Abstract
BACKGROUND
In papillary thyroid cancer (PTC), the role of lymph node dissection remains controversial, and staging systems consider metastatic lymph nodes as a binary entity. The purpose of this study was to determine a threshold lymph node ratio (LNR) that impacted disease-specific mortality (DSM).
METHODS
We utilized the Surveillance, Epidemiology, and End Results (SEER) database to analyze adult patients who underwent thyroidectomy with lymph node dissection. A LNR (metastatic lymph nodes to total lymph nodes) was calculated after eliminating patients with less than three nodes harvested. Kaplan-Meier estimates for DSM were plotted for LNRs and compared by the log-rank test. The Cox-proportional hazards model was used to evaluate LNR with other known clinical and pathologic determinants of prognosis.
RESULTS
10,955 cases contained data on lymph nodes. Median follow-up time was 25 months (range 0–59 months), and the mean LNR was 0.28 ± 0.37. After comparing Kaplan-Meier survival estimates and overall DSM rates, we found that a LNR ≥ 0.42 best divided those with lymph node metastasis based on DSM (p<0.01). Those with a LNR ≥ 0.42 experienced a DSM rate of 1.72% while those with a LNR < 0.42 had a DSM rate of 0.65% (p<0.01). In addition, patients with a LNR ≥ 0.42 experienced a 77% higher DSM rate compared to those with metastatic lymph nodes as a whole. When considered with other known determinants of prognosis, we found that LNR was strongly associated with DSM (hazard ratio 4.33, 95% C.I. 1.68–11.18, p<0.01).
CONCLUSIONS
LNR is a strong determinant of DSM, and a threshold LNR of 0.42 can be used to risk-stratify patients with metastatic lymph nodes.
Introduction
The incidence of papillary thyroid cancer (PTC) has increased nearly 2.4 fold since 1973 (1). Improved detection accounts for the vast majority of this increase, and the mortality rate has remained relatively stable (2). Yet, the extent of initial surgery for papillary thyroid cancer remains controversial. The debate stems from the prognostic significance of metastatic cervical lymph nodes in PTC. Lymph node metastases are relatively common, occurring in 20–50% of cases of PTC (3, 4). This wide range depends on how lymph node metastases are defined because up to 90% of patients will have micrometastatic disease (5, 6). Lymph node metastases have been associated with disease recurrence. Wada et al reported that patients with metastatic lymph nodes (defined by routine histology) experienced a recurrence rate of 16.3% while those without metastatic lymph nodes had a recurrence rate of zero (7).
The relationship between clinically positive lymph node metastases and recurrence is well accepted, but the significance of lymph node metastases on survival remains debatable. Historically, many felt that disease in the lymph nodes did not influence mortality (8). More recent population-based data utilizing the Surveillance Epidemiology and End Results (SEER) database suggests that metastatic lymph nodes negatively influence survival (9, 10). Critics argue that these studies demonstrate relatively small differences in survival and cannot control for the adequacy of surgical resection.
Amidst the uncertainty regarding the importance of lymph node metastases on survival, some have supported routine prophylactic central (level 6) neck dissection. Proponents of prophylactic central neck dissection argue that it reduces the chances of a central neck recurrence since ultrasound has limited ability to evaluate these central compartment lymph nodes preoperatively and radioactive iodine ablation of micrometastic disease in the central neck carries its own risks (11, 12). Those against prophylactic neck dissection note the potential for increased complications, the efficacy and low risk of radioactive iodine ablation, and the questionable significance of clinically unapparent disease in the lymph nodes (13).
Numerous staging or prognostic scoring systems exist for differentiated thyroid cancers, but some of these do not even consider the status of cervical lymph nodes (14). When lymph nodes are included, they are considered in a binary sense (presence vs. absence of metastases) based on anatomic location, but not on the extent of nodes involved (15–19). For example, in the AJCC staging system, patients with cervical lymph node metastases are labeled as either N1a (level VI) or N1b (levels 1–V, VII), and a patient with two of 48 lymph nodes positive is grouped with a patient who has 46 of 48 lymph nodes positive (15).
As the significance and surgical treatment of cervical lymph nodes is debated, there is a need to understand how the extent of lymph node metastases impacts disease-specific mortality (DSM). The purpose of this study was to determine how the lymph node ratio (LNR) may be used to risk stratify patients by DSM.
Methods
We utilized the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute for this study. Adult patients (≥18 years old) with PTC treated with total, subtotal, or near total thyroidectomy and lymph nodes removed between 1988 and 2007 were selected for further analysis. To select patients treated with total, near total, or subtotal thyroidectomy, we selected cases with codes 30, 40, or 50 for the “surgery primary site” variable. Although the SEER data collection began in 1973, we selected cases diagnosed in 1988 or later because this was the first year that SEER collected more detailed information on lymph nodes. Cases were lymph nodes were harvested at the time of thyroidectomy were identified using codes 01 through 89 for the variable “Reg LN Exam.” The following ICD-0-3 histology codes were selected for papillary thyroid tumors and sub-types: 8050, 8260, 8340, 8341, 8342, 8343, and 8344. Patients with distant metastases at the time of diagnosis, known secondary cancers, or tumors identified at autopsy were excluded. Also excluded were 146 patients with an unknown number of regional lymph nodes examined (codes 97–99). A variable for “unfavorable histology” was created for tumors greater than 1 cm that were columnar cell, or tall cell, papillary carcinomas.
A LNR was calculated for each subject by dividing the number of metastatic lymph nodes by the total number of lymph nodes harvested. Kaplan-Meier survival curves were plotted and compared by the log-rank test. Regression analysis was used to determine each patient’s probability of thyroid-cancer specific death. The optimal LNR that created the greatest difference in DSM was identified. For this analysis, we only considered cases where three or more lymph nodes were harvested (n = 6,103) to control for low lymph node yields which bias the LNR to zero or one. A Cox proportional hazards model was constructed to determine the relative impact of LNR when adjusting for other known clinical factors associated with thyroid cancer specific survival. Again, only patients with three or more nodes harvested were included in this multivariate analysis. P < 0.05 was considered significant. Statistical analyses were conducted using STATA v. 10.1 software (StataCorp, College Station, TX).
Results
Patient and Tumor Characteristics
The SEER database contained 23,189 adult (≥18 years old) patients treated with total thyroidectomy for PTC between 1988 and 2007. Of these, 10,955 cases contained data on lymph nodes and the patient and tumor features of this subset are listed in Table 1. The average age was 48.8 ± 3.6 years old, and 75.0% of patients were female as expected (Table 1). Over 86% of the patients were Caucasian (Table 1).
TABLE 1.
Patient and Tumor Characteristics (n = 10,955)
| Variable | Number (%) |
|---|---|
| Age | 48.8 ± 3.6 |
| Female | 8,221 (75.0) |
| RACE | |
| Caucasian | 9,452 (86.3) |
| Black | 337 (3.1) |
| Native American, Pacific Islander | 1,177 (10.7) |
| Other | 42 (0.4) |
| Tumor size (mm) | 19.4 ± 4.1 |
| HISTOLOGY | |
| Papillary | 7,863 (71.8) |
| Microcarcinoma | 260 (2.4) |
| Follicular variant | 2,626 (23.9) |
| Columnar cell variant | 206 (1.9) |
| Extrathyroidal extension | 4,398 (40.1) |
| Median follow up time (months) | 25 ± 4.2 |
Data are presented as the mean ± the standard error of the mean unless otherwise stated. Numbers in parentheses are the percentage of the total.
The mean tumor size was 19.4 ± 4.1 mm, and 72% of tumors in this cohort were well-differentiated papillary cancers (Table 1). The second most common variant was follicular variant papillary cancer (23.9%), followed by microcarcinomas (2.4%), and columnar cell variant (1.9%, Table 1). 40.1% of patients had extrathyroidal extension (Table 1).
Disease-Specific Mortality and Status of Cervical Lymph Nodes
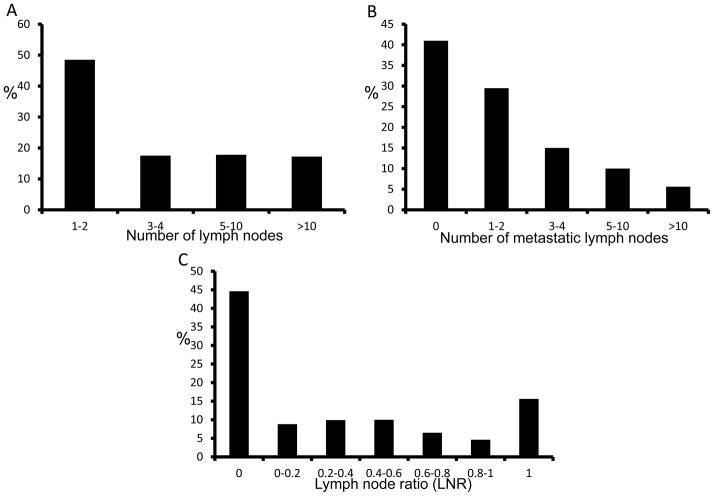
The disease-specific mortality (DSM) rate among those with metastatic lymph nodes was 1.77% while only 0.17% for those without metastatic lymph nodes. Median follow-up time was 25 months (Table 1). Further analysis of the 10,955 patients who had lymph nodes dissected at the time of their thyroidectomy revealed that the median number of lymph nodes examined was 3 (range 1–90, Figure 1A), and the median number of metastatic lymph nodes was 2 (range 0–68, Figure 1B). 48.5% of these patients had only one or two regional lymph nodes harvested (Figure 1A). As shown in Figure 1B, over 70% of patients had less than three lymph nodes with metastatic disease. It is impossible to determine the type or intent of lymph node dissection performed by the surgeon using the SEER database, but 15.0% of patients had metastatic nodes in the lateral neck. The mean LNR was 0.27 ± 0.34, and, as expected, the distribution of LNR was bimodal: 44.6% had a LNR of zero while 15.7% had a LNR of one. LNRs of zero and one accounted for over 60% of cases (Figure 1C).
Figure 1.
Histograms depicting the distribution of cervical lymph nodes harvested (A), the number of metastatic lymph nodes (B), and the lymph node ratios (C).
Threshold LNR
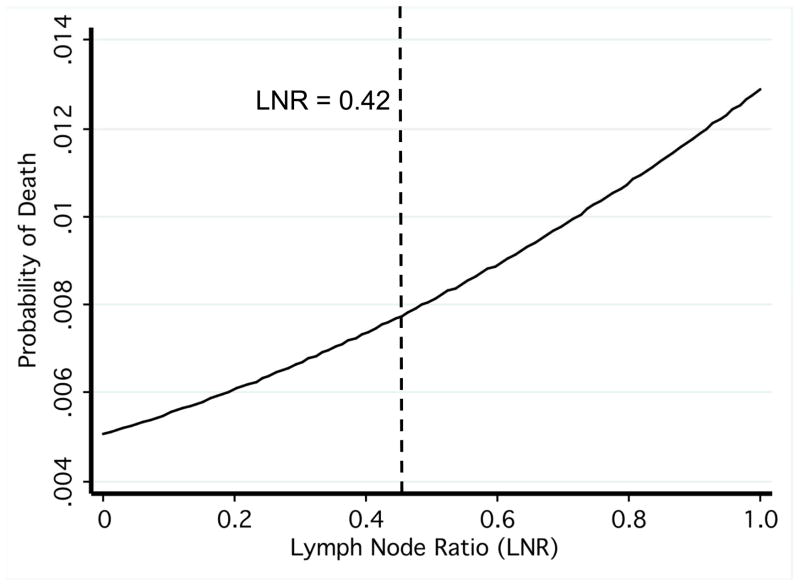
We aimed to determine a threshold LNR that best divided the cases based on DSM, the relevant oncologic outcome contained in the SEER database. Kaplan-Meier disease-free survival estimates were plotted for cases where at least three lymph nodes were removed (n = 6,103), beginning with quartile divisions in LNR. From these divisions, we converged upon a LNR of 0.42 that best divided the cases based on DSM (p = 0.02, Figure 2). To aid in this determination, we also used regression analysis to derive each patient’s probability of thyroid cancer specific mortality by LNR (Figure 3). The probability function displayed an exponential pattern. That is, the probability of death from thyroid cancer increased exponentially with increasing LNR (Figure 3). Our threshold LNR of 0.42 was near the inflection point in this curve (Figure 3). The group with a LNR greater than or equal to 0.42 experienced a DSM of 1.72% while those with a LNR less than 0.42 had a DSM of 0.65% (p < 0.01).
Figure 2.
Kaplan-Meier estimates of disease-free survival according to the threshold lymph node ratio.
Figure 3.
Probability of thyroid cancer-specific death by lymph node ratio. The dashed line indicates the threshold lymph node ratio.
Multivariate Analysis
To consider the relative impact of LNR when combined with other known clinical and pathologic determinants of thyroid cancer specific mortality, we performed a multivariate analysis using a Cox proportional hazards model. As with our derivation of a threshold LNR, we excluded cases where less than three lymph nodes were harvested to control for the heterogeneity in surgical technique and to eliminate a large number of patients whose LNR was zero (Figure 1C). LNR was significantly associated with DSM, with a hazard ratio of 4.33 (95% confidence interval 1.68–11.18, p < 0.01, Table 2). In this model, age and tumor size were marginally associated with DSM (Table 2). The number of negative lymph nodes was also included in the model to further control for lymph node yield. The number of positive nodes did not independently predict DSM (p = 0.32, Table 2). We attempted to include extrathyroidal extension and unfavorable histology (columnar cell, and tall cell tumors > 1 cm) into the model, but these two terms were dropped because of collinearity.
TABLE 2.
Cox Proportional Hazards Model
| Variable | Hazard Ratio | 95% C.I. | p value |
|---|---|---|---|
| Age | 1.09 | 1.07–1.11 | < 0.01 |
| Male gender | 0.78 | 0.39–1.55 | 0.48 |
| Size | 1.01 | 1.00–1.02 | 0.01 |
| Lymph node ratio (LNR) | 4.33 | 1.68–11.18 | < 0.01 |
| # Positive lymph nodes | 1.03 | 0.97–1.09 | 0.32 |
| # Negative lymph nodes | 1.05 | 1.03–1.07 | < 0.01 |
| Radioactive iodine ablation | 0.84 | 0.44–1.61 | 0.59 |
C.I. = confidence interval. Extrathyroidal extension and unfavorable histology were also included in the model but dropped because of collinearity.
This same analysis was performed after excluding the subset of patients with lateral lymph node metastases (N1b). LNR was no longer a significant factor associated with DSM. In this model, only age was significantly associated with DSM (HR 1.10, p<0.01, 95% C.I.1.07–1.12).
Discussion
In this study, we derived a threshold LNR that best divided patients with PTC and lymph node metastases by thyroid cancer specific mortality. Although the differences in mortality for PTC are quite small, we found that a LNR of 0.42 was the optimal LNR that divided patients with at least three lymph nodes removed based on DSM. Furthermore, LNR was significantly associated with mortality when controlling for other known clinical and pathologic predictors of thyroid cancer specific mortality (Table 2).
The concept of LNR for assessing surgical quality and predicting DSM exists for many other cancers where lymph node dissection is performed (20, 21). In contrast to previous studies that have examined LNR in the SEER dataset and elsewhere, we have derived a single, threshold LNR rather than studying arbitrary divisions in LNR (22). Furthermore, we have chosen DSM as our primary outcome rather than overall mortality (22) or stimulated thyroglobulin levels (23). Current staging systems for thyroid cancer only consider lymph nodes as a binary entity (metastases present versus absent)(18, 19). As in other cancers, LNR provides a measure of surgical quality since it also depends on the surgeon performing an adequate lymph node dissection with a “margin” of negative nodes. This obviously depends on good preoperative nodal evaluation to determine the appropriate nodal compartments to be dissected (24).
SEER provides limited data on treatment. The extent of lymph node dissection, is not coded, and one cannot determine the type or intent of lymph node dissection performed. This contrasts with previous studies of LNR in PTC which examined a specific type of lymph node dissection (23). Therefore, our analysis likely combines patients with central or lateral compartment-oriented dissections, lymph node sampling or “berry-picking,” and cases where intra-thyroidal or peri-thyroidal nodes were incidentally removed with the gland.
Many have suggested that the number of metastatic (positive) nodes alone predicts survival. Zaydfudim et al analyzed the impact of lymph node metastases on thyroid cancer specific survival. In patients with PTC, who were 45 years of age or older, lymph node metastases were associated with an increased risk of death whereas lymph node involvement did not influence survival in patients younger than 45 years old (10). Given that our study only examined the subset of adult (≥18 years old) patients with lymph node metastases, the results presented here are consistent with this study and demonstrate the utility of LNR in risk stratifying patients with lymph node metastases.
Given the current controversy regarding the utility of prophylactic central neck dissection, the results of this study must be interpreted with caution. Since SEER cannot provide data on the surgeon’s intention, we do not know what portion of these lymph node dissections were therapeutic or prophylactic. The data presented here cannot support any particular indication for lymph node dissection. Rather, this study supports the notion of compartment-oriented dissection rather than lymph node sampling or “berry-picking” to obtain a sufficient “margin” of negative nodes such that the LNR is lower. Since the relationship between LNR and DSM disappeared when the analysis was performed without patients with lateral compartment lymph node metastases (N1b), this suggests that lateral neck metastases hold relatively more importance over DSM than central compartment metastases alone.
Certainly, LNR indicates surgical quality since it depends on the adequacy or completeness of lymph node dissection and preoperative lymph node evaluation of both the central and lateral compartments. The very large numbers in SEER and the various controls on this analysis also reveal some underlying truths about thyroid cancer biology: the extent and location (N1b) of lymph node metastases influences DSM. Nonetheless, one cannot deny the importance of both the surgeon and the pathologist in determining the LNR since both the numerator and denominator depend on the thoroughness of both parties.
This study has several drawbacks. As with all population-based studies, the very large sample size makes it quite easy to achieve statistical significance with questionable clinical significance. We feel that our strategies to limit heterogeneity, control for lymph node yield, and including other known risk factors in our multivariate analysis still exposed the importance of LNR in determining DSM. Nevertheless, the differences in mortality reported here remain relatively small. Because of the intention to limit heterogeneity in surgical technique, the utility of LNR only applies to cases where at least three lymph nodes were harvested. As discussed, the indications and surgical technique for lymph node dissection remain unknown in the SEER database, making it difficult to draw definitive conclusions about any particular treatment strategy. Additionally, a large number of patients had zero nodes positive. This limits the interpretation of a LNR of zero since zero of three is quite different than zero of 33. Finally, the only relevant oncologic outcome contained in the SEER database is DSM. A median follow-up time of 25 months probably is not long enough to truly represent DSM in PTC, a slow-growing cancer that can recur decades after initial treatment.
Despite these limitations, we utilized population level data to show how LNR impacts thyroid cancer specific mortality when controlling for traditionally cited clinical and pathologic determinants of mortality. We derived a threshold LNR that serves as a simple tool to alert surgeons and patients when the extent of disease in the lymph nodes portends a worse prognosis.
Acknowledgments
This work was supported by NIH T32 CA009614-23.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer database report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Grubbs EG, Evans DB. Role of lymph node dissection in primary surgery for thyroid cancer. J Natl Compr Canc Netw. 2007;5:623–30. doi: 10.6004/jnccn.2007.0053. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid: developing pattern of metastasis. Cancer. 1970;2 doi: 10.1002/1097-0142(197011)26:5<1053::aid-cncr2820260513>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131:249–56. doi: 10.1067/msy.2002.120657. [DOI] [PubMed] [Google Scholar]
- 6.Arturi F, Russo D, Giuffrida D, Ippolito A, Perrotti N, Vigneri R, et al. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol. 1997;82:1638–41. doi: 10.1210/jcem.82.5.4062. [DOI] [PubMed] [Google Scholar]
- 7.Wada N, Suganuma N, Nakayama H, Masudo K, Rino Y, Masuda M, et al. Microscopic regional lymph node status in papillary thyroid carcinoma with and without lymphadenopathy and its relation to outcomes. Langenbeck’s Arch Surg. 2007;392:417–22. doi: 10.1007/s00423-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferri EL, Young RL, Oertel JE, Kemmerer WT, Page CP. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine. 1977;56:171–96. [PubMed] [Google Scholar]
- 9.Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731–4. doi: 10.1177/000313480507100907. [DOI] [PubMed] [Google Scholar]
- 10.Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144:1070–8. doi: 10.1016/j.surg.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Hughes DT, Doherty GM. Central neck dissection for papillary thyroid cancer. Cancer Control. 2011;18:83–8. doi: 10.1177/107327481101800202. [DOI] [PubMed] [Google Scholar]
- 12.Hughes DT, White ML, Miller BB, et al. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–6. doi: 10.1016/j.surg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–9. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Atlas. New York: Springer; 2010. [Google Scholar]
- 16.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–95. [PubMed] [Google Scholar]
- 17.Byar DP, Green SB, Dor P, Williams D, Colon J, van Gilse H, et al. A prognostic index for thyroid carcinoma: a study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 18.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos T, Cooper DS, et al. Prospective multicenter study of thyroid carcinoma treatment. Cancer. 1988;83:1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 20.Mocellin S, Pasquali S, Rossi CR, Nitti D. Validation of the prognostic value of lymph node ratio in patients with cutaneous melanoma: a population-based study of 8,177 cases. Surgery. 2011;150:83–90. doi: 10.1016/j.surg.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman SC, McMasters KM, Scoggins CR, Martin RC, Chagpar AB. Lymph node ratio: a proposed refinement of current axillary staging in breast cancer patients. J Am Coll Surg. 2011;213:45–53. doi: 10.1016/j.jamcollsurg.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Beal SH, Chen SL, Schneider PD, Martinez SR. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76:28–32. [PubMed] [Google Scholar]
- 23.Lang BH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19:1257–63. doi: 10.1245/s10434-011-2105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poehls JL, Chen H, Sippel RS. Preoperative ultrasonography findings predict the need for repeated surgery in papillary thyroid cancer. Endocr Pract. 2012;18(3):403–9. doi: 10.4158/EP11221.OR. [DOI] [PubMed] [Google Scholar]
- 25.Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006;13:119–28. doi: 10.1177/107327480601300206. [DOI] [PubMed] [Google Scholar]
- 26.Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19:1373–80. doi: 10.1089/thy.2009.1606. [DOI] [PubMed] [Google Scholar]
- 27.Institute NC, editor. SEER cancer statistics review 1975–2005. Bethesda, MD: [Google Scholar]