Mann-Whitney U z=2.09, p=0.04) and GluA1 S831 (right, Mann-Whitney U z=2.09, p=0.04) in tissue punches taken from the core of the Nucleus Accumbens (n=6 slices each). (f) Anpirtoline did not potentiate TA-CA1 fEPSPs in slices from GluA1 S831A knock-in mice (n=7 slices from 4 animals), but produced strong potentiation in slices from wild-type littermate mice (n=4 slices from 4 animals). *, p<0.05; **, p<0.01 compared with before anpirtoline. Full-length blots are presented in Supplementary Figure 7.
Multiple phosphorylation sites in AMPA receptors, including two serines in the C terminal of the GluA1 subunit are important for synaptic long-term potentiation (LTP)27,28. Anpirtoline produced a time-dependent increase in the phosphorylation of GluA1 at serine 831 (142±11% of untreated, n=11, t(10)=3.17, p=0.01), the target of protein kinase C and CaMK, but not at the PKA site, serine 845 (116±8% of untreated, n=4, p>0.05), as revealed in Western blots of SLM tissue wedges (Fig. 3a, b, d). Consistent with the lack of potentiation of SC-CA1 synapses, anpirtoline had no effect on CaMK autophosphorylation or GluA1 S831 phosphorylation in isolated tissue from SR (Supplementary Fig. 4a) or in SLM in tissue from 5-HT1BR−/− mice (Supplementary Fig. 4b). Consistent with the persistent potentiation induced by acute application of antidepressants (Fig. 1), acute fluoxetine produced an activation of CaMK and phosphorylation of GluA1 S831 that persisted after fluoxetine washout, unlike anpirtoline (Supplementary Fig. 4c). Comparable time-dependent increases in CaMK activation and GluA1 S831 phosphorylation in response to anpirtoline were obtained with tissue from the NAc core (Fig. 3e) and medial prefrontal cortex (Supplementary Fig. 4d), suggesting that local regulation of the strength of synaptic excitation by serotonin may occur in multiple brain regions important for cognitive function.
We next tested the hypothesis that CaMK-mediated phosphorylation of S831 is required for anpirtoline-induced potentiation using transgenic mice in which GluA1 S831 has been mutated to alanine (GluA1 S831A mice), rendering it incapable of being phosphorylated by CaMK29. Neither anpirtoline (Fig. 3f) nor fluoxetine (Supplementary Fig. 4e) had any effect on TA-CA1 fEPSP slope in slices prepared from these mice, although they potentiated TA-CA1 fEPSP slope in slices from wild-type littermate mice. We conclude that serotonin enhances AMPA receptor function by activating CaMK and increasing GluA1 phosphorylation at serine 831.
Serotonin action is involved in memory consolidation
Activation of CaMK and phosphorylation of GluA1 are required not only for serotonin-induced potentiation of TA-CA1 synapses, but are also involved in conventional LTP at SC-CA1 synapses28,30. Indeed, induction of LTP at TA-CA1 synapses prevented subsequent potentiation by anpirtoline (Fig. 4a, b) and TA-CA1 LTP could not be induced after potentiating fEPSPs with anpirtoline (Fig. 4c). In contrast, anpirtoline did not potentiate SC-CA1 fEPSPs or occlude SC-CA1 LTP (Fig. 4d). We conclude that serotonin-induced potentiation and conventional synaptic LTP occlude each other at TA-CA1 excitatory synapses, probably because they share common signaling and expression mechanisms.
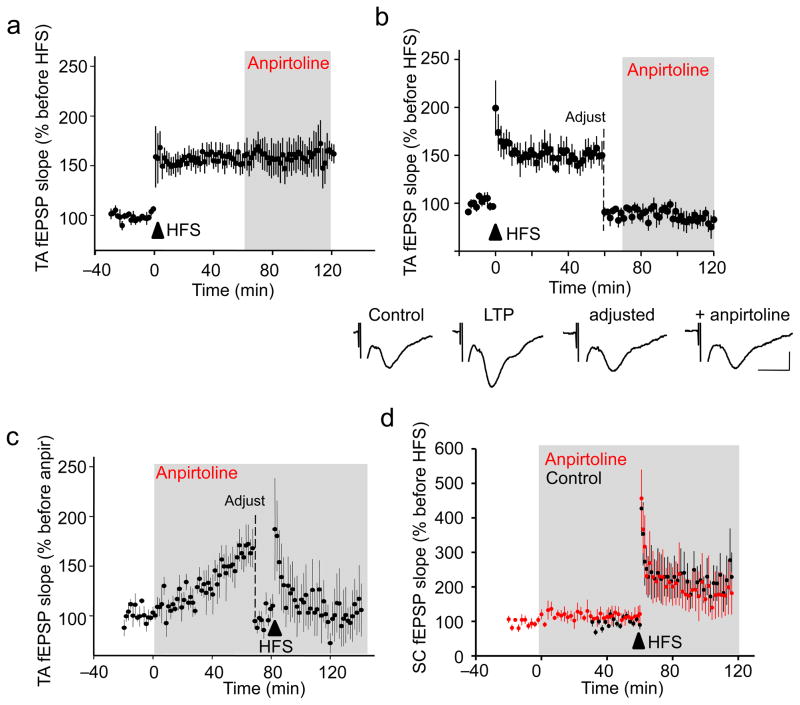
Figure 4. 5-HT1BR-mediated potentiation occludes LTP at TA-CA1 synapses and influences memory consolidation.
(a) After induction of LTP with high frequency stimulation (4 trains, 1 sec per train at 100 Hz, 5 min interval), bath application of anpirtoline (50 μM) failed to induce further potentiation of TA-CA1 fEPSP slope (n=8 slices). (b) The effect of anpirtoline on TA-CA1 fEPSP slope remained occluded after induction of LTP, even when the stimulation intensity was decreased to return the fEPSP to the baseline level (n=5 slices). Representative traces are shown below. Scale bar = 0.1 mV, 20 ms. (c) Anpirtoline-induced potentiation of TA-CA1 fEPSP slope occluded tetanus-induced LTP at TA-CA1 synapses (n=4 slices). (d) Anpirtoline neither enhanced SC-CA1 fEPSPs nor occluded LTP of SC-CA1 synapses (red; n=7 slices). LTP of SC-CA1 fEPSPs in control slices shown in black (n=5 slices). Only every other data point is plotted to allow the two data sets to be distinguished.
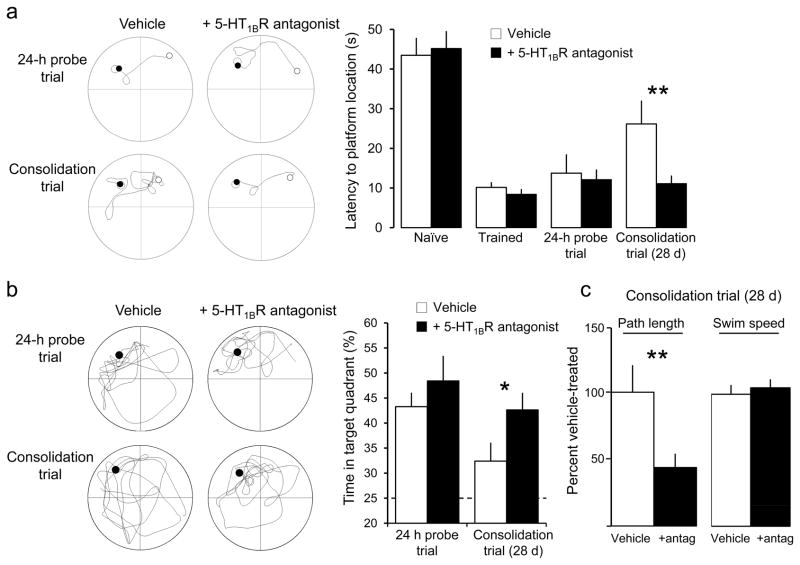
TA-CA1 synapses are strongly activated during cognitive tasks, including both spatial and nonspatial tasks involving working and associative memory in primates31. In rodents, TA-CA1 synapses support learning in the Morris water maze32 and are required for long-term memory consolidation12. In order to determine whether serotonin activates 5-HT1BRs at TA-CA1 synapses in intact animals, we asked whether a 5-HT1BR antagonist would have any influence on consolidation of spatially cued memory using the Morris water maze (Fig. 5). The time required for rats to locate a hidden platform became significantly shorter after six days of training. Successful memory of the location was verified in a probe trial 24 hrs after the final training session. Animals were then divided into two groups, one group administered the 5-HT1BR antagonist SB216641 (4 mg/kg i.p, daily for two weeks starting immediately after the probe trial)33 and another as controls (saline i.p. daily). The animals underwent a second probe trial to test consolidation 28 days after the end of training. Both groups of animals performed better in this trial than they had before training, indicating that they had consolidated some memory of the platform location. Rats treated with SB216641, however, displayed a significantly shorter latency to find the platform location than the untreated controls. Furthermore, the latency of the SB216641-treated rats to the platform location was not significantly different 28 days after training than it was in the 24 hour probe trial. Treated rats also crossed through the platform area significantly more times than the control animals (p < 0.05), and spent more time in the target quadrant, indicating a more accurate search pattern. Consistent with previous reports that 5-HT1BR−/− mice have improved short-term learning in the Morris water maze34, we conclude that activation of 5-HT1BRs by endogenous serotonin interacts with the memory consolidation process, perhaps through interaction with LTP at TA-CA1 synapses.
Figure 5. Spatial memory consolidation is affected by endogenous activation of 5-HT1BRs.
Latency to find the target platform in the Morris water maze (a) decreased significantly between the first (naïve) and last training trial (trained), demonstrating that the rats learned the location of the platform; remained short during a probe trial administered 24 hrs after the last training trial, demonstrating that the rats remembered the platform location; and remained significantly shorter than naïve when tested 28 days after the final training trial, demonstrating memory consolidation. Rats administered a 5-HT1BR antagonist (SB216641, 4 mg/kg, i.p.) daily for 14 days starting after the 24 hr probe trial displayed a significantly shorter escape latency in the consolidation trial than the control group (0.9% NaCl, i.p.). Representative examples of the swim path in the maze from the start (white) to stop (black) positions are shown at left for control and SB-treated individuals. SB-treated animals also spent more time in the target quadrant during the 28 day consolidation probe trial than controls (b), and swam a significantly shorter distance than controls (c). Both groups had identical swim speeds (c), however, indicating that the difference in performance was not due to altered motor function. Full swim paths for two different individuals in the probe trials are shown at left. Dashed line indicates random performance. **, p<0.05; *, p=0.06; n= 8 animals per group; post-hoc t-test. Further statistical information can be found in the Methods.
Potentiation is altered in a depression model
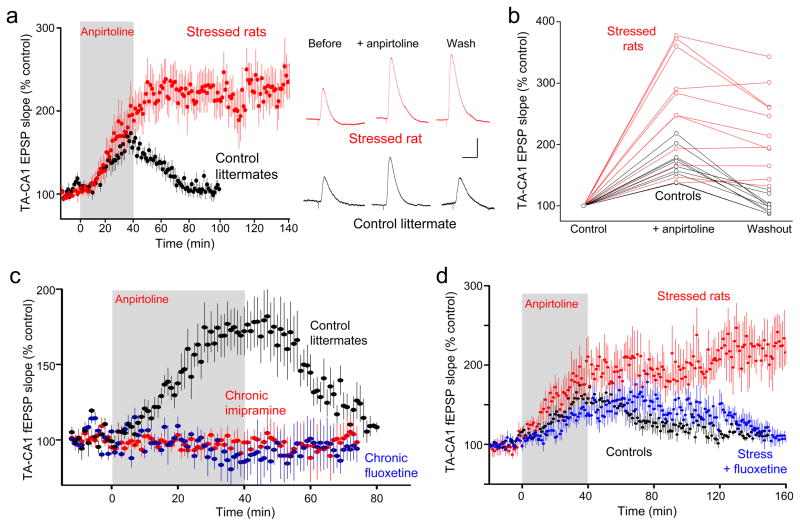
The serotonin hypothesis of depression postulates that changes in the actions of serotonin contribute to its pathology. Depression is a purely human condition, but a depressive-like behavioral state can be induced in rodents. We therefore investigated serotonin-induced potentiation in rats subjected to the chronic unpredictable stress (CUS) model of depression, which has face, construct, and predictive validity35. After three weeks of CUS, animals displayed both a significant decrease in sucrose preference and increased latency in novelty suppressed feeding (Supplementary Fig. 5a, b), as reported previously for rodent models of depression35,36. We observed that the anpirtoline-induced potentiation of TA-CA1 EPSPs was much greater in slices from CUS animals than in unstressed littermates, and that EPSP slope remained enhanced even after 100 minutes of washout in rats subjected to CUS, but not controls, in whole-cell (Fig. 6a, b) and extracellular (Supplementary Fig. 5c, d) recordings. The persistence of the potentiation was independent of its magnitude (Fig. 6b) and was resistant to isamoltane (Supplementary Fig. 5e). Despite the increased magnitude of the response in CUS animals, the potentiation remained specific to the TA-CA1 synapse (Supplementary Fig. 5d). Elevation of extracellular serotonin with acute application of imipramine also produced a large enhancement of TA-CA1 fEPSP slope in slices from animals subjected to CUS (230±46% in naïve vs. 370±88 in CUS, n=4,3; 2×2 ANOVA main effect of imipramine F(1,5)=18.22, p=0.008; main effect of group naïve vs. CUS F(1,5)=2.264, p=0.2). We conclude that endogenous serotonin can still be released from dorsal raphe afferents within the hippocampus in animals subjected to CUS, and that the resting level of extracellular serotonin is still regulated by the serotonin transporter, but that serotonin’s downstream signaling at excitatory synapses is increased in animals subjected to CUS.
Figure 6. Chronic stress enhances, whereas chronic antidepressants eliminate, the effect of anpirtoline on TA-CA1 synaptic transmission.
(a) Changes in TA-CA1 EPSP slope in slices from CUS animals (red, n=11 cells) and control littermates (black, n=10 cells) showing the significantly larger amplitude and persistence of potentiation in CUS animals, compared to controls. Representative traces before and after anpirtoline application and after 60 min of washout shown at right. Scale bars = 2 mV, 50 ms. (b) Changes in TA-CA1 EPSP slope induced by anpirtoline in slices from CUS animals (red) are larger and more persistent than the potentiation seen in slices from controls (black)(controls: peak change in fEPSP slope = 162±3%, fEPSP slope after 60 min wash = 106±3%; CUS: peak change = 226±23%, 218±21% after 60 min wash)(n=10,11 slices). (c) Anpirtoline produced no significant effect on fEPSP slope in slices from animals treated for 21–28 days with antidepressant imipramine (100 mg/L, red) or fluoxetine (80 mg/L, blue), whereas it doubled fEPSP slope in slices from controls in interleaved experiments (black) (imipramine: n=9 slices; fluoxetine: n=5 slices; controls: n=7 slices). (d) Administration of fluoxetine for the final 3 weeks in animals subjected to six weeks of CUS (blue, n=12 slices) resulted in a restoration of transient anpirtoline-induced potentiation (black, n=11 slices), clearly different from the irreversible potentiation in CUS animals (red, n=5 slices).
Many antidepressants elevate extracellular serotonin levels and this chronic elevation of serotonin increases AMPA receptor phosphorylation in the hippocampus37. We therefore asked what effect chronic antidepressant treatment of naïve rats might have on 5-HT1BR-mediated potentiation. In hippocampal slices from rats given imipramine or fluoxetine via their drinking water for 21–28 days, anpirtoline-induced potentiation of TA-CA1 fEPSP slope was absent, but was present in slices from untreated littermate controls (Fig. 6c). In contrast, anpirtoline produced normal potentiation in slices from animals treated with imipramine for only 36 hrs (not shown), insufficient time for most behavioral effects in rodents38 and depressed humans. Chronic antidepressant treatment thus prevents 5-HT1BR activation from potentiating TA-CA1 synapses; an effect directly opposite to the enhancement of 5-HT1BR-induced potentiation seen after CUS.
Finally, we observed that restoration of normal sucrose preference and novelty suppressed feeding behaviors in animals subjected to CUS for 3 weeks followed by an additional 3 weeks of CUS and fluoxetine administration (Supplementary Fig. 5a, b) was accompanied by restoration of normal magnitude, reversible potentiation of TA-CA1 synapses in response to anpirtoline (Fig. 6d). A brief period of fluoxetine administration (36 hrs), in contrast, failed to restore either sucrose preference or the reversible anpirtoline response (Supplementary Fig. 6). The response of TA-CA1 synapses to 5-HT1BR activation thus correlates with the affective state of the animals under several conditions.
Potentiation required for antidepressant action
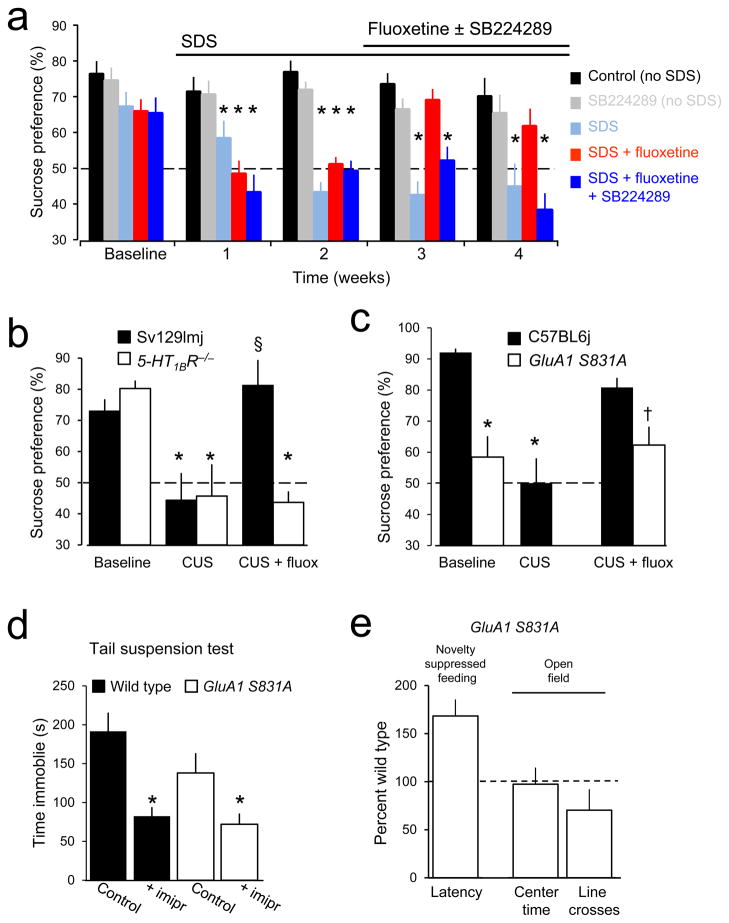
Does the 5-HT1BR-dependent potentiation of excitatory synaptic transmission observed in brain slices in response to acute elevation of serotonin play any role in the action of chronically administered SSRIs in assays of antidepressant response? The sucrose preference test was chosen as a behavioral endpoint because of its predictive validity. To answer this question we first used a pharmacological approach in C57BL6j mice subjected to the social defeat stress (SDS) model of depression39 (Fig. 7a). Sucrose preference was high in control mice, and remained high in the absence of SDS, but became significantly decreased after one week of SDS and remained low during succeeding weeks of SDS. Administration of fluoxetine via the drinking water restored a high level of sucrose preference to SDS animals after 1 week. Administration of the 5-HT1BR antagonist SB224289 (4 mg/l)40 had no effect on the high sucrose preference of naïve mice, but prevented the increase in sucrose preference produced by chronic fluoxetine administration in mice that had lost their sucrose preference after SDS. Similarly, naïve 5-HT1BR−/− mice had a high sucrose preference41 that was lost after 3–5 weeks of CUS (Fig. 7b). Three weeks of fluoxetine administration restored sucrose preference in wildtype Sv129Imj mice subjected to CUS, but not in 5-HT1BR−/− mice. We conclude that 5-HT1BR activation is necessary for the efficacy of SSRIs in this behavioral test, consistent with previous evidence that acute activation of 5-HT1BRs is sufficient for an antidepressant-like effect of SSRIs in the forced swim test42.
Figure 7. 5-HT1BR-dependent potentiation of GluA1 receptors is necessary for the therapeutic action of antidepressants.
(a) Sucrose preference was high in vehicle-treated control C57BL6j mice (black) and remained high. SDS eliminated sucrose preference after one week (light blue). Fluoxetine (80 mg/l) restored sucrose preference in SDS animals in which it had been lost (red). The 5-HT1BR antagonist SB224289 (4 mg/liter in drinking water) had no effect on sucrose preference in non-SDS mice (gray) but prevented restoration of sucrose preference in SDS mice administered fluoxetine (dark blue). * = significantly less than corresponding baseline. (b) Sucrose preference was high in naïve 5-HT1BR−/−mice (blue) and wild-type mice (Sv129Imj, black), and was decreased in both strains after CUS. Fluoxetine restored sucrose preference in wild-type, but not 5-HT1BR−/− mice. * = significantly different than baseline in same genotype (Bonferroni corrected post-hoc p<0.05). § = significantly different than after CUS (Bonferroni corrected post-hoc p<0.05). We conclude that 5-HT1BR activation is thus required for fluoxetine to exert its therapeutic action. (c) CUS decreased sucrose preference in control C57BL6j mice (Bonferroni corrected post-hoc p<0.02), which was restored by fluoxetine [baseline vs. fluoxetine p>0.5]. Sucrose preference was lower in GluA1 S831A mice (red) than in wild-type mice (C57BL6j, black)[Student’s t(19)= 3.37, p<0.005]. Sucrose preference in GluA1 S831A mice was not affected by fluoxetine [t(14)= 0.335, p>0.7 with paired t-test], and antidepressant treated GluA1 S831A mice had lower sucrose preference than controls [Student’s t(25)=2.42, p<0.05 with Bonferroni adjustment for multiple comparisons]. * = significantly less than C57BL6j baseline, †, significantly different than C57BL6j baseline, but not GluA1 S831A baseline. (d) In the tail suspension test, C57BL6J littermates (black) treated with imipramine spent less time immobile than wild-types treated with saline [t(10)= 4.10, p<0.005]. S813A mice (red) treated with imipramine spent less time immobile than GluA1 S831A mice treated with saline [t(13)= 2.35, p<0.05]. * = significantly different than control in same genotype. A behavioral response to acute administration of antidepressants was thus preserved in this assay, unlike the response to chronic antidepressants in the sucrose preference test. (e) Latency to feeding in the novelty-suppressed feeding test was longer in GluA1 S831A mice (n=14) than in wild-type C57BL6j mice (n=13)[t(27)= −2.10, p<0.05]. GluA1 S831A mice did not differ from wild-type littermates with regard to time spent in the center in the open field test, but completed fewer line crossings than littermates, consistent with a hypo-locomotor phenotype, but not increased anxiety [t(13)= −2.29, p<0.05]. * = significantly different than wildtype. Taken together with lower sucrose preference, GluA1 S831A mice display a depressive-like behavioral phenotype. Further statistical information can be found in the Methods.
Is 5-HT1BR-mediated potentiation of AMPARs also required? GluA1 S831A mice displayed a low preference for 1% sucrose during the baseline period (Fig. 7c). Administration of fluoxetine for three weeks failed to induce a significant increase in preference for 1% sucrose in GluA1 S831A mice, but did restore sucrose preference in wild-type C57BL6j mice that had lost their sucrose preference after CUS. GluA1 S831A knock-in mice did respond to acute imipramine administration in the tail suspension test (Fig. 7d), in contrast, indicating that serotonin uptake remains sensitive to inhibition by tricyclics in these mice and highlighting the importance of serotonin-induced plasticity of glutamatergic transmission in the ability of antidepressants to reverse anhedonia. The lack of preference for 1% sucrose in naïve GluA1 S831A mice suggested that they might have a depressive-like phenotype. We therefore compared novelty suppressed feeding in these mice with their wild-type controls (Fig. 7e). The GluA1 S831A mice required almost twice as long as wild-type C57BL6j mice to begin eating food in the brightly lit, novel arena, consistent with behavioral changes induced in several animal models of depression36. They also displayed a hypo-locomotor phenotype in the open field test, without evidence of anxiety (Fig. 7e).
Taken together, we conclude that 5-HT1BR activation and subsequent phosphorylation of GluA1Rs by CaMK is required for the ability of chronically administered SSRIs and tricyclic antidepressants to reverse anhedonia in these assays. Because the sucrose preference test does not depend on the hippocampus, these experiments support the suggestion that serotonin-induced potentiation of excitatory synaptic transmission occurs in multiple brain regions involved in hedonic behaviors.
DISCUSSION
We have observed that SSRIs, tricyclic antidepressants, agonists of 5-HT1BRs, and conventional LTP all potentiate excitatory synaptic transmission at TA-CA1 synapses and occlude each other, suggesting that the molecular events triggered by serotonin and conventional antidepressants overlap with those underlying activity-dependent synaptic plasticity to a surprising degree5. Activation of 5-HT1BRs enhanced AMPA receptor-mediated synaptic excitation by activating CaMK and causing phosphorylation of GluA1 at serine 831. We also observed that long-term memory consolidation, which depends upon intact TA-CA1 synaptic circuits12,32, was enhanced by inhibiting 5-HT1BRs. We suggest that release of endogenous serotonin and activation of 5-HT1BRs occurs during memory consolidation, after learning has taken place, and influences the ability of TA-CA1 synapses to participate in the consolidation process. Unlike LTP, 5-HT1BR-mediated potentiation was reversed rapidly after withdrawal of agonist. Given the similarity of the biochemical pathways, this difference is surprising and cannot be readily explained. Induction of LTP requires CaMK activity, whereas maintenance of LTP does not43. It thus appears that LTP induction is accompanied by recruitment of some additional signaling process(es) that are not recruited by 5-HT1BR activation. A better understanding of the second-messenger pathways downstream of this novel form of potentiation may reveal key biochemical steps distinguishing the induction of potentiation, which seems to occur via a common mechanism in both forms of potentiation, from the maintenance of potentiation, which only occurs after induction of LTP.
TA-CA1 synapses were potentiated by serotonin, but nearby SC-CA1 synapses were not. The signaling processes underlying this phenomenon must therefore be highly localized in postsynaptic CA1 cell dendrites. Serotonin release can be predicted to change the flow of information through the hippocampal circuitry, favoring the so-called direct pathway at the expense of the indirect pathway. Regulating information flow through a neural circuit is a unique mechanism of action for neuromodulatory transmitters, and may be generally applicable in other brain regions. Conversely, a dysregulation of information flow due to altered serotonin receptor signaling or altered regulation of excitatory synapses may contribute to the cognitive dysfunction of depression.
Animals subjected to chronic stress exhibit elevated glucocorticoid levels, and elevated glucocorticoids damage neuronal structure and function in the hippocampus44, including impairing Schaffer collateral LTP45. We observed that serotonin-induced potentiation of TA-CA1 synapses was altered in two distinct ways in chronically stressed rats: the potentiation was increased in magnitude and became irreversible. We do not yet know the explanation for these changes. A decrease in initial synaptic strength6 could potentially account for the increase in the magnitude of the potentiation, but the persistence of the potentiation in CUS animals was independent of its magnitude (Fig. 6). Chronic antidepressant administration in naïve animals decreased the potentiation and restoration of sucrose preference in CUS animals treated chronically with antidepressants was accompanied by a restoration of the normal transient potentiation. Short-term administration of antidepressants had no effect on serotonin-induced potentiation in either naïve or CUS animals. The response of TA-CA1 synapses to 5-HT1BR activation correlates well with the affective state of the animals under five experimental conditions, and thus represents a unique endophenotype of this model of depression.
Evidence is increasing that excitatory synapses are altered in models of depression4,5. Depression of AMPAR-mediated excitation of neurons in the reward circuitry of the nucleus accumbens (NAc) is required for the genesis of some depressive-like behaviors after chronic restraint stress, including anhedonia in the sucrose preference test10. The source of the inputs that become depressed was not identified, but the NAc receives prominent projections from the hippocampus and frontal cortex. The serotonin-mediated plasticity we describe here may act to counteract this depression of NAc excitatory synapses in two ways. First, serotonin-induced potentiation of TA-CA1 synapses increases hippocampal action potential discharge (suppl. Fig. 3b) and should increase the excitatory drive onto NAc cells, promote de-depression of NAc inputs, and thereby act to reverse anhedonia and restore the rewarding values of peripheral stimuli, such as sucrose. Consistent with this suggestion, activation of hippocampal 5-HT1BRs has been shown to increase dopamine levels in the NAc46 and increased dopamine alleviates depressive-like behaviors47. The enhanced and persistent action of serotonin in the CUS animals (Fig. 6) may be interpreted not only as a unique stress-induced form of plasticity of excitatory synapses, but also as a potential compensatory response, acting to promote the de-depression of hippocampal-NAc inputs. Second, medium spiny cells in the NAc express high levels of mRNA for 5-HT1BRs. If the plasticity we describe here also occurs in NAc D1-expressing neurons, then direct potentiation of excitatory synapses should counteract stress-induced depression of excitatory synapses10. Consistent with this possibility, we observed a 5-HT1BR-induced activation of CaMK and phosphorylation of S831 of GluA1 receptors in the NAc.
Our results link antidepressant-induced elevation of serotonin to functional potentiation of excitatory synapses in vivo for the first time. We found that pharmacological inhibition or genetic deletion of 5-HT1BRs prevented the ability of chronically administered fluoxetine to restore sucrose preference in two chronic stress models. Furthermore, sucrose preference was low in GluA1 S831A mice and was not increased with three weeks of fluoxetine administration. We therefore suggest that conventional antidepressants exert at least some of their therapeutic actions by promoting on-going serotonin-induced, 5-HT1BR-mediated potentiation of excitatory synaptic transmission. It is noteworthy that acute application of SSRIs produced a rapid potentiation of excitatory synaptic transmission in brain slices, whereas the therapeutic action of SSRIs in depressed humans and in animal models of depression requires several weeks. We do not yet have an explanation, but suggest that induction of potentiation is a critical first step that improves affective state by promoting action potential firing which subsequently triggers other slower, synergistic activity-dependent processes1, such as growth factor signaling, neurogenesis, and changes in gene expression. Repeated bouts of potentiation may be required to produce lasting changes, as is observed with deep brain stimulation and electroconvulsive therapy.
Given the plethora of behavioral changes that define depression, it is unlikely that there is one specific brain region that would be critical to the ability of antidepressants to restore these functions. The plasticity we describe here for TA-CA1 synapses is attractive as a potential explanation for some of serotonin’s therapeutic actions because it is generalizable: like LTP for learning and memory, this form of plasticity may, in principle, take place at any excitatory synapse at which 5-HT1BRs are expressed and may thus re-normalize activity in almost any region in the depressed brain. This is in contrast to the well documented ability of serotonin to promote dentate gyrus neurogenesis, which does not occur in any other brain region. The TA-CA1 synapse serves as a useful archetype to study the actions of serotonin on excitatory synaptic transmission and the correlates of a depression-like state.
Elevating monoamine levels improves the affective state of depressed patients- the findings that led to the serotonin hypothesis of depression3- but the evidence of a deficiency in serotonin synthesis or release in depression is modest. Instead, our results lead us to propose an excitatory synapse hypothesis of depression. We postulate that changes in excitatory synapses are fundamental to the genesis of depression and that their restoration is critical to the relief of depression. We found that induction of serotonin-mediated potentiation was abnormally enhanced and prolonged after CUS and that GluA1 S831A mice, like GluA1−/− mice48, displayed a depressive-like phenotype. Similarly, glutamate receptor expression is decreased6 and excitatory synaptic transmission is depressed10 in other systems after repeated stress. Serotonin-mediated potentiation would be predicted to restore the normal strength of synapses weakened by chronic stress. Indeed, we observed that 5-HT1BR activation and GluA1 phosphorylation at S831 were necessary for fluoxetine to restore sucrose preference in chronically stressed animals, providing evidence that this action may be critical to the therapeutic efficacy of SSRIs. It is noteworthy that these effects of conventional, slowly acting antidepressants, such as SSRIs and tricyclics, are shared by newly identified, fast-acting antidepressants. For example, ketamine potentiates excitatory synapses in hippocampal slices and its immediately effective therapeutic actions have been attributed to enhanced activation of glutamatergic circuits49. Potentiation of excitatory synaptic transmission by serotonin in multiple brain regions involved in cognitive function, and its bidirectional alteration by stress and chronic antidepressants, provides a novel link between the pathology of depression and its treatment, and thus represents a new perspective on depression and antidepressant action. (4334 words)
Full Methods are available on the Nature Neuroscience website.
METHODS
All protocols were approved by the University of Maryland School of Medicine and St. Mary’s College of Maryland Institutional Animal Care and Use Committees.
Acute slice electrophysiology
Hippocampal slices were prepared from 3- to 6-week-old male Sprague-Dawley rats or mice (C57BL6j or Sv129Imj, as described). Dissection was done in ice-cold artificial cerebrospinal fluid (ACSF, in mM: 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1.5 MgCl2, 2.5 CaCl2, 26 NaHCO3, and 10 glucose) bubbled with 95%O2/5%CO2. Brain slices (400 μM) were cut on a vibratome and kept in a holding chamber at room temperature at the interface of ACSF and humidified 95% O2-5% CO2 for >1 hr. The slices were then transferred to a submersion-type recording chamber and perfused at room temperature with ACSF (flow rate = 1–2ml/min). Picrotoxin (0.1 mM) and CGP52432 (2μM) were included to block GABAA and GABAB receptors. Area CA3 and the dentate gyrus were cut from the slice to prevent spontaneous epileptiform discharge (Suppl. Fig. 1a). Concentric bipolar tungsten electrodes were placed either in SLM to stimulate TA afferents or in SR to stimulate SC afferents (Suppl. Fig. 1a). Extracellular recording pipettes were filled with ACSF (3–5 MΩ) and placed 100–150 μm from the stimulating electrodes. Stimuli (100 μs duration) were delivered at 0.05 Hz. The stimulus intensity was set at 150% of threshold intensity, resulting in an fEPSP of 0.1 – 0.2 mV. All compounds were applied by perfusion. fEPSPs were recorded using Axoclamp 2B (Molecular Devices) or n.p.i. (n.p.i., Tamm, Germany) amplifiers, filtered at 10 kHz, and amplified 100× prior to digitization. Whole-cell intracellular recordings were obtained with patch pipettes (tip resistances = 3–6 MΩ) filled with: (in mM) Cs- or K-methanesulphate 135 (for voltage- or current-clamp recording, respectively), HEPES 10, NaCl 10, MgCl2 1, K4BAPTA 0.1, Mg2+-ATP 2, and phosphocreatine 10 (adjusted to pH 7.3 with KOH). All experiments were performed at room temperature. Whole-cell recordings were made from cell bodies and were made “blind”.
Photolysis
As in our previous work (Cai et al., 2004), an argon ion laser fitted with UV optics was used to produce a continuous 300 mW beam of UV light at the 355 and 361 nm lines. The light was focused into a 25 μm multimode quartz fiber and delivered to the preparation via a dichroic mirror, so as to permit simultaneous wide-field excitation with an HBO lamp. Laser flash duration was controlled by a high speed electromechanical shutter. The proximal end of the fiber was focused via a relay lens assembly in a conjugate image plane with respect to the cell and positioned with micromanipulators near the center of the field of view through the 60× (1.0 N.A.) water immersion objective of an upright microscope (Nikon). The location and focus of the UV spot within the tissue was determined from excitation of a dye-filled dendritic shaft by an attenuated beam. Intracellular Alexa 568 fluorescence was imaged with a CCD camera (Hamamatsu Orca ER II, effective pixel size = 0.108 μm2) controlled by Simple PCI software (Compix, Inc). Caged glutamate (0.5 mM N-Ncm-Glu) and the antioxidant Trolox (0.1 mM) were added via perfusion.
Western blotting
Protein expression was quantified using Western blotting. Area SLM or SR tissue wedges were dissected from hippocampal slices (3–4) under ice cold saline, pooled and homogenized in ice cold lysis solution containing phosphatase and protease inhibitors (PPI, Sigma, Saint Louis, MO) and sample buffer (Laemmli, Sigma), boiled, and loaded into 4–12% Bis-Tris gel (Invitrogen). After running in 1X NuPage MOPS SDS buffer, the gel was transferred onto polyvinylidene difluororide membranes in 1X Nupage transfer buffer (in 10% methanol). The membrane was blocked with 5% nonfat dry milk in buffer containing 1M Tris-buffered saline and 0.05% Tween, and probed with antibodies against Ser845-phosphorylated GluR1 (1:1000; Millipore AB5849 ), Ser831-phosphorylated GluR1 (1:1000; Sigma A4352), and Thr286-phosphorylated CaMKII (1:5000; Cell Signaling Technology #3361) at 4°C overnight. After rinses in TBS-Tween, the membrane was incubated for 1 hr at room temperature in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1000, Cell Signaling Technology #7074). The immunoblot was developed with enhanced chemiluminescence (Amersham). Membranes were then stripped, blocked, and reprobed with antibodies against GluR1 (0.5 μg/ml; Thermo Scientific PA1-37776), CaMKII (1:2000; Cell Signaling Technology #3362), or β-actin (1:2000; Cell Signaling Technology #4967). Levels of phosphorylation, expressed as the ratio of phospho-specific intensity divided by total protein intensity and computed with ImageJ, were used for statistical analysis. For display purposes, blots were cropped (see supplemental material) and brightness and contrast were adjusted globally using Photoshop.
Serotonin depletion
Rats were given two injections of the tryptophan hydroxylase inhibitor p-chlorophenylalanine (300 mg/kg, i.p., 24 hrs apart)(Dewar et al., 1992) before preparing slices for electrophysiological recording. Tissue from the same animals was fixed, mounted in gelatin, sectioned at 50 μm, and stained with a polyclonal antibody against serotonin (1:1000; Millipore AB125), followed by labeling with a secondary antibody conjugated with the fluorophore Cy2 and epifluorescence microscopy.
Chronic unpredictable stress (CUS) procedure
Adult Sprague-Dawley rats (3–4 weeks old) were randomly divided into control and CUS groups. CUS animals were treated as following: Day 1, cage rotation (3 h), forced swim (5 min), food deprivation (16h). Day 2, strobe light (30 min), restraint (30 min), food and water deprivation (16 h). Day 3, strobe light (30 min), social isolation (16 h). Day 4, strobe light (30 min), restraint (30 min). Day 5, cage rotation (3 h), water deprivation (16 h). Day 6, restraint (3 h), social isolation (16 h). Day 7, cage rotation (3 h), restraint (30 min). The cycle was repeated over 3–5 weeks. Electrophysiological experiments were then performed and analyzed with the experimenter blinded to the experimental condition of the animals.
Social Defeat Stress (SDS) procedure
Individual male test mice (>4 weeks old) were introduced into the home cage of an unfamiliar resident mouse for 5 min and observed to ensure that they were physically attacked and defeated, as indicated by freezing behavior and submissive posture. Resident mice were CD1 breeders selected for short attack latencies. After 5 min of physical interaction, the test mouse was kept for 1 hr in the resident’s cage while separated by a perforated screen that protected the test mouse from physical contact, but permited olfactory, visual and auditory contact. The SDS protocol was maintained for five weeks. To avoid individual differences in intensity of defeat, the test animals were confronted with a different resident each day.
Sucrose preference
After 3 hours of water deprivation, rats were given a choice between two bottles for 3–4 hrs, one with 1% sucrose solution and another with normal drinking water. To prevent the possible effects of side preference in drinking, the position of the bottles was reversed after 2 hrs. Mice were tested over 12 hours (6 hrs light/6hrs dark) with bottle positions switched after 6 hrs. The consumption of water and sucrose were measured by weighing the bottles. Preference for sucrose was calculated as a percentage of consumed sucose-containing solution relative to the total amount of liquid intake. 50% means that they drank equally from both bottles, i.e. they had no preference for sucrose. Naïve animals were tested repeatedly in a group housing until the cage had a consistent preferences for 1% sucrose, but were always tested individually thereafter. For experiments with only wild type animals, any individual that did not demonstrate a sucrose preference >65% was discarded. In some experiments, animals were first trained to the task using 2% sucrose. Tests were repeated once per week during the course of CUS or antidepressant administration.
Novelty supressed feeding
Novelty suppressed feeding tests were performed as previously (Santarelli et al., 2003). The test apparatus was a brightly lit arena (60 × 60 × 35 cm for rats, 50 × 40 × 25 cm for mice) with a solid floor placed in a dimly lit room. The floor of the box was covered with a layer of bedding. Two laboratory chow pellets were placed on a white paper circle platform positioned in the center of the box. Rats that had been food deprived for 24 hours, and mice food depreived for 3 hours, were gently placed in a corner of the arena. The latency to begin eating, defined as active chewing of the pellet, was recorded. A maximum time allowance was set at 600 s. Immediately after the test, animals were returned to their home cage and allowed to feed for 5 min. Food pellets were weighed before and after the 5 min, and the amount of food consumed was calculated. Rats that ate less than 0.3 g of food within this 5-minute period were removed from all analyses, in order to ensure that only sufficiently hungry animals were included. Similarly, mice that failed to actively chew on a pellet were not included.
Open field test
Mice were placed in a 60 × 60 × 35 cm plexiglass box for 5 min. The box was divided into 12 squares using tape on the bottom of the box. Mice were video recorded and a blinded experimenter calculated the time spent in center two squares and the number of line crossings for each animal.
Tail Suspension Test
Mice were intraperitoneally injected with saline or 30 mg/kg imipramine 30 min prior to testing. Each mouse was taped to a wooden horizontal bar 2 in from the base of its tail. A blinded experimenter recorded the amount of time spent immobile for a 6 min period.
Antidepressant treatment
Animals were given antidepressants via their drinking water in order to minimize stress. The concentrations of antidepressants were: imipramine, 100 mg/liter; fluoxetine, 80 mg/liter. Animals were housed singly and drinking water was changed every 3 days. Animals were given antidepressants continually for 3 – 4 weeks. Control animals received water only. Experiments were then performed and analyzed with the experimenter blinded to the experimental condition of the animals.
Data analysis
Experiments were performed with the investigators blinded to the genotype of the animals or the identity of the substance being applied whenever possible. The blind was not broken until data analysis was complete. For quantification of anpirtoline and antidepressant actions in electrophysiological experiments, fEPSP or EPSC slope values were averaged and quantified over a three minute period preceding application of the substance (control) and a three minute period at the end of the substance application (effect).
Memory consolidation
Sixteen male 70–80 day-old Sprague Dawley rats were housed two per cage in a temperature controlled room. Rats were kept on a 12:12 light-dark schedule and all testing was done during the light phase. All rats were handled for approximately 5-min per day for the four days prior to testing. The rats were given unlimited access to food and water throughout the testing periods. The water maze (139.7 cm in diameter) was filled with black-colored water created with non-toxic black paint. During training, a clear Plexiglas platform (10.16 cm in diameter) was submerged 0.5 cm below the water surface. Three black and white geometric spatial cues were placed around the maze and remained in a fixed location throughout training and testing.
Each rat was randomly assigned a quadrant (NW, NE, SE, SW) in which the platform would always be located. Following previous protocols (Remondes and Schuman, 2004), rats were then given 10 training blocks in the water maze across 6 days. Days 1 and 6 contained one training block. Days 2–5 contained two training blocks separated by a minimum of 2 hours. Each training block consisted of 4 training trials in which the animal was placed in a random starting location in the pool (NW, NE, SE, SW) and given 120s to swim to the platform. The animal was allowed to remain on the platform for 30 seconds. If the rat did not reach the platform in 120 seconds, it was guided to the platform and left to sit on the platform for 30 seconds. Animals were given a 2-minute inter-trial interval. Two probes tests were conducted in which the platform was removed, animals were placed in a random starting location, and were allowed to swim in the maze for 60 seconds. One probe test was given 24 hours after the completion of the final training block. The second probe was given 28 days after the original probe trial. Latency to the target, distance to target, time spent in the target quadrant, and swim speed were recorded by HVS Image software (Hampton, UK).
Following completion of the first probe test, animals were treated with either the 5-HT1BR antagonist SB216641 (Tocris) or 0.9% physiological saline for 14 consecutive days. Intraperitoneal injections of SB216641 (4 mg/kg; 4mg/ml) or saline were given at the same time each day immediately after light onset in the housing room.
The animals did not differ in performance during the original training blocks. A 2 ×2 mixed Analysis of Variance (Block × Group) was used to examine performance on the original and final training block. The escape latency decreased significantly from Block 1 (M = 44.34, SEM = 3.1) to Block 10 (M = 8.1, SEM = 0.9), F (1, 13) = 107.4, p < 0.001. There was no difference between untreated (M = 26.4, SEM = 2.1) and future SB-treated (M = 26.0, SEM = 2.1) animals, F (1, 13) = 0.02, p > 0.05. There was no interaction, F (1, 13) = 0.36, p > 0.05.
Examination of performance on the probe trial given 24 hours after the final training block also showed no group differences. There was no difference in the percentage of time spent in the target quadrant, t (14) = 0.91, p > 0.05. There was no difference in path taken to the target area, t (14) = 0.46, p > 0.05. There was no difference in latency to the target area, t (14) = 0.3, p > 0.05, and no difference in swim speed between the animals, t (14) = 0.51, p > 0.05.
Twenty-eight days after the end of training in the water maze, the animals were tested for consolidation of original training. Animals treated with SB216641 demonstrated significantly improved retention of spatial information. During the probe trial, animals treated with SB (M = 42.66, SEM = 3.4) spent an increased percentage of time in the target quadrant compared to the untreated animals (M = 32.41, SEM = 3.69), t (14) = 2.04, p = 0.06. Animals treated with SB (M = 1.77, SEM = 0.42) used a shorter path to the target area than the untreated animals (M = 4.05, SEM = 0.82), t (14) = 2.48, p = 0.27. The SB treated animals (M = 11.11, SEM = 2.06) also demonstrated a significantly shorter latency to the target area than the untreated animals (M = 26.26, SEM = 5.89), t (14) = 2.41, p = 0.03. There were no differences in swim speed between the two groups, t (14) = 0.5, p > 0.05.
Therapeutic actions of 5-HT1BRs
SB-224289 was used as the 5HT1BR antagonist and was given to mice via drinking water. The antagonist was administered at 4 mg/liter. Water levels were checked daily to ensure each mouse was receiving the intended SB dose throughout the allotted period.
Preference for sucrose was analyzed in the experiments of Fig. 7a by a 5 × 5 Mixed Analysis of Variance (ANOVA; Time × Group). There was a significant effect of Time, F (4, 100) = 9.33, p < 0.001 as the overall sucrose preference decreased across weeks. There was a significant effect of Group, F (4, 25) = 47.3, p < 0.001, and there was a significant interaction between Time and Group, F (16,100) = 2.99, p <0.001. Each time-point was then analyzed by a between-subjects one-way ANOVA. There were no differences between the groups in sucrose preference at week 1 (baseline), F (4,32) = 2.26, p > 0.05.
The groups were significantly different in sucrose preference levels at week 2, F (4,32) = 9.2, p < .001. At week 2, all socially defeated animals (SDS, SDS + fluoxetine, fluoxetine + SB224289) had a significantly lower sucrose preference than both control groups as measured by independent t-tests, all p’s < 0.003. This pattern remained at week 3, as the overall groups were significantly different, F (4,26) = 36.2, p < 0.001 and all socially defeated animals had significantly lower sucrose preferences than the controls, all p’s < 0.001.
At week 4, the groups were significantly different, F (4,26) = 6.78, p = 0.001. Independent t-tests indicated that the control animals had a significantly higher sucrose preference than the SDS group, t (8) = 9.95, p < 0.001, and a significantly higher sucrose preference than the SB + fluoxetine group, t (12) = 2.5, p = 0.03. The SB group had a significantly higher sucrose preference than the SDS group, t (8) = 4.57, p = 0.002. However, the SDS + fluoxetine group had a significantly higher sucrose preference than the SDS group, t (9) = 5.6, p < .001, but was not different than the SB + fluoxetine group, t (13) = 1.889, p = .081.
Groups differed in overall sucrose preference at week 5, F (4,25) = 6.9, p = 0.001. The control animals had a significantly higher sucrose preference than the SDS group, t (8) = 5.02, p = 0.001, and the SB + fluoxetine group, t (11) = 4.19, p = 0.002. The SB group had a significantly higher sucrose preference than SB + fluoxetine group, t (11) = 2.77, p = 0.018, but was not different from the SDS group, t (8) = 2.09, p = 0.07. The SDS + fluoxetine group had a significantly higher sucrose preference than the SDS group, t (9) = 3.34, p = 0.009 and the SB + fluoxetine group, t (12) = 3.28, p = 0.007.
Finally, dependent t-tests indicated that sucrose preference significantly increased in the SDS + fluoxetine group from week 3 to week 4, t (6) = 4.28, p = 0.005, and remained higher at week 5 compared to week 3, t (6) = 3.11, p = 0.02. However, no increase in sucrose preference was seen in the SB + fluoxetine animals between week 3 and 4, t (7) = 1.77, p = 0.12, or between week 3 and week 5, t (6) = 1.7, p = 0.15.
In the experiment with 5-HT1BR−/− mice (Fig. 7b), two-factor ANOVA found a significant effect of genotype: F(1,12)= 2.965, p=0.111; effect of condition: F(2,24)= 12.594, p<0.001; and interaction between genotype and condition: F(2,24)= 8.090, p=0.002. In the experiment with GluA1 S831A mice (Fig. 7c), there was a significant effect of condition in the control mice by repeated-measures ANOVA [F(2,10)= 8.67, p<0.01]). Finally, a one-way ANOVA showed a significant group effect in the tail suspension test (Fig. 7d). [F(3,23)= 7.70, p=0.001].
Supplementary Material
Supplementary Figure 1. Serotonin effects in the hippocampus
Supplementary Figure 2. Serotonin-induced potentiation requires 5-HT1BRs and is selective for TA-CA1 synapses.
Supplementary Figure 3. Promotion of action potential firing and enhancement of TA-SC interaction by 5-HT1B receptor activation.
Supplementary Figure 4. 5-HT1BR activation phosphorylates serine 831 at TA-CA1 synapses but not SC-CA1 synapses and is required for potentiation by fluoxetine.
Supplementary Figure 5. Altered behavior in animals subjected to CUS is accompanied by a qualitative and quantitaive change in responses to anpirtoline.
Supplementary Figure 6. Effects of brief administration of fluoxetine.
Supplemental Figure 7. Uncropped Western blots
Acknowledgments
We thank Drs. Susanne Ahmari and René Hen (Columbia University) for providing the 5-HT1BR−/− mice; Drs. Bradley Alger, Thomas Blanpied, Todd Gould, Jimok Kim, and Margaret McCarthy for their comments; Stephanie Aungst for advice on immunohistochemistry; Hannah Zimmerman, Leepeng Mok, and Michael Taylor for technical assistance; the Mr. and Mrs. Robert and Lee Peterson Southwest Florida NARSAD Young Investigator Award (X.C.), the Howard Hughes Medical Institute (R.L.H.), and the National Institutes of Health (R.L.H., H.-K.L., X. C., S.M.T.) for financial support.
Footnotes
The authors declare they have no conflicting financial interests.
Author contributions X.C., A.J.K., M.D.K., A.M.B., and S.M.T. designed the research; X.C., A.J.K., M.D.K., S.G., K.G., and A.M.B. performed the experiments and analyzed the data; H.-K.L. and R.L.H. contributed the GluA1 S831A mice; X.C., A.J.K., and S.M.T. prepared the manuscript; all authors discussed the results and commented on the manuscript.
Additional details of statistical analyses for some experiments and supplemental figure legends
References
- Cai X, Liang CW, Muralidharan S, Kao JPY, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Dewar KM, Grondin L, Carli M, Lima L, Reader TA. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem. 1992;58:250–257. doi: 10.1111/j.1471-4159.1992.tb09303.x. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
Reference List
- 1.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings AG, Cronkite RC, Moos RH. Social-environmental factors in unipolar depression: comparisons of depressed patients and nondepressed controls. J Abnorm Psychol. 1983;92:119–133. doi: 10.1037//0021-843x.92.2.119. [DOI] [PubMed] [Google Scholar]
- 3.Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- 4.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- 8.Hickie I, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 9.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 10.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihara N, Ueda S, Kawata M, Sano Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the mammalian hippocampal formation. Acta Anat (Basel) 1988;132:335–346. doi: 10.1159/000146599. [DOI] [PubMed] [Google Scholar]
- 12.Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- 13.Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28:6272–6280. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ait AD, Segu L, Naili S, Buhot MC. Serotonin 1B receptor regulation after dorsal subiculum deafferentation. Brain Res Bull. 1995;38:17–23. doi: 10.1016/0361-9230(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 16.Sari Y, et al. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 17.Saudou F, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 18.Svenningsson P, et al. Alterations in 5-HT1B receptor function by p11 in depressive-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 19.Gothert M, et al. 5-HT3 receptor antagonism by anpirtoline, a mixed 5-HT1 receptor agonist/5-HT3 receptor antagonist. Br J Pharmacol. 1995;114:269–274. doi: 10.1111/j.1476-5381.1995.tb13222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewar KM, Grondin L, Carli M, Lima L, Reader TA. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem. 1992;58:250–257. doi: 10.1111/j.1471-4159.1992.tb09303.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharp T, Bramwell SR, Grahame-Smith DG. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- 23.Cai X, et al. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Hsu EH, Lochan AC, Cowen DS. Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK. J Pharmacol Exp Ther. 2001;298:825–832. [PubMed] [Google Scholar]
- 25.Leone AM, Errico M, Lin SL, Cowen DS. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J Neurochem. 2000;75:934–938. doi: 10.1046/j.1471-4159.2000.0750934.x. [DOI] [PubMed] [Google Scholar]
- 26.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 27.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluA1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Takamiya K, Hen K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1(GluA1) phosphorylation sites in regulation synaptic plasticity in the CA1 region of the hippocampus. J Neurophysiol. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 31.Sybirska E, Davachi L, Goldman-Rakic PS. Prominence of direct entorhinal-CA1 pathway activation in sensorimotor and cognitive tasks revealed by 2-DG functional mapping in nonhuman primate. J Neurosci. 2000;20:5827–5834. doi: 10.1523/JNEUROSCI.20-15-05827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 33.Tatarczy3ska E, Klodzi3ska A, Stachowicz K, Chojnacka-Wójcik E. Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav Pharmacol. 2004;15:523–534. doi: 10.1097/00008877-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Buhot MC, Wolff M, Benhassine N, Costet P, Hen R, Segu L. Spatial learning in the 5-HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn Mem. 2003;10:466–477. doi: 10.1101/lm.60203. [DOI] [PubMed] [Google Scholar]
- 35.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacol (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 36.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svenningsson P, et al. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci U S A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacol. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 39.Malatynska E, Knapp R. Dominant-submissive behavior as models of mania and depression. Neurosci & Biobehavioral Rev. 2005;29:715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Gaster LM, Blaney FE, Davies S, Duckworth DM, Ham P, et al. The selective 5-HT1B receptor inverse agonist 1′-methyl-5-[[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro-spiro[furo[2,3-f]indole-3,4′-piperidine](SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. J Med Chem. 1998;41:1218–1235. doi: 10.1021/jm970457s. [DOI] [PubMed] [Google Scholar]
- 41.Bechtholt AJ, Smith K, Gaughan S, Lucki I. Sucrose intake and fasting glucose levels in 5-HT(1A) and 5-HT(1B) receptor mutant mice. Physiol Behav. 2008;93:659–665. doi: 10.1016/j.physbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill MF, Conway MW. Role of 5-HT1A and 5-HT1B receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacol. 2001;24:391–398. doi: 10.1016/S0893-133X(00)00196-2. [DOI] [PubMed] [Google Scholar]
- 43.Buard I, et al. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krugers HJ, Lucassen PJ, Karst H, Joëls M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:1–10. doi: 10.3389/fnsyn.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 46.Boulenguez P, Rawlins JNP, Chauveau J, Joseph MH, Mitchell SN, Gray JA. Modulation of dopamine release in the nucleus accumbens by 5HTlB agonists: Involvement of the hippocampo-accumbens pathway. Neuropharmacol. 1996;35:1521–1529. doi: 10.1016/s0028-3908(96)00099-8. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chourbaji S, et al. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- 49.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Serotonin effects in the hippocampus
Supplementary Figure 2. Serotonin-induced potentiation requires 5-HT1BRs and is selective for TA-CA1 synapses.
Supplementary Figure 3. Promotion of action potential firing and enhancement of TA-SC interaction by 5-HT1B receptor activation.
Supplementary Figure 4. 5-HT1BR activation phosphorylates serine 831 at TA-CA1 synapses but not SC-CA1 synapses and is required for potentiation by fluoxetine.
Supplementary Figure 5. Altered behavior in animals subjected to CUS is accompanied by a qualitative and quantitaive change in responses to anpirtoline.
Supplementary Figure 6. Effects of brief administration of fluoxetine.
Supplemental Figure 7. Uncropped Western blots