Abstract
Stressors impact dopamine-dependent behaviors such as motivation, though the underlying neurobiological mechanism is not well defined. We report that corticotropin-releasing factor (CRF) acts within the ventral tegmental area (VTA) to reduce the motivation to work for food rewards. CRF in the VTA regulates dopamine output in a stimulus- and pathway-specific manner, collectively offering a mechanism by which acute stress selectively regulates information transmission via the VTA to reprioritize motivated behavior.
Stress can exacerbate the motivational disturbances found in psychiatric disorders such as drug addiction and depression1,2, but the neural mechanisms by which stress influences goal-directed behavior are not well-characterized. A wealth of experimental evidence highlights that motivated behavior is facilitated by activity of the mesolimbic dopamine projections from the VTA to the nucleus accumbens core (NAcc)3. Dopamine levels in the NAcc are elevated during appetitive behavior4,5 and also in response to a variety of stressors6,7, highlighting that mesolimbic VTA dopamine neurons are well-positioned to mediate the interaction between stress and motivation. During stressor exposure, the neuropeptide CRF initiates the hypothalamic-pituitary-adrenal axis, but is also released into the VTA in an activity-dependent manner8. Electrophysiological studies demonstrate a functional diversity in CRF’s postsynaptic effects on VTA dopamine neurons with both inhibitory9 and excitatory10,11 actions. However, it is unknown whether CRF acts in the VTA in vivo to mediate the effect of acute stress on the motivation to work for natural rewards. In the current study, we addressed this question and investigated the net effect of CRF in the VTA on mesolimbic dopamine transmission in vivo with fast-scan cyclic voltammetry during motivated behavior.
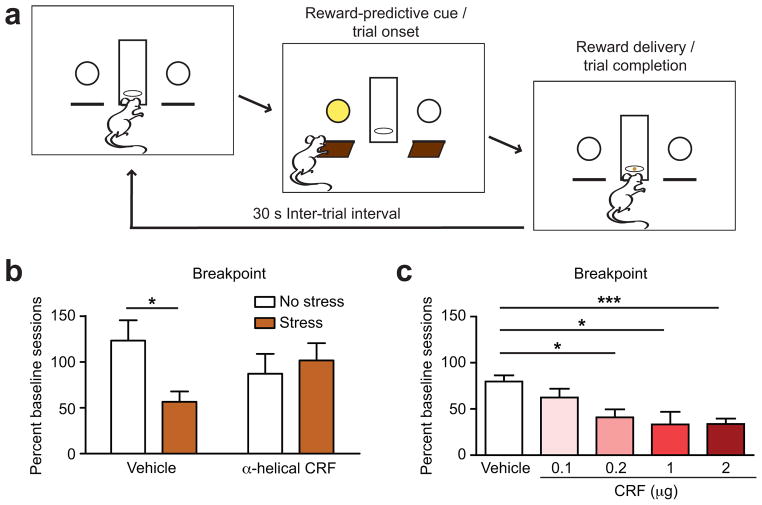
Motivation was assessed by determining the amount of work (breakpoint) that rats exerted to obtain food rewards in an operant task (Fig. 1a) under a progressive ratio (PR) reinforcement schedule, using a training regimen that elicits stable behavior across multiple days of testing12(Supplementary Table). To examine whether acute stress modulates the motivation to work for food rewards in a CRF-dependent manner, rats received a bilateral intra-VTA injection of the CRF receptor antagonist (500 ng α-helical CRF) or vehicle control and underwent 20 minutes of acute restraint stress prior to assessing motivation in the PR session. Stressor exposure significantly reduced the breakpoint relative to baseline sessions, an effect that was blocked by administering the CRF receptor antagonist into the VTA (two-way ANOVA: stress × drug interaction: F1,43 = 4.4, P < 0.05; post-hoc Bonferroni t-test, effect of stress t43 = 2.4, P < 0.05; n = 11 for Stress-Vehicle group and n = 12 rats for other groups; Fig. 1b). Furthermore, a bilateral microinjection of exogenous CRF into the VTA reduced the breakpoint in a dose-dependent manner (Kruskal-Wallis H4 = 23.2, P < 0.001; post-hoc Mann-Whitney comparisons relative to vehicle, n = 18; 0.1 μg CRF: U18,5 = 33, P > 0.05, n = 5; 0.2 μg CRF: U18,5 = 11.5, P < 0.05, n = 5; 1 μg CRF: U18,4 = 7, P < 0.05, n = 4; and 2 μg CRF: U18,23 = 45.5, P < 0.001, n = 23; Fig. 1c). Neither stress nor CRF administration elicited gross motor impairments (Supplementary Fig. 1). This action of CRF in the VTA to suppress the motivation to obtain food rewards was also observed following unilateral microinjections (Supplementary Fig. 2), but was absent in rats where the cannula placement was not in the VTA (Supplementary Fig. 3). Collectively, these results demonstrate that CRF acts in the VTA to attenuate the motivation to work for natural rewards.
Figure 1.
Effect of stress and CRF in the VTA on motivation to work for food rewards during PR sessions. (a) Schematic of operant task. (b) Acute restraint stress reduced the breakpoint in PR sessions, which was blocked by intra-VTA injections of α-helical CRF, post-hoc Bonferroni t-test, * P < 0.05). (c) The breakpoint in PR sessions was dose-dependently attenuated by intra-VTA CRF injections, post-hoc Mann-Whitney test relative to vehicle treatment, * P < 0.05, *** P < 0.001. Data presented as mean + s.e.m.
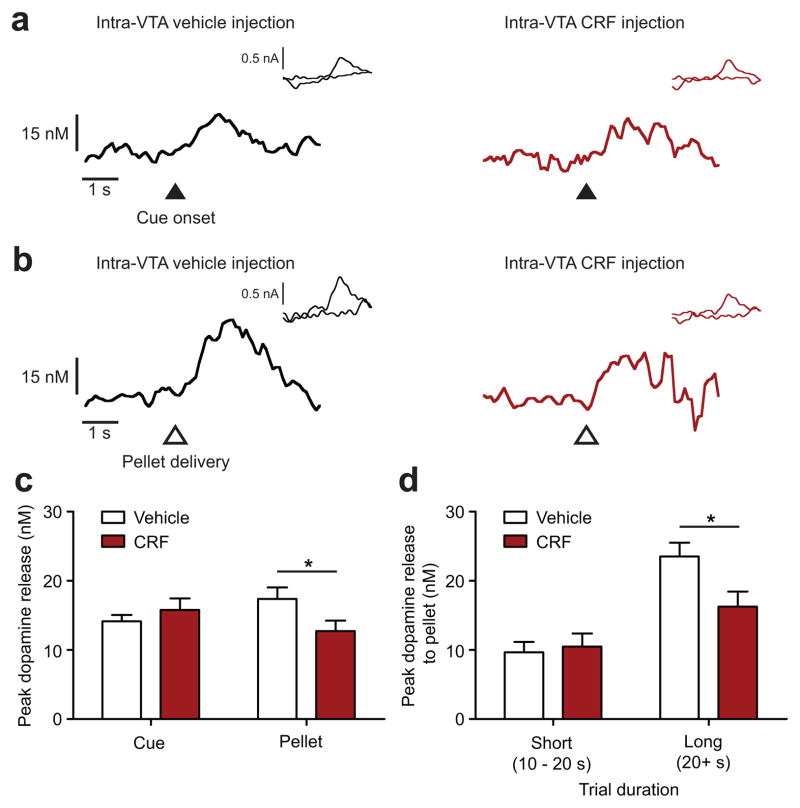
We next ascertained whether there was a corollary change in mesolimbic dopamine transmission during the suppression of motivation by CRF. We used fast-scan cyclic voltammetry in rats during PR sessions to monitor dopamine release in the NAcc to rewards and reward-predictive cues, both of which are sensitive to manipulations of reward magnitude (Supplementary Fig. 4). Intra-VTA injections of CRF (2 μg) reduced motivation in the rats used for voltammetry experiments (Supplementary Fig. 5), but interestingly, had no effect on dopamine release to reward-predictive cues (unpaired t-test with Welch’s correction, t149 = 0.9, P > 0.05, n = 9 rats with 116 trials for vehicle injection and 95 trials for CRF injection; Fig. 2a, c). In contrast, this manipulation significantly inhibited dopamine release to reward delivery (unpaired t-test, t77 = 2.0, P < 0.05; n = 9 rats with 43 trials for vehicle injection and 36 trials for CRF injection; Fig. 2b, c). Consequently, the effect of CRF in the VTA on reward-evoked dopamine release was significant for longer duration trials where dopamine release is greatest (two-way ANOVA: trial duration F1,75 = 24.7, P < 0.001; trial duration × drug interaction F1,75 = 4.2, P < 0.05; post-hoc Bonferroni t-test, long duration trials t75 = 2.5, P < 0.05; Fig. 2d). Intra-VTA injections of CRF also attenuated dopamine release to an unexpected food pellet delivery given at the end of the PR session (Supplementary Fig. 6). These data demonstrate that when CRF acts in the VTA to reduce motivation to work for food rewards, it produces a selective abrogation of dopamine release to rewards, without affecting dopamine release to reward-predictive cues.
Figure 2.
CRF in the VTA attenuates NAcc dopamine release to rewards but not to reward-predictive cues. Representative change in extracellular dopamine concentration in response to the presentation of (a) reward-predictive cues or (b) reward delivery in PR sessions after receiving intra-VTA injections of vehicle (left) or 2 μg CRF (right). Insets present cyclic voltammograms identifying the detected electrochemical signal as dopamine. (c) Intra-VTA injections of CRF did not affect the average release to reward-predictive cues per trial, though significantly attenuated the average dopamine release to reward delivery per trial (unpaired t-test, * P < 0.05). (d) Intra-VTA CRF injections principally affected reward-evoked dopamine release in long duration trials (post-hoc Bonferroni t-test, long duration trials * P < 0.05). Data presented as mean + s.e.m.
The ability of CRF in the VTA to affect phasic dopamine release in a stimulus-specific manner suggests that CRF regulates information transmitted through a subset of synaptic inputs to the VTA. To probe this hypothesis, we assessed how CRF in the VTA affected dopamine release in the NAcc in response to stimulation the pedunculopontine tegmental nucleus (PPT) or the bed nucleus of the stria terminalis (BNST). Activation of either the PPT or the BNST evokes phasic dopamine release in a VTA-dependent manner 13–15(Supplementary Fig. 7). Intra-VTA CRF injections decreased dopamine release in the NAcc when stimulating the PPT (two-way ANOVA: drug F1,170 = 88.8, P < 0.001; time F15,170 = 2.0, P < 0.05; drug × time interaction F15,170 = 1.9, P < 0.05, n = 6/7 rats for Vehicle/CRF groups; Fig. 3a), but increased dopamine levels when stimulating the BNST (two-way ANOVA: drug F1,224 = 18.7, P < 0.001, n = 8 rats for both groups; Fig. 3b), together illustrating the pathway-selective effects of CRF on dopamine release (Supplementary Fig. 7). We next assessed whether the behavioral effect of CRF in the VTA on motivation could be occluded by inactivating the PPT with the GABA receptor agonists baclofen and muscimol (B/M, 0.3 nmol and 0.03 nmol, respectively) (Fig. 3c). CRF infusions into the VTA reduced motivation after vehicle infusions into the PPT, but was blocked when the PPT was inactivated with B/M injections (two-way ANOVA: CRF F1,34 = 16.9, P < 0.001; CRF × B/M interaction F1,34 = 4.7, P < 0.05; post-hoc unpaired t-test adjusted for planned comparisons with Welch’s correction, effect of CRF, t8 = 3.6, P < 0.05; n = 9 rats for Vehicle-Vehicle and B/M-CRF groups and n = 10 rats for Vehicle-CRF and B/M-Vehicle groups; Fig. 3c). Reducing motivation through overnight ad libitum food access did not block the behavioral effect of intra-VTA CRF injections (Supplementary Fig. 8), suggesting that the occlusion by PPT inactivation was not due to a non-specific manipulation of motivation. These results collectively highlight the involvement of PPT activity in the avolition elicited by CRF acting within the VTA.
Figure 3.
CRF in the VTA affects NAcc dopamine release in a pathway-specific manner. (a) Schematic of experimental procedure (left). Intra-VTA CRF (2 μg) injections decreased dopamine release in the NAcc core when stimulating the PPT (two-way ANOVA drug effect, *** P < 0.001; right). (b) Schematic of experimental procedure (left). Intra-VTA CRF injections increased dopamine release in the NAcc core when stimulating the BNST (two-way ANOVA drug effect, *** P < 0.001; right). (c) Schematic of experimental procedure (left). Intra-VTA injections of 2 μg CRF did not alter motivation following inactivation of the PPT with injections of 0.3 nmol baclofen / 0.03 nmol muscimol (B/M) (post-hoc unpaired t-test, * P < 0.05, right). Data presented as mean + s.e.m.
Stress can reduce reward-seeking behaviors16 and alter decision-making processes17, which illustrates a reprioritization of behavior thought to arise from a reduction in dopamine transmission18. Here we demonstrate that the motivational suppressant effects of acute stress are mediated by endogenous CRF acting in the VTA and these effects can be recapitulated by exogenous VTA application of CRF. Notably, in stark contrast to its effects in the VTA, CRF acts in the NAcc of stress-naïve animals to increase dopamine release and promote appetitive behavior19. Moreover, CRF positively affects drug-seeking following an experience-dependent neuroadaptation in CRF’s capacity to regulate glutamate release within the VTA8,20. Taken together, these studies illustrate the diverse effects of CRF on behavior and highlight the involvement of CRF in models of psychiatric disorders. Collectively, our results demonstrate that CRF selectively gates afferent inputs to the VTA in a stimulus- and pathway-specific manner, as well as offer a mechanism by which acute stress selectively regulates information transmission via the VTA to reprioritize motivated behavior.
ONLINE METHODS
Subjects and surgery
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River, CA) were pair-housed upon arrival and given ad libitum access to water and lab chow and maintained on a 12-hour light/dark cycle. Recovery surgeries were performed under isoflurane anesthesia on rats weighing 300–350g (~60–70 days old), after which rats were single-housed. Carbon-fiber electrodes targeting the NAcc (relative to bregma: 1.3 mm anterior; ±1.3 mm lateral; 7.0 mm ventral) and a Ag/AgCl reference electrode were implanted for voltammetry experiments. Implantation of guide cannulas for microinjection experiments targeted the VTA (relative to bregma: 5.6 mm posterior; 0.5 mm lateral, 7.0 mm ventral) and/or the PPT (relative to bregma: 8.0 mm posterior; 1.5 mm lateral, 5.8 mm ventral). For non-recovery voltammetry surgeries, rats were anesthetized with urethane (1.5 g/kg), additional holes were drilled above the PPT (relative to bregma: 8.0 mm posterior; 2.0 mm lateral) or the BNST (relative to bregma: 0.3 mm posterior; 1.5 mm lateral), and only the guide cannula above the VTA was cemented into place.
Behavioral training
After at least 2 weeks of recovery from surgery, rats were placed and maintained on mild food restriction (~15 g/day of standard lab chow) to target 90% free-feeding weight, allowing for an increase in weight of 1.5% per week. Operant training was performed as described previously12. Behavioral sessions were performed in operant chambers (Med Associates, VT) that had sloped floors, a house light, and contained a food tray and two cue lights above two retractable levers on a single wall. The cue lights and their corresponding levers were located on either side of the food tray. Rats were exposed to PR or FR experimental sessions (one session per day) according to the schedule presented in the Supplementary Table, which was previously shown to elicit stable behavior12 and was designed to minimize inflexible behaviors by alternating the side of the active lever across sessions. This behavioral schedule also accommodated at least two days of recovery time between intra-VTA pharmacological manipulations. Behavioral sessions began with both levers extending, and illumination of the house light and the cue light over the active lever. Completion of the correct number of lever presses led to the delivery of food rewards (45-mg food pellets, BioServ, NJ), retraction of the levers, and the cue and house lights turning off for a 30-s inter-trial interval (ITI). Food rewards were earned on a FR4 reinforcement schedule during FR sessions that consisted of 60 trials. PR sessions were identical to FR4 sessions except that the operant requirement on each trial (T) is the integer (rounded-down) of 1.4(T-1) lever presses, starting at 1 lever press (i.e. 1, 1, 1, 2, 3, 5, 7, 10, 14, 20, 28, 40, 56, 79, 111, 155, 217, 304, 426, etc). PR sessions ended after 15 min elapsed without completion of the response requirement in a trial. Rats completed at least two baseline PR sessions before the reward magnitude was changed or drugs were administered intra-cerebrally. Acute stress was administered by placing the rat in a tail vein restrainer for 20 minutes. No experimental manipulation was performed on the PR session following exposure to restraint stress. All manipulations were performed in a counterbalanced manner and only during PR sessions.
Microinjections
Intra-cerebral injectors extended 1 mm past the end of the guide cannula, targeting a final depth below the skull surface of 8.0 mm for intra-VTA injections and 6.8 mm for intra-PPT injections. Infusions were performed 15 min prior to the start of the behavioral session and were visually monitored to ensure successful infusion. Injectors remained in place for 1-minute post-injection to minimize backflow of the drug. Injections of αhelical CRF (500 ng in 1% acetic acid in saline vehicle) and CRF (100 ng, 200 ng, 1 μg, or 2 μg in artificial cerebral spinal fluid) were administered into the VTA in a volume of 0.5 μl. Doses of CRF were based upon previous work using site-specific injections21. Intra-PPT injections of baclofen/muscimol (0.3 nmol / 0.03 nmol) were delivered in saline vehicle at a volume of 0.3 μl. All drug treatments were administered in a counterbalanced manner across PR sessions. Drugs were purchased from Bachem (CRF), Tocris (baclofen and muscimol) and Sigma (α-helical CRF).
Voltammetry recording sessions
During experimental recording sessions in behaving rodents, the chronically-implanted carbon-fiber microelectrodes were connected to a head-mounted voltammetric amplifier for dopamine detection by fast-scan cyclic voltammetry as described elsewhere22. The potential applied to the carbon fiber was ramped from −0.4 V (vs Ag/AgCl) to +1.3 V and back at a rate of 400 V/s during a voltammetric scan and held at −0.4 V between scans at a frequency of 10 Hz. To confirm that electrodes were capable of detecting dopamine, unexpected food pellets were delivered before and after a recording session to elicit dopamine release. Chemical verification of dopamine was achieved by obtaining high correlation of the cyclic voltammogram (electrochemical signature) to that of a dopamine standard (correlation coefficient r2 ≥ 0.75 by linear regression). The voltammetry data for a session were not analyzed if food pellet delivery did not elicit dopamine release that satisfied the chemical verification criteria. For anesthetized experiments, dopamine release was evoked by stimulating the PPT (relative to bregma: 8.0 mm posterior; 2.0 mm lateral, 6.5–7.5 mm ventral) or the BNST (relative to bregma: 0.3 mm posterior; 1.5 mm lateral, 6.5–7.5 mm ventral) with a bipolar stimulating electrode (60 pulses delivered at 60 Hz, ≤ 200 μA). Stimulations were performed every 5 min until a stable baseline for 20 min was achieved (< 10% deviation from the mean peak response of dopamine). Intra-VTA drug injections were performed as described above and stimulations commenced immediately after completion of the infusion.
Data analysis
Dopamine was isolated from the voltammetric signal using chemometric analysis23 with a standard training set of stimulated dopamine release detected by chronically implanted electrodes, as has been previously reported12. Dopamine concentration was estimated based upon the average post-implantation sensitivity of electrodes22. A within-animal design was used for voltammetry data analysis, so that data was included only from rats where dopamine release satisfied the chemical verification criteria on both the control and treatment sessions. Voltammetric data analysis was carried out using software written in LabVIEW and low-pass filtered at 2,000 Hz. Data was smoothed using a 0.5 s moving average. Analysis of extracellular dopamine concentration was restricted to a period of 3 s following cue onset or reward delivery. The analysis of cue-evoked dopamine release omitted the first trial of a session, i.e., prior to the first reward in the session. Reward-evoked dopamine release in individual trials was analyzed only for trials lasting greater than 10 s in order to minimize any carry-over contribution of cue-evoked dopamine release in the detected signal, as described previously12. The number of animals used per experiment was determined by a power analysis with an alpha level of 0.05 and power level of 0.8, using the effect size and variance estimated from preliminary data. To assess normality, the Kolmogorov-Smirnov test was performed on the residuals after data were fitted to a Gaussian curve with the mean and standard deviation of each data set. If data failed this test, non-parametric statistical tests were performed. The Welch’s correction was used for post-hoc tests under conditions with unequal variances between groups. Statistical analyses of voltammetry data used unpaired Student’s t-tests, or two-way ANOVAs with repeated measures when appropriate, followed by post-hoc t-tests. Statistical analyses of behavioral data used Kruskil-Wallis tests followed by Mann-Whitney post-hoc tests corrected for inflated alpha, Student’s t-tests (unpaired or paired as appropriate), or two-way ANOVAs, followed by post-hoc t-tests. Data were normalized to the behavior observed on the baseline sessions before experimental manipulations to reduce inter-animal variability. Rats were also excluded from behavioral studies if they did not complete >50% of planned experiments due to technical complications. Rats were excluded for analysis if cannulas did not target the region of interest, save for those illustrating the lack of an effect of CRF when administered outside of the VTA (Supplementary Fig. 3). General motor activity was assessed using the rate of head entries into the food tray. Data were analyzed using Excel, Prism and SPSS.
Histology
Following completion of the experimental sessions, animals were anesthetized with ketamine/xylazine (100 mg/kg) and the recording site was marked by making a small electrolytic lesion at the electrode tip by passing a current (~70 μA) through the carbon fiber microelectrode for 20 seconds. Animals were subsequently perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline at pH = 7.4 before the brains were removed and post-fixed in the paraformaldehyde solution. The brains were then placed in 30% sucrose solution in phosphate-buffered saline for 48 hours, flash frozen, and sectioned coronally (60 μm). All sections were mounted and stained with cresyl violet. Histology is presented in Supplementary Fig. 9.
Supplementary Material
Supplementary Figure 1. Acute restraint stress and intra-VTA injections of CRF did not elicit gross impairments of motor function.
Supplementary Figure 2. Behavioral effects of unilateral or bilateral injections of CRF on motivated behavior during PR sessions.
Supplementary Figure 3. The effect of CRF on motivated behavior was absent when guide cannula did not target the VTA.
Supplementary Figure 4. NAcc dopamine release in response to rewards and their predictions is sensitive to rewards size in PR sessions.
Supplementary Figure 5. Behavioral data from the rats included in voltammetry experiments assessing the effect of CRF in the VTA on motivation.
Supplementary Figure 6. CRF in the VTA reduced dopamine release to unexpected reward delivery after completion of the PR session.
Supplementary Figure 7. Dopamine release in the NAcc elicited by stimulating VTA afferent pathways and its modulation by CRF.
Supplementary Figure 8. Intra-VTA injections of CRF further attenuated motivation in rats in a reduced motivational state.
Supplementary Figure 9. Histology.
Supplementary Table. Training schedule.
Acknowledgments
We would like to thank S. Ng-Evans for invaluable technical support and C. Akers for technical assistance. We thank N. Hollon, J. Clark, and S. Sandberg for scientific discussion. This work was funded by the National Institutes of Health (R01-MH079292, P.E.M.P.; R01-DA016782, A.B. and P.E.M.P.; T32-AA009455 and F32-DA026273, M.J.W.) and NARSAD (P.E.M.P).
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS:
M.J.W., A.B., and P.E.M.P. designed the experiments. M.J.W. collected and analyzed the data. M.J.W. and P.E.M.P. wrote the manuscript.
References
- 1.Sinha R. Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 2.Meyer SE, Chrousos GP, Gold PW. Dev Psychopathol. 2001;13:565–580. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- 3.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Frontiers in Behavioral Neuroscience. 2009;3:1–12. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 6.Inglis FM, Moghaddam B. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- 7.Tidey JW, Miczek KA. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, et al. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckstead MJ, et al. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungless MA, et al. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 11.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanat MJ, Kuhnen CM, Phillips PE. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georges F, Aston-Jones G. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarnati E, Campana E, Pacitti C. Brain Res. 1984;304:351–361. doi: 10.1016/0006-8993(84)90339-1. [DOI] [PubMed] [Google Scholar]
- 15.Zweifel LS, et al. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zacharko RM, Anisman H. Neurosci Biobehav Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- 17.Shafiei N, Gray M, Viau V, Floresco SB. Neuropsychopharmacology. 2012;37:2194–2209. doi: 10.1038/npp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabib S, Puglisi-Allegra S. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 19.Lemos JC, et al. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blacktop JM, et al. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalivas PW, Duffy P, Latimer LG. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- 22.Clark JJ, et al. Nat Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heien ML, et al. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Acute restraint stress and intra-VTA injections of CRF did not elicit gross impairments of motor function.
Supplementary Figure 2. Behavioral effects of unilateral or bilateral injections of CRF on motivated behavior during PR sessions.
Supplementary Figure 3. The effect of CRF on motivated behavior was absent when guide cannula did not target the VTA.
Supplementary Figure 4. NAcc dopamine release in response to rewards and their predictions is sensitive to rewards size in PR sessions.
Supplementary Figure 5. Behavioral data from the rats included in voltammetry experiments assessing the effect of CRF in the VTA on motivation.
Supplementary Figure 6. CRF in the VTA reduced dopamine release to unexpected reward delivery after completion of the PR session.
Supplementary Figure 7. Dopamine release in the NAcc elicited by stimulating VTA afferent pathways and its modulation by CRF.
Supplementary Figure 8. Intra-VTA injections of CRF further attenuated motivation in rats in a reduced motivational state.
Supplementary Figure 9. Histology.
Supplementary Table. Training schedule.