Abstract
The neurotrophin, brain-derived neurotrophic factor (BDNF), is recognized as a key component in the regulation of central nervous system ontogeny, homeostasis and adult neuroplasticity. The importance of BDNF in central nervous system development and function is well documented by numerous reports from animal studies linking abnormal BDNF signaling to metabolic disturbances and anxiety or depressive-like behavior. Despite the diverse roles for BDNF in nearly all aspects of central nervous system physiology, the regulation of BDNF expression, as well as our understanding of the signaling mechanisms associated with this neurotrophin, remains incomplete. However, links between sex hormones such as estradiol and testosterone, as well as endogenous and synthetic glucocorticoids, have emerged as important mediators of BDNF expression and function. Examples of such regulation include brain region-specific induction of Bdnf mRNA in response to estradiol. Additional studies have also documented regulation of the expression of the high-affinity BDNF receptor TrkB by estradiol, thus implicating sex steroids not only in the regulation of BDNF expression, but on mechanisms of signaling associated with it. In addition to gonadal steroids, further evidence also suggests functional interaction between BDNF and glucocorticoids, such as in the regulation of corticotrophin-releasing hormone and other important neuropeptides. In this review, we provide an overview of the roles played by selected sex or stress hormones in the regulation of BDNF expression and signaling in the central nervous system
Introduction
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of proteins, is translated from the BDNF gene into a small precursor protein, proBDNF. This precursor protein is subsequently cleaved to BDNF and then secreted from both neurons and glial cells. As with other members of the neurotrophin family of proteins, BDNF binds with low affinity to p75, a neurotrophin receptor subunit common to all neurotrophin receptors, to exert a variety of downstream effects (Barrett, 2000). However, the majority of trophic effects mediated by BDNF are thought to occur following binding to the high-affinity, protein kinase receptor, Tropomyosin-Related Kinase B (TrkB). Interactions between BDNF and TrkB result in homodimerization and phosphorylation of the receptor followed by activation of either mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K), or phospholipase C (PLC) (Huang and Reichardt, 2003). Activation of the MAPK, PI3K, or PLC subsequently leads to a variety of downstream events characterized by neuronal survival (Yoshii and Constantine-Paton, 2010), neurite growth (Davies et al., 1986), cell migration (Behar et al., 1997), glutamate release (Zhang et al., 2012), synapse formation (Hofer and Barde, 1988), and neuropeptide expression (Jeanneteau et al., 2012). The wide variety of signaling pathways and downstream effects mediated by BDNF thus emphasize the critical role that this neurotrophin plays in the maintenance of homeostatic mechanisms in the central nervous system. However, despite the recognized importance for BDNF in maintaining neuronal homeostasis, essential aspects governing the control of the expression of BDNF and TrkB, as well as its downstream activity are still not fully understood.
Deciphering the regulation of BDNF expression and action is hindered by the complexity of the Bdnf gene in both rodents and humans (Figure 1). In both species, the Bdnf gene consists of at least eight non-translated exons which form a variety of splice variants with the final exon, which is ultimately translated into the precursor protein proBDNF (Aid et al., 2007). Distinct promoter regions upstream of each non-translated exon drive expression of the gene and are thought to be key components of tissue- and cell-specific expression of BDNF splice variants (Pattabiraman et al., 2005; Aid et al., 2007). Of the eight non-coding exons currently recognized, exon IV, which is heavily expressed in cultured embryonic neurons, has received the most extensive scrutiny regarding molecular events which regulate expression, revealing multiple Ca2+ and cAMP response elements (Lyons and West, 2011). By contrast, regulation of the remaining non-coding exons remains poorly understood. In addition to regulation through a variety of transcription factor response elements, Bdnf gene expression is also controlled through epigenetic mechanisms including methylation of at least 11 CpG islands dispersed along the entire gene, as well as histone methylation (Boulle et al., 2012).
Figure 1.
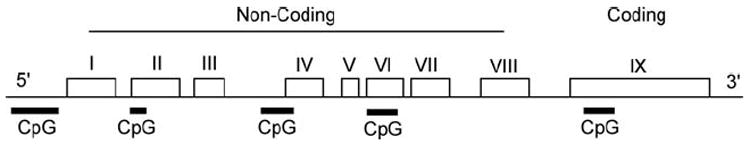
The structure of rodent Bdnf includes eight 5’ non-coding exons spliced to a common 3’ exon IX. CpG islands within exons I, II, IV, VI, and IX are all subject to methylation. Adapted from Aid et al. (2007) and Lubin et al. (2008). Each individual exon is represented by a box and CpG islands are represented by dark lines underlying the corresponding region that they occur.
Investigation into the regulation of Bdnf gene expression and the signaling mechanisms through which this neurotrophin exerts its biological effects has revealed interactions between hormone signaling and BDNF. Such examples include the regulation of BDNF expression by gonadal steroid hormones, which may influence the role played by BDNF in sexually dimorphic development of the brain (Arevalo et al., 2012) or in sexually dimorphic brain functions and behaviors. Evidence demonstrates that the gonadal steroid hormone, estradiol, regulates region-specific developmental expression of BDNF in the brain (Solum and Handa, 2002). Besides gonadal steroids, additional research has demonstrated a balance between BDNF and glucocorticoid (GC) signaling is responsible for the expression of hypothalamic neuropeptides such as corticotrophin-releasing hormone (CRH) (Jeanneteau et al., 2012). Mechanisms behind GC/BDNF functional interactions have been reported to include convergence at a single molecular “control point”, such as cAMP-mediated signaling (Jeanneteau et al., 2012), while additional evidence suggests that GC may directly bind with and modulate the activity of TrkB (Numakawa et al., 2009). In the present review, we provide an overview of the interactions between BDNF and selected gonadal and stress hormones, with discussion of affected biological processes and potential mechanisms of action.
Gonadal Steroid Hormones Regulate BDNF expression
A role for Estrogen in BDNF expression
In rodents, sexual differentiation of the brain is largely governed by the intracellular transformation of testosterone into estradiol by the aromatase enzyme and, subsequently, through activation of estrogen receptors, resulting in the programming of a male behavioral and physiological phenotype. Lacking circulating testosterone, developing female rodents are shielded from maternal estrogen by circulating -fetoprotein, a liver-derived protein which binds estradiol with high affinity. Thus, in rodents, the absence of estrogen receptor stimulation leads to a default pattern of feminized behaviors, whereas the presence of androgens and estrogen during development lead to masculinization and defeminization of the brain (Lenz et al., 2012). Recently, a direct role for sex chromosomes have also been implicated in some aspects of brain sexual differentiation (McCarthy and Arnold, 2011). Some brain regions that are most influenced by estrogens during development include the midbrain (Beyer and Karolczak, 2000), cerebellum (Minano et al., 2008), hippocampus (Bender et al., 2010), hypothalamus (Lenz and McCarthy, 2010) and preoptic area (Simerly et al., 1985a; Simerly et al., 1985b). Within the hypothalamus, estradiol promotes the formation of sex differences in the neuronal circuitry controlling feeding, growth, and reproductive behaviors (Arevalo et al., 2012). Although mechanisms underlying estrogen receptor-mediated brain development and growth are not completely understood, evidence exists suggesting that interactions with BDNF and other neurotrophic factors are crucial (Arevalo et al., 2012).
Early studies demonstrating an interaction between estradiol and neurotrophins employed dual in situ hybridization to colocalize estrogen receptor mRNA with Bdnf, TrkB, or p75 mRNA throughout multiple brain regions in tissue harvested from Sprague Dawley rats (Miranda et al., 1993). This hypothesized interaction between estradiol and BDNF was subsequently proven by a study showing the rapid induction of Bdnf mRNA following the administration of estradiol to ovarectomized rats (Sohrabji et al., 1995). Despite the induction of Bdnf mRNA by estradiol reported by Sohrabji et al (1995), expression of this gene was not suppressed following ovariectomy, suggesting that regulation of Bdnf mRNA by estradiol constitutes only one component of a multifaceted regulatory system. Additional evidence that estradiol regulates BDNF mRNA was reported by Dittrich et al., (1999) who demonstrated that the induction of this gene occurred in the high vocal center of male zebra finches following the administration of estradiol. Interestingly, similar induction was not observed in the females, indicating possible sex differences in the regulation of BDNF by estradiol (Dittrich et al., 1999).
Additional studies demonstrating estrogen responsive BDNF expression have been reported in mice. BDNF protein has been shown to be elevated in the cerebellum following injections of estradiol benzoate (Sasahara et al., 2007). This same study also reported that estradiol-mediated induction of BDNF was prevented if the mice were co-treated with the estrogen receptor antagonist, tamoxifen (Sasahara et al., 2007). Thus, estrogen receptor activity appears to be critical for the induction of BDNF by estradiol. Besides the cerebellum, reports also indicate that hippocampal BDNF expression mirrors the changes in estradiol that occur across the estrous cycle in rodents, although inconsistency exists between the various studies. For example, it was reported by one study, which tracked hippocampal Bdnf mRNA using in situ hybridization, that expression of this gene was minimal during late proestrus and into estrus (Gibbs, 1998), shortly after the peak of circulating estradiol. However, another report indicates that the expression of BDNF protein in the hippocampus is induced on the day of proestrus and into estrus (Scharfman et al., 2003). Aware of this discrepancy, the report by Scharfman et al. (2003) suggested that the contrasting results reported between their study and that of Gibbs (1998) may be due to methodological differences, since they were measuring BDNF protein, which may be influenced by antibody specificity, while Gibbs measured mRNA (Scharfman et al., 2003). Furthermore, the discrepancy between these studies may also be due to the population of neurons studied. Because the majority of BDNF protein in hippocampus is located in the mossy fiber axons, inclusion of other regions in the analysis, or a focus on regions outside the mossy fiber axons, may result in variable quantification. Additional observations reported by Scharfman et al., (2003), also linked the pattern of BDNF protein expression in the hippocampus across the estrous cycle with corresponding changes in hippocampal excitability, thus suggesting a physiological role for fluctuating BDNF. Reports correlating estradiol levels to hippocampal BDNF expression also include those indicating that ovariectomy of Sprague Dawley rats results in reduced Bdnf mRNA expression in the frontal and temporal cortices and the CA2 CA3, CA4, and dentate gyrus regions of the rat hippocampus (Singh et al., 1995). However, Singh et al (1995) also demonstrated that the estrogen-mediated Bdnf expression is region specific since estradiol replacement following ovariectomy only induced Bdnf mRNA in the CA3, CA4, and dentate gyrus regions of the hippocampus, while no induction was observed in the CA2 region of the hippocampus nor in the frontal or temporal cortices (Singh et al., 1995). This distribution of estrogen sensitivity is not necessarily consistent with the distribution of ERs in the hippocampus (Simerly et al., 1990). Nonetheless, the concept that estrogen influences BDNF expression in a region-specific manner is reinforced by studies indicating the presence of ER, ER in mossy fibers of the hippocampus which also contain high amounts of BDNF (Mitterling et al., 2010). Moreover, studies report that estrogen replacement therapy in ovariectomized rats resulted in increased BDNF expression in the olfactory bulb, but decreased expression in the cingulate cortex (Jezierski and Sohrabji, 2000) further emphasizing the region specific nature of this interaction.
Given the documented influence of estradiol on BDNF signaling in adult animals, it has been hypothesized that estrogen can influence sexually dimorphic brain development through a BDNF-mediated mechanism (Solum and Handa, 2002). This study built off previous work which demonstrated that estrogen binding and estrogen receptor (ER) mRNA (O’Keefe and Handa, 1990; O’Keefe et al., 1995), and later that the estrogen receptor (ER) alpha subtype, are all heavily expressed in the rat hippocampus during the early neonatal period of hippocampal development (Solum and Handa, 2001). Gonadectomy of male offspring on the day of birth followed by measurement of the expression of hippocampal Bdnf and TrkB mRNA using quantitative real-time PCR (qRT-PCR) showed reduced expression of Bdnf from PD 0-10, although by PD15 mRNA expression had recovered to the level observed in intact offspring (Solum and Handa, 2002). The administration of a single injection of estradiol benzoate immediately following castration, resulted in recovery of Bdnf mRNA levels to those observed in intact animals thus demonstrating the requirement for estradiol in developing hippocampal Bdnf expression.
Regulation of TrkB Expression by BDNF
Because TrkB is required for the trophic effects mediated by BDNF, the expression of this receptor in response to estradiol has also been explored. However, results vary based on the model used and the brain region examined. Specifically, the administration of a TrkB antisense oligonucleotide prevented estradiol-induced axon elongation in cultured primary hypothalamic neurons (Brito et al., 2004). Additional studies conducted in primary hypothalamic neurons demonstrated that estradiol induced TrkB protein in the presence of astrocyte-conditioned media (Cambiasso et al., 2000). This report also showed a sexual dimorphism in the observed response, where estradiol only potentiated TrkB protein expression in neurons isolated from male rats, and had no effect on TrkB expression in neurons from females in the presence or absence of the conditioned media. Therefore, it is still questionable whether estradiol alone is responsible for induction of TrkB. However, although a role for estrogen in TrkB expression is suggested by a variety of in vitro studies, estradiol administration to castrated rat pups was not shown to affect TrkB gene or protein levels in the hippocampus (Solum and Handa, 2002). Therefore, whether or not TrkB expression is influenced by estradiol remains controversial, with the possibility that such regulation occurs in a region specific manner, as observed with BDNF. Additionally, these data further emphasize the possibility that the effects of estrogen on BDNF expression and function may differ between adult and developing organisms.
Molecular Mechanisms of BDNF gene regulation by estrogen
To address the molecular mechanisms for estradiol regulation of BDNF expression, a scan of the rat Bdnf gene by Sohrabji et al., (1995) for the canonical estrogen response element (ERE; GGTCAnnnTGACC) revealed a putative ERE in Exon IX. Although the sequence of this putative ERE-motif (GGTGAGAAGAGTGATGACC) differs from the canonical ERE in that the pentamers are separated by a longer spacer, it was also demonstrated that the incubation of DNA containing the putative ERE, with nuclear protein extract, resulted in a band shift using a gel shift assay. Such responses suggest that the ER protein does in fact interact with this DNA region (Sohrabji et al., 1995). Moreover, the notion that this DNA sequence was bound by ER is supported by the observation that no gel shift occurred if oligonucleotides containing the known ERE of the vitellogenin gene, a common marker of estrogen signaling, were included in the incubation (Sohrabji et al., 1995). These data suggest a classical mechanism of Bdnf induction by an ERE in its promoter region, which would require that the gene and ER are present in the same cell. Dual imaging immunohistochemical analyses were therefore conducted using brain tissue from Fischer 344 rats in which BDNF and ER alpha were found to be co-expressed in various regions throughout the brain (Blurton-Jones et al., 2004). Interestingly, the hippocampal regions showing BDNF/ER dual labeling only included the CA3 region, whereas no dual labeling was observed in the CA2 region or dentate gyrus (Blurton-Jones et al., 2004), although expression of Bdnf mRNA was previously demonstrated to be increased in these regions by estrogen (Solum and Handa, 2002). This lack of co-localization goes against the findings from a number of earlier studies co-localizing ER with Bdnf (Miranda et al., 1993), but may be explained by differences in the choice of model, since rodents used by Blurton-Jones et al., (2004) were conducted at random points in the estrous cycle using mature female rats, while Miranda et al., (1993) measured Bdnf mRNA in rats approximately two weeks old, the latter eliminating any effects from the estrous cycle.
The discrepancy between estrogen-mediated induction of Bdnf and co-expression of the gene and estrogen receptor suggests alternate mechanisms by which this regulation occurs. Although cellular colocalization was not found extensively throughout the regions profiled by Blurton-Jones et al. (2004), cells expressing Bdnf or ER (both and) were observed with high frequency. This observation suggests that cross-talk between BDNF expressing neurons and other cell types, such as astrocytes, may play a role in this process. Such cross-talk is supported by reports that astrocytes express both forms of estrogen receptor (Al-Bader et al., 2011), as well as observations that glial-conditioned media influence neuronal BDNF activity (Martin et al., 2012). Another explanation for the regulation of BDNF by estrogen was described in a report by Blurton-jones and Tuszynski (2004), in which the administration of 17- -estradiol to ovariectomized rats resulted in the inhibition of GABAergic interneurons in the perirhinal cortex (Blurton-Jones et al., 2004). Consequently, it was proposed by the authors that inhibition of these inhibitory interneurons resulted in increased tone on BDNF-expressing neurons, leading to increased expression of this neurotrophin.
Functional Interactions Between Glucocorticoid and BDNF
Stress hormones, BDNF, and depression
Activation of the hypothalamic-pituitary-adrenal (HPA) axis is associated with the neuroendocrine response to stressful events, which may also influence BDNF activity. Reactivity of the HPA axis is characterized by the release of corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) by hypophysiotrophic neurons of the paraventricular nucleus of the hypothalamus (PVN). Upon release, these hormones travel through the hypothalamo-hypophyseal portal vasculature to act upon corticotrophs of the pituitary gland to promote the secretion of adrenocorticotropic hormone (ACTH). ACTH subsequently enters the general circulation and ultimately stimulates the release of glucocorticoid by the adrenal cortex (Fernandez-Guasti et al., 2012). Activation of the HPA axis is typically self-limiting due to the inhibitory effects of GC on the stress response. However, sustained stimulation of the HPA axis due to chronic stress or through dysregulation of GC-mediated negative feedback is thought to be neuroendangering and cause damage to the central nervous system, and has been linked to mood disorders such as depression (McEwen, 2005).
Associations between HPA hyperactivity and depression have been well established. Following an initial report indicating a correlation between elevated CRH in the cerebrospinal fluid and the incidence of depression in humans (Nemeroff et al., 1984) came findings of increased numbers of CRH and vasopressin neurons within the postmortem hypothalamus of depressed patients (Purba et al., 1995; Raadsheer et al., 1995). Additional clinical evidence which suggests dysregulation of the HPA as a factor in depression include observations of elevated ACTH and cortisol secretion following the administration of a combination of a synthetic glucocorticoid, dexamethasone (DEX), and CRH to patients diagnosed with depression (Heuser et al., 1994; Kunugi et al., 2006). Because this challenge is designed to measure GC-mediated negative feedback on the HPA axis, the results of these studies collectively indicate impaired negative regulation of the HPA by GC in depressed patients.
The connection between HPA hyperactivity and depression may be explained in part by reports that chronic elevated GC results in structural remodeling of the brain. Magnetic resonance imaging studies in depressed humans have revealed significant volume reduction in regions of the brain including the prefrontal cortex (Coffey et al., 1990). Additional evidence also exists demonstrating reduced volume of the hippocampus, although this effect was more pronounced in chronically-depressed individuals when compared to those with remitted depression (Shah et al., 1998). Similar evidence has also been reported in animal models of chronic stress, which results in decreased hippocampal volume and a depressive-like phenotype (Joels et al., 2004). Mechanistically, it is unclear what link exists between HPA hyperactivity and brain remodeling, but an additional study has also indicated that BDNF may play a role in the neuropathology resulting from chronic stress, based on observations that hippocampal plasticity was restored by BDNF in a model of depressive-like behavior following chronic mild stress in male mice (Ye et al., 2011). A role for BDNF in depressive behaviors has also been suggested based on reports of decreased serum BDNF in patients with major depressive disorder (MDD), although it is unclear from such studies whether serum BDNF accurately reflects the levels of this neurotrophin in the central nervous system (Oral et al., 2012; Yoshida et al., 2012). Furthermore, although these studies enrolled both male and female participants, the data from the two sexes were pooled, thus making a comparison between BDNF expression in male and female populations impossible. Given that females are diagnosed with MDD more frequently than males, the omission of such a comparison was surprising. Additional data implicating BDNF in depression include postmortem evaluation of brains from individuals diagnosed with MDD, which show reduced levels of BDNF throughout the hippocampus and prefrontal cortex (Karege et al., 2005; Dunham et al., 2009). However, as with studies measuring serum BDNF, although BDNF expression was measured in both male and female brains, no attempt to distinguish a sex effect was reported.
A role for BDNF in depression is also suggested by results from animal studies where forebrain BDNF knockout alters the antidepressant actions of desipramine in the forced swim test (Monteggia et al., 2004) and causes an increased incidence in depression like behaviors in females (Monteggia et al., 2007). Given the higher prevalence of depression in women, these latter data are particularly interesting if transferrable to humans in that a sex specific role for BDNF in the pathogenesis of MDD is suggested. Furthermore, GR(+/-) mice, which show high levels of depressive-like behaviors, also show impaired processing of BDNF in hippocampal synaptosomes following stress (Molteni et al., 2010). Additional evidence suggests that GC not only influences the expression of Bdnf and TrkB (reviewed by Jeanneteau and Chao, 2012), but also modulates the expression of BDNF-driven genes, such as CRH, through competition within signaling pathways (Jeanneteau et al., 2012). Taken together, these data indicate that abnormal BDNF expression may play an important role in the pathogenesis of mood disorders such as depression, and further suggests links between GC signaling and BDNF.
Glucocorticoid and BDNF Regulate CRH in the PVN
Abnormal GC and BDNF signaling are linked to CNS pathology, but interactions between these components are not well understood. Evidence suggesting functional crosstalk between GC and BDNF indicate that BDNF and GC signaling converge to regulate expression of some neuropeptides such as CRH in the PVN (Jeanneteau et al., 2012). CRH neurons in the PVN control HPA axis activity and other autonomic functions through projections to the hypothalamo-hypophyseal portal vasculature or centrally to forebrain and brainstem regions (Swanson et al., 1987). Although not completely understood, the expression of PVN CRH is regulated predominantly through the negative feedback action of GR and MR, Other possibilities exist where additional signaling pathways can influence the expression of CRH such as that which has been shown for BDNF (Jeanneteau et al., 2012). Using a series of novel transgenic mice, they demonstrated that CRH expression is regulated through both GC and BDNF signaling. Specifically, conditional knockout mice which lacked a GC receptor in the PVN had increased levels of CRH, likely due to reduced negative feedback by GC, and increased levels of BDNF and phosphorylated TrkB, an indicator of activation of BDNF signaling (Jeanneteau et al., 2012). The ensuing hypothesis, that BDNF signaling is involved in CRH expression, was tested using another transgenic mouse which overexpressed BDNF. These animals showed increased CRH expression and ultimately, the balancing point was shown to exist at the level of cAMP response-element binding protein (CREB) mediated signaling, since signaling through TrkB induced CREB resulting in elevated CRH expression, while GR stimulation resulted in neutralization of the CREB co-activating protein, CRTC2 (Jeanneteau et al., 2012). The data by Jeanneteau et al., (2012) thus suggest a mechanism whereby GC influences the physiological effects of BDNF by opposing its actions at the level of a target gene, rather than directly influencing the expression of BDNF or TrkB.
Interactions Between GC and TrkB
Aside from the functional interactions at the level of CRH regulation mentioned above, modulation of BDNF-mediated synaptic plasticity and glutamate signaling by GC have also been reported by Kumamaru et a., 2008). Using primary hippocampal neurons taken from fetal brain, BDNF-mediated neurite outgrowth and synaptic protein expression were suppressed by pretreatment with the synthetic GC, DEX. Further, DEX-pretreatment also reduced postsynaptic Ca2+ influx in these cells, a function normally attributed to TrkB signaling (Kumamaru et al., 2008). Because DEX has a greater affinity for the GR than the MR (Gaeggeler et al., 2005), these data suggest that stimulation of GR is responsible for the observed effects. This possibility was confirmed by the use of the GR antagonist RU-486 or use of small interfering RNA to silence the GR, which caused a blockade of DEX-mediated suppression of Ca2+ influx (Kumamaru et al., 2008). Moreover, the study by Kumamaru et al., (2008) indicated that DEX inhibited BDNF-mediated functions in primary hippocampal neuron cultures through blockade of MAPK signaling (Kumamaru et al., 2008). Additional studies demonstrated that the mechanism behind DEX-mediated suppression of BDNF activated MAPK signaling involved blockade of interactions between the protein Src homology-2 domain-containing phosphatase-2 (Shp2) and TrkB (Kumamaru et al., 2011).
Although it remains unclear why DEX pretreatment prevented interaction between Shp2 and TrkB, the data are consistent with a similar report indicating that chronic treatment of primary cortical neurons with DEX prevents the association of TrkB with PLC- (Numakawa et al., 2009). This same study also demonstrated that direct interactions between GR and TrkB result in enhanced BDNF-triggered glutamate release. Chronic DEX treatment has also been shown to inhibit glutamate release, however, it was concluded that this was likely due to downregulation of the GR resulting from chronic DEX stimulation of the GR.
Programming of BDNF by Early-Life GC Exposure
The possibility that imprinting of adult metabolic and behavioral functions occur as a result of the fetal environment has recently received considerable attention as a way to explain individual susceptibility to disease in adulthood. Although links between adverse fetal environments and adult disease are well established in both humans and animals models (Barker et al., 1989; Hales and Barker, 1992; Coe and Lubach, 2005; Painter et al., 2005; Barker, 2006; O’Regan et al., 2008), mechanisms explaining this phenomenon remain unclear. However, given the role for BDNF signaling in mitigating a variety of metabolic and mood disorders, permanent changes in the expression of this gene in response to adverse fetal environments represents a possible scenario. A recent study implicating abnormal programming of BDNF in response to the fetal environment revealed that maternal hypothyroidism resulted in decreased hippocampal BDNF expression in the offspring, a fact that correlates with cognitive and neurodevelopmental deficits observed in these offspring (Chakraborty et al., 2012). Supporting a role for GC in programming BDNF expression come from studies where pregnant Sprague-Dawley rats were administered DEX from gestational day (GD) 14 until parturition. The results of such studies show a blunted induction of Bdnf and TrkB mRNA in the PVN following an acoustic startle in adulthood (Hossain et al., 2008). These data further suggest the possibility that reduced expression of Bdnf mRNA after prenatal DEX exposure is due to epigenetic modification of the gene.
The effect of prenatal DEX exposure on programming of the Bdnf gene was tested in our laboratory by administering DEX (0.4 mg/kg) subcutaneously to pregnant Sprague Dawley rats between gestational days (GD)18-21. BDNF mRNA and promoter methylation levels were measured in the PVN after the offspring had reached postnatal day (PD) 60. Although no effect on Bdnf mRNA expression was observed under normal conditions (Figure 2A), the application of a metabolic stressor, consisting of a high-fat diet (60% fat-derived calories; Research Diets, Inc., New Brunswick, NJ, USA) caused a significant main effect of fetal DEX exposure [F(1, 8)=13.44; p=0.0063] and sex [F(1, 8)=23.11; p=0.0013], as well as a significant interaction between these variables [F(1, 8)=6.646; p=0.0327] when compared using two-way ANOVA (Figure 2B). Post hoc analysis showed a significant decrease in Bdnf mRNA in the PVN of female, but not male offspring (Figure 2B), although the lack of significance in the male offspring may be due to low levels of Bdnf expression in the male which was near the detection limits of the assay. Because Bdnf mRNA was measured when the female rats were in diestrus, the observed effect was likely independent of adult estradiol levels.
Figure 2.
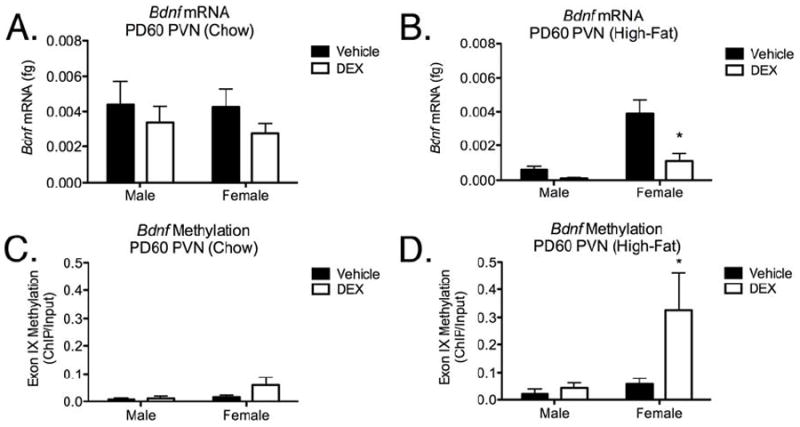
Bdnf mRNA expression (Panels A-B) and exon IX methylation (Panel C-D) measurements at PD60 in the PVN following fetal DEX exposure during GD18-21 in rats. Bdnf mRNA was measured using qRT-PCR. Animals were weaned at 21 days of age and placed on a high fat diet. Animals were euthanized at 60 days of age, females were all in diestrus. Data are expressed as the mean ± SEM of 3-7 individual rats per group. * = (p<0.05) between vehicle and DEX-exposed groups.
We employed chromatin immunoprecipitation (ChIP) using an antibody against 5-methylcytosine (Epigentek, Farmingdale, NY, USA) to measure methylation of the CpG island flanking exon IX (Lubin et al., 2008). Although fetal DEX exposure appeared to increase methylation of the Bdnf exon IX in the PVN of female offspring that were not challenged with the high fat diet, this did not reach statistical significance (Figure 2C). Although this may have been due to the small sample size (n=3) in some groups of offspring weaned onto the high-fat diet, two-way ANOVA revealed a sex specific effect in methylation [F(1, 12)=5.109; p=0.0432] of exon IX and post hoc analysis indicated a significant increase in methylation restricted to female offspring which were exposed during gestation to DEX when compared with non-exposed females (Figure 2D).
Our data are consistent with those from previous studies indicating that fetal DEX exposure causes a dysregulation of Bdnf mRNA expression in the adult PVN (Hossain et al., 2008). We have also shown that this may be programmed through an epigenetic methylation event, similar to the effect reported by Lubin et al., (2008). Additionally, we demonstrate that fetal DEX exposure appears to “prime” the female offspring for pathology in response to a metabolic stressor later in life (high fat diet). These results are consistent with previous studies also demonstrating elevated susceptibility to diet-induced metabolic pathology in female rat offspring following either fetal DEX exposure (Carbone et al., 2012), or offspring born to dams which were subject to a random stress paradigm during late gestation (Tamashiro et al., 2009).
Conclusion
The diverse roles for BDNF in nearly all aspects of neuronal homeostasis and development, as well as the highly variable expression of this neurotrophin in multiple tissues and cell types is reflected by the complex nature of Bdnf gene regulation and modulation of downstream activity. As such, our understanding of the regulation of BDNF signaling is still incomplete, but sex and stress hormones have been recognized to have powerful influences on the expression and activity of this neurotrophin. This action may occur through genomic and/or epigenetic mechanisms. In this review, we have examined various mechanisms by which hormones acutely regulate BDNF at both the transcriptional and post-translational levels. Additionally, we present novel data to suggest that aberrations in the hormonal milieu during development have the potential to permanently program the brain of offspring to express a phenotype characterized by a blunted BDNF response to metabolic stressors in adulthood. Given the link between deficits in BDNF and multiple disease processes, further understanding of such programming events warrants further research.
Table 1.
Regulation of BDNF expression by estrogen.
| Reference | Year | Species | mRNA/Protein | Observations |
|---|---|---|---|---|
| Miranda et al. | 1993 | Rat | mRNA | Colocalizatian of ER with Bdnf, TrkB, and p75 |
| Sohrabji et al. | 1995 | Rat | mRNA | Estradiol administration induces Bdnf. |
| Gibbs | 1998 | Rat | mRNA | Minimal hippocampal Bdnf expression during proestrus/estrus. |
| Dittrich et al. | 1999 | Finch | mRNA | Estradiol induces BDNF in male, but not female finch. |
| Cambiasso et al. | 2000 | Rat | Protein | Estradiol potentiates TrkB protein expression in neurons isolated from male, but not female, rats. |
| Jezierski and Sohrabji | 2000 | Rat | Protein | Estrogen replacement induces BDNF in olfactory bulb, but |
| Solum and Handa | 2002 | Rat | mRNA | Estrogen influences brain development through a BDNF-mediated |
| Scharfman et al. | 2003 | Rat | Protein | Hippocampal BDNF protein is induced during proestrus/estrus. |
| Brito et al. | 2004 | Rat | NA | TrkB antisense oligonucleotide blocks estradiol-mediated elongation in primary hypothalamic neurons. |
| Singh et al. | 2005 | Rat | mRNA | Estrogen-mediated Bdnf expression is region specific. |
| Sasahara et al. | 2007 | Mouse | Protein | Estrogen receptor is required for estrogen-mediated BDNF induction, decreases expression in cingulate cortex. |
Highlights.
BDNF plays a critical role in the development and maintenance of the CNS.
BDNF expression and activity are regulated by estrogen.
Glucocorticoid may influence the activity of BDNF.
Abnormalities in fetal hormone exposure may program atypical BDNF expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader MD, Malatiali SA, Redzic ZB. Expression of estrogen receptor alpha and beta in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation. Physiol Res. 2011;60:951–960. doi: 10.33549/physiolres.932167. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Ruiz-Palmero I, Scerbo MJ, Acaz-Fonseca E, Cambiasso MJ, Garcia-Segura LM. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: Recent advances. J Steroid Biochem Mol Biol. 2012;131:52–56. doi: 10.1016/j.jsbmb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, Brown E, Scott C, McKay RD, Barker JL. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Bender RA, Zhou L, Wilkars W, Fester L, Lanowski JS, Paysen D, Konig A, Rune GM. Roles of 17ss-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cereb Cortex. 2010;20:2985–2995. doi: 10.1093/cercor/bhq047. [DOI] [PubMed] [Google Scholar]
- Beyer C, Karolczak M. Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signalling. J Neurosci Res. 2000;59:107–116. [PubMed] [Google Scholar]
- Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Brito VI, Carrer HF, Cambiasso MJ. Inhibition of tyrosine kinase receptor type B synthesis blocks axogenic effect of estradiol on rat hypothalamic neurones in vitro. Eur J Neurosci. 2004;20:331–337. doi: 10.1111/j.1460-9568.2004.03485.x. [DOI] [PubMed] [Google Scholar]
- Cambiasso MJ, Colombo JA, Carrer HF. Differential effect of oestradiol and astroglia-conditioned media on the growth of hypothalamic neurons from male and female rat brains. Eur J Neurosci. 2000;12:2291–2298. doi: 10.1046/j.1460-9568.2000.00120.x. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology. 2012;153:295–306. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty G, Magagna-Poveda A, Parratt C, Umans JG, MacLusky NJ, Scharfman HE. Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU) Endocrinology. 2012;153:1311–1316. doi: 10.1210/en.2011-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29:227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- Davies AM, Thoenen H, Barde YA. The response of chick sensory neurons to brain-derived neurotrophic factor. J Neurosci. 1986;6:1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci U S A. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JS, Deakin JF, Miyajima F, Payton A, Toro CT. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J Psychiatr Res. 2009;43:1175–1184. doi: 10.1016/j.jpsychires.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44:607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;810:294. doi: 10.1016/s0006-8993(98)00945-7. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hofer MM, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hossain A, Hajman K, Charitidi K, Erhardt S, Zimmermann U, Knipper M, Canlon B. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A. 2012;109:1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau FD, Chao MV. Are bdnf and glucocorticoid activies calibrated? J Neurosci. 2012 doi: 10.1016/j.neuroscience.2012.09.017. http://dx.doi.org/10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed]
- Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Kumamaru E, Numakawa T, Adachi N, Kunugi H. Glucocorticoid suppresses BDNF-stimulated MAPK/ERK pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett. 2011;585:3224–3228. doi: 10.1016/j.febslet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Kumamaru E, Numakawa T, Adachi N, Yagasaki Y, Izumi A, Niyaz M, Kudo M, Kunugi H. Glucocorticoid prevents brain-derived neurotrophic factor-mediated maturation of synaptic function in developing hippocampal neurons through reduction in the activity of mitogen-activated protein kinase. Mol Endocrinol. 2008;22:546–558. doi: 10.1210/me.2007-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H, et al. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a Multicenter Study. Neuropsychopharmacology. 2006;31:212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32:2096–2104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci. 2012;6:26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Brown AL, Balkowiec A. Glia determine the course of brain-derived neurotrophic factor-mediated dendritogenesis and provide a soluble inhibitory cue to dendritic growth in the brainstem. Neuroscience. 2012;207:333–346. doi: 10.1016/j.neuroscience.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Minano A, Xifro X, Perez V, Barneda-Zahonero B, Saura CA, Rodriguez-Alvarez J. Estradiol facilitates neurite maintenance by a Src/Ras/ERK signalling pathway. Mol Cell Neurosci. 2008;39:143–151. doi: 10.1016/j.mcn.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Presumptive Estrogen Target Neurons Express mRNAs for both the Neurotrophins and Neurotrophin Receptors: A Basis for Potential Developmental Interactions of Estrogen with the Neurotrophins. Mol Cell Neurosci. 1993;4:510–525. doi: 10.1006/mcne.1993.1063. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, Riva MA. Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. J Psychopharmacol. 2010;24:595–603. doi: 10.1177/0269881108099815. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci U S A. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe JA, Handa RJ. Transient elevation of estrogen receptors in the neonatal rat hippocampus. Brain Res Dev Brain Res. 1990;57:119–127. doi: 10.1016/0165-3806(90)90191-z. [DOI] [PubMed] [Google Scholar]
- O’Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain Res Mol Brain Res. 1995;30:115–124. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E, Canpolat S, Yildirim S, Gulec M, Aliyev E, Aydin N. Cognitive functions and serum levels of brain-derived neurotrophic factor in patients with major depressive disorder. Brain Res Bull. 2012;88:454–459. doi: 10.1016/j.brainresbull.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Purba JS, Raadsheer FC, Hofman MA, Ravid R, Polman CH, Kamphorst W, Swaab DF. Increased number of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of patients with multiple sclerosis. Neuroendocrinology. 1995;62:62–70. doi: 10.1159/000126989. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. Reversal of the sexually dimorphic distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus by treatment with perinatal androgen. Brain Res. 1985a;340:91–98. doi: 10.1016/0006-8993(85)90777-2. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985b;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Lind RW, Rho JH. The CRH motoneuron: differential peptide regulation in neurons with possible synaptic, paracrine, and endocrine outputs. Ann N Y Acad Sci. 1987;512:12–23. doi: 10.1111/j.1749-6632.1987.tb24948.x. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett. 2011;503:15–19. doi: 10.1016/j.neulet.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K. Decreased Serum Levels of Mature Brain-Derived Neurotrophic Factor (BDNF), but Not Its Precursor proBDNF, in Patients with Major Depressive Disorder. PLoS One. 2012;7:e42676. doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fan J, Ren Y, Zhou W, Yin G. The release of glutamate from cortical neurons regulated by BDNF via the TrkB/Src/PLC-gamma1 pathway. J Cell Biochem. 2012 doi: 10.1002/jcb.24311. [DOI] [PubMed] [Google Scholar]


