Abstract
CKD is associated with premature death from cardiovascular disease which is, in part, driven by HDL deficiency and dysfunction. One of the main causes of HDL deficiency in CKD is diminished plasma apoliprotein A-I level. Plasma ApoA-I is reduced in dialysis patients and hepatic ApoA-I mRNA is decreased in the uremic rats. This study explored the mechanism of uremia-induced down-regulation of ApoA-I. HepG2 cells were incubated in media containing whole plasma or plasma subfractionation from normal subjects and ESRD patients pre- and post-hemodialysis. Cells and culture media were isolated to measure ApoA-I protein and mRNA. ApoA-I promoter activity was measured using transfection with a luciferase promoter construct containing the −2096 to +293 segment of ApoA-I gene. Finally effect of uremic and control plasma was assessed on ApoA-I RNA stability. Exposure to uremic plasma significantly reduced ApoA-I mRNA expression and ApoA-I protein production. These effects were reversed by replacing uremic plasma with normal plasma. While no difference in ApoA-I promoter activity was found between cells exposed to uremic and normal plasma, uremic plasma significantly reduced ApoA-I RNA stability. Experiments using plasma sub-fractions revealed that the inhibitory effect of uremic plasma on ApoA-I mRNA expression resides in fractions containing molecules larger but not smaller than 30kd. The pre- and post-dialysis plasma exerted an equally potent inhibitory effect on ApoA-I mRNA abundance.
Uremia lowers ApoA-I production by reducing its RNA stability. The inhibitory effect of uremic milieu on ApoA-I mRNA expression is mediated by non-dialyzable molecule(s) larger than 30 kd.
Keywords: End stage renal disease, HDL, cardiovascular disease, apolipoprotein A-I, dyslipidemia
Introduction
Approximately 9% of the United States (US) population have chronic kidney disease (CKD), translating into 20 million adults (1). Out of this staggering number, approximately 400,000 patients have advanced (stage V) CKD requiring maintenance dialysis (1). Five year survival for patients with stage V CKD is about 35%, a mortality rate that is worse than that associated with many cancers (2–3). Almost half of these deaths are attributed to premature cardiovascular disease (2–3). Furthermore, there is an independent and graded association between reduced creatinine clearance and the risk of cardiovascular events, hospitalization and death (4). Cardiovascular disease in CKD is associated with and, in part, due to profound dysregulation of lipoprotein metabolism which leads to a highly pro-atherogenic plasma lipid profile. This is marked by impaired clearance and accumulation of oxidation-prone intermediate density lipoprotein (IDL) particles, chylomicron remnants and small dense low density lipoprotein (LDL) coupled with HDL deficiency and dysfunction (5,6). The CKD-induced HDL abnormalities include reduction of plasma HDL cholesterol concentration and impaired maturation, antioxidant, anti-inflammatory and reverse cholesterol transport capacities of HDL (7–10). Several factors contribute to HDL deficiency and dysfunction including reduced plasma levels of ApoAI and lecithin cholesterolacyltransferase (LCAT), oxidative modification of HDL limiting its binding to the gateway of cholesterol efflux, and upregulation of acyl-CoA cholesterolacyltransferase (ACAT) impeding the release of free cholesterol from lipid laden cells in the artery wall (9–15). Together accumulation of oxidation prone, highly atherogenic and pro-inflammatory lipoprotein remnants and HDL deficiency and dysfunction as opposed to the traditional hypercholesterolemia and elevated LDL cholesterol are the primary features of CKD-induced dyslipidemia (16–20). These lipid abnormalities most likely make a significant contribution to the atherogenic diathesis in this population. Accordingly, interventions aimed at alleviating inflammation and oxidative stress and enhancing HDL-mediated reverse cholesterol transport may confer greater cardiovascular protection in CKD population than the cholesterol-lowering therapies.
The critical role of HDL as the vehicle of reverse cholesterol transport and it’s effectiveness in prevention and impeding atherosclerosis have been demonstrated in many clinical studies (21–24). The biosynthesis and maturation of HDL is a complex process involving production and release of its major apolipoprotein components, extraction of phospholipids and cholesterol from the target cells, and exchange of its lipid and apoprotein cargo with Apo B-containing lipoproteins in the circulation. The assembly of these different components leads to the generation of mature HDL particles. The major apolipoprotein of HDL is Apolipoprotein A-I (ApoA-I) which constitutes approximately 70% of the HDL protein content (25–26). ApoA-I is synthesized in the liver and intestine. Deletion of the ApoA-I gene results in a significant reduction of HDL and increased risk of atherosclerosis (27–29) Whereas its over-expression significantly increases serum HDL levels and inhibits progression of atherosclerosis and even causes regression of existing atherosclerotic plaques (30–33).
Several studies in patients with CKD and end stage renal disease have demonstrated the association of CKD with ApoA-I deficiency (34–36). However the available data on the underlying mechanisms of CKD-induced ApoA-I deficiency are limited. The present study was performed to determine the mechanisms by which uremia leads to down regulation of ApoA-I gene expression.
Material and Methods
Pooled plasma preparations
Pre- and post-dialysis plasma samples from a group of 50 patients with end-stage renal disease were collected in heparinized tubes and pooled. Patients receiving HMG-CoA reductase inhibitors and other lipid-altering medications and those with viral and bacterial infections or intercurrent acute illnesses were excluded from the study. The protocols for collection and pooling of plasma samples were reviewed and approved by University of California, Irvine institutional review board (IRB). The urea nitrogen, concentrations in the pooled samples was measured by QuantiChrom™ Urea Assay Kit (BioAssay Systems, Hayward, CA, U.S.A.) and found to be 67 mg/dL in pre-dialysis and 30 mg/dL in post-dialysis pooled samples. Gender-matched pooled plasma from healthy controls was purchased from a vendor (Innovative Research, MI, USA) and the urea nitrogen was measured and found to be 14 mg/dL. In addition plasma concentrations of sodium, potassium, phosphorus and glucose were measured in the pooled plasma samples and the results summarized in table. Amicon filters were used to fractionate uremic and control plasma per manufacturer’s protocol (Amicon Ultra-4 Centrifugal Filter Units – 10,000 and 30,000 NMWL (10 and 30 kilodaltons), EMD Millipore Corporation, Billerica, MA, USA).
Table 1.
Sodium, potassium, phosphorus, glucose and urea nitrogen (BUN) concentrations in the pooled plasma samples from gender-matched controls and dialysis patients.
| Sodium mEq/L |
Potassium mEq/L |
Phosphorus mg/dL |
Glucose mg/dL |
BUN mg/dL |
|
|---|---|---|---|---|---|
| Pooled Dialysis Samples | 137 | 5.1 | 5.3 | 132 | 67 |
| Pooled Control Samples | 144 | 3.7 | 2.7 | 103 | 14 |
Cell Culture
The human-derived hepatic HepG2 cells (passage 20; American Type Culture Collection, Manassas, VA) derived from a 15-yr-old Caucasian male were grown in DMEM supplemented with 10% (vol/vol) FBS, glutamine (0.29 g/l), sodium bicarbonate (2.2 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l) in 75-cm2 plastic flasks at 37°C and 5% CO2-95% air. The cells were plated at a density of 2 × 105 cells/well in 12-well plates and media were changed every 2 or 3 days.
Quantitative real-time PCR (qPCR) and ELISA
Quantitative PCR (qPCR) was performed using the Bio-Rad iCycler (Hercules, CA) and a Qiagen Quantitect SYBR Green PCR kit (Valencia, CA). RNA from HepG2 cells was isolated using TriZOL (Invitrogen, Carlsbad, CA), following the manufacturer's instructions. The RNA was DNase treated and first-strand cDNA was made from 5 µg of the isolated total RNA primed with oligo dT using an Invitrogen Superscript synthesis system. The RT products were then used in the subsequent qPCR with primers specific for the human ApoA-I (forward, 5′-AGCTTGCTGAAGGTGGAGGT -3′ and reverse, 5′-ATCGAGTGAAGGACCTGGC -3′) (22) and the human β-actin (forward 5′-AGCCAGACCGTCTCCTTGTA-3′ and reverse, 5′-TAGAGAGGGCCCACCACAC-3′) genes. The qPCR consisted of a 15-s 95°C melt follo wed by 40 cycles of 95°C melt for 30 s, 58°C annealing for 30 s, and 72°C extension a nd data collection for 1 min. To compare the relative relationship between ApoA1 levels, we used a calculation method provided by the iCycler manufacturer (Bio-Rad) described previously (38). The approach determines the relative relationship among various samples by first determining the threshold cycle (the number of cycles in the PCR that were required to achieve a specific level of product) for our gene of interest and a housekeeping gene in each sample (β-actin). All samples are then normalized to the housekeeping gene. Next the sample with the lowest expression level is set to a relative value of one and all samples are calculated according to the expression level over that sample. Each unit any gene is expressed above another represents a doubling of the expression level. ApoA-I protein concentration was determined after exposing HepG2 cells to 10% uremic versus control plasma for 48 hours. Cells were subsequently washed four times with PBS and fresh serum-free media was added and incubated for 6 hours. Subsequently the concentration of ApoA-I secreted into the media was measured using an ELISA kit following the manufacturer’s protocol (Catalog# SEL3664, R&D systems, Minneapolis, U.S.A.).
Cloning of the 5′-regulatory region for the ApoA-I gene
In order to determine whether uremic down-regulation of ApoA-I expression is also mediated at the promoter level the −2096 to +293 segment of this gene was cloned into a pGL3-luciferase reporter plasmid as previously described with minor modifications (39).To obtain the genomic DNA fragment that contained the 5′-regulatory region of the ApoA1 gene, we used the sequence information deposited in GenBank (accession no. M20656.1) for the ApoA-I gene and flanking sequence. Primers were designed with the incorporation of Mlu I and Bgl II restriction sites. A PCR was then performed using mentioned primers and 100 ng of human genomic DNA (Invitrogen). A reaction buffer and polymerase specially developed to allow amplification through GC-rich regions in the DNA sequence was used (Advantage GC Genomic PCR Kit; Clontech). PCR conditions were denaturation at 95°C for 5 min, followed by 30 cycles of denaturati on at 95 C for 30 s, annealing at 50°C for 30 s, extension at 72°C for 4 min, and then a f inal extension at 72°C for 15 min. The 2389 base pair product was run on a 0.7% agarose gel and purified. The purified DNA was then cut with the restriction enzymes Mlu I and Bgl II (sequence encoded in the primers) and subcloned into the pGL3-basic vector (Promega, Madison, WI), cut with the same enzymes. The entire DNA sequence was verified via sequencing (Laragen, Los Angeles, CA).
Cell culture, transfection, and luciferase assay
Four micrograms of the full-length promoter-luciferase constructs for ApoA-I was transfected into HepG2 cells using the Lipofectamine 2000 reagent (Invitrogen, San Diego, CA) following the manufacturer's protocol. To normalize for transfection efficiency, the cells were cotransfected with 100 ng of pRL-TK (Promega, Madison, WI) plasmid along with the promoter constructs. Total cell lysate was prepared from cells 24 h posttransfection, and firefly luciferase activity was assayed using the dual luciferase kit (Promega) and a Turner Design 20/20 Luminometer (Sunnyvale, CA). The activity was normalized to the Renilla luciferase activity from pRL-TK in the same extract. A control plasmid (SV-40 pGL3) was also used to rule out a general effect on transcription from the uremic toxins. Data presented are the means ± SEM of at least three independent experiments and given as fold expression over pGL3-basic expression set arbitrarily at one. Statistical analysis was performed using the Student's t-test.
RNA Stability Assay
A RNA decay rate assay (i.e., RNA stability assay) was performed as previously described (40–41). HepG2 cells were maintained in control or uremic growth medium and then analyzed for RNA stability by the addition of actinomycin D (1 µM, Sigma) to the growth medium followed by an isolation of total RNA using TRIzol (Invitrogen) and the manufacturer's procedures at specific time points of 30 minutes, 1, 4, 6, and 22 h. RNA was then reverse transcribed with oligo-dT, and qPCR analysis was performed with specific primers to either ApoA-I or β-actin using the conditions and primers described above.
Results
Effect of uremic plasma on expression and secretion of ApoA-I
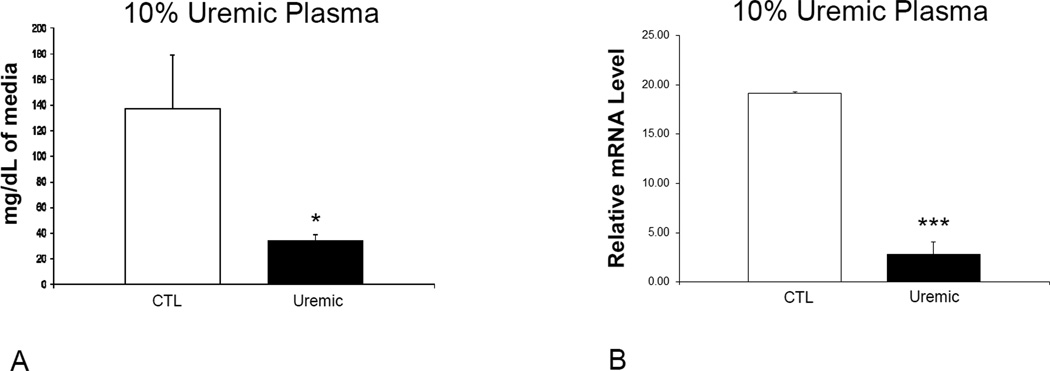
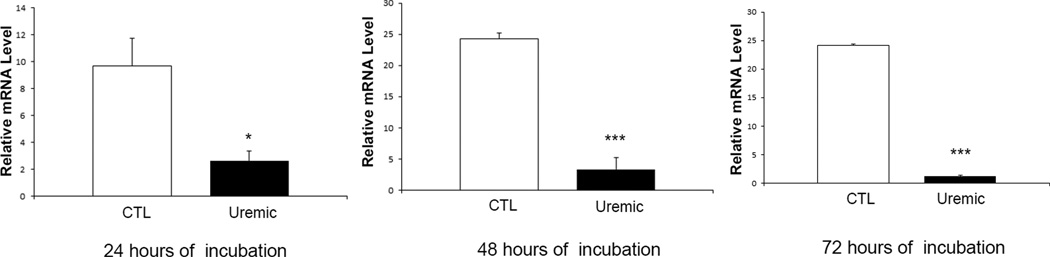
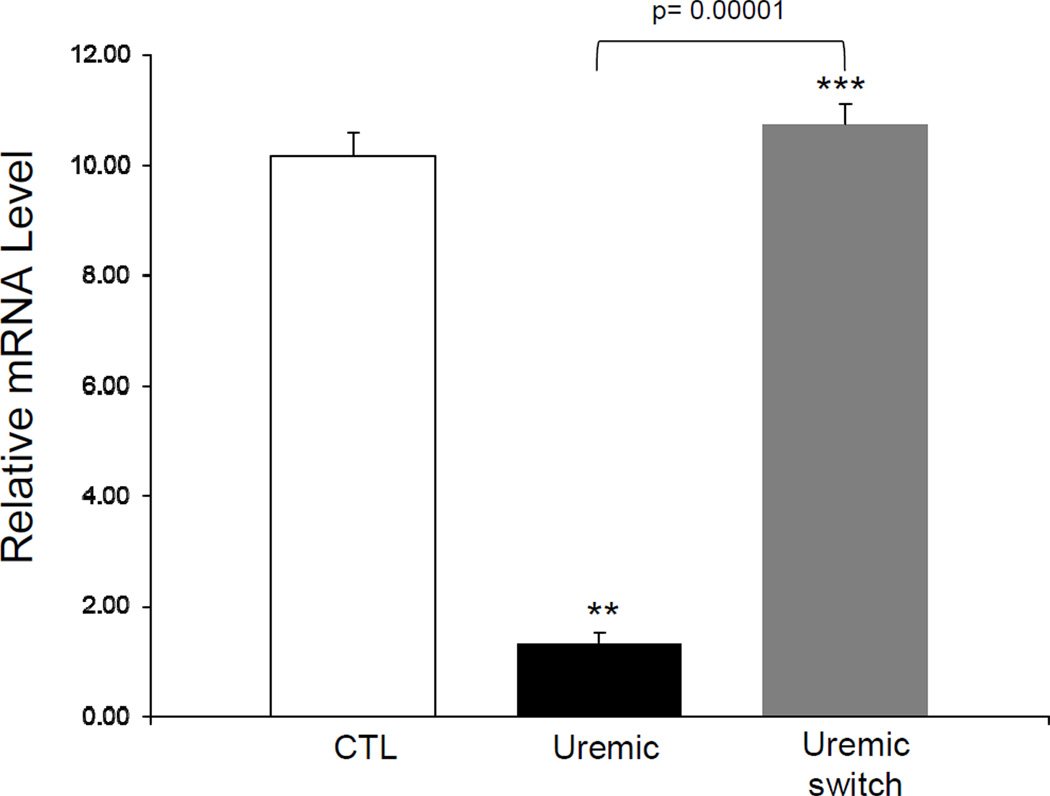
Exposure to uremic plasma resulted in a marked reduction in ApoA-I release in the media by HepG2 cells when compared to cells exposed to the normal plasma (Figure 1A). To assess whether this was mediated via a transcriptional mechanism, cells were incubated in serum-free media for 24 hours and then exposed to media containing different concentrations of uremic and normal plasma. To this end cells were exposed to DMEM containing 10% uremic or control plasma for 48 hours. We found a significant decrease in the ApoA-I mRNA abundance in cells exposed to uremic plasma at 10 % concentration when compared to cells treated with the normal plasma (Figure 1B). In addition, a time course study was conducted by exposing cells to 10% uremic and control plasma for 24, 48 and 72 hours. There was a significant decrease in ApoA-I mRNA expression with exposure to uremic plasma which was most significant at 48 hours (Figure 2). To assess the reversibility of this phenomenon, we exposed cells to 10% uremic plasma for two days after which the media was removed, cells were washed and then exposed to control media for one day. While there was a significant decrease in ApoA-I mRNA concentration in cells exposed to the media containing uremic plasma, this effect was fully reversed when the uremic plasma was replaced with the control plasma (Figure 3).
Figure 1.
A. ApoA-I mRNA expression in HepG2 cells exposed to 10% uremic or control plasma. Data represent means ± SEM of at least 3 independent experiments. * p<0.05, ** p<0.01, ***p<0.001. B. ApoA-I protein concentration in media of HepG2 cells exposed to 10% uremic or control plasma for 48 hours and subsequently washed with PBS and incubated with plasma-free media. Data represent means ± SEM of at least 3 independent experiments. * p<0.05
Figure 2.
ApoA-I mRNA expression in HepG2 cells exposed to 10% uremic or control plasma for 24, 48 and 72 hours. Data represent means ± SEM of at least 3 independent experiments. * p<0.05, ** p<0.01, ***p<0.001
Figure 3.
ApoA-I mRNA expression in HepG2 cells exposed to 10% uremic or control plasma, followed by switching one group of uremic cells from uremic plasma to control. Cells were exposed to uremic media for 48 hours which was then removed and replaced with control media for another 24 hours. Data represent means ± SEM of at least 3 independent experiments. ** p<0.01, ***p<0.001
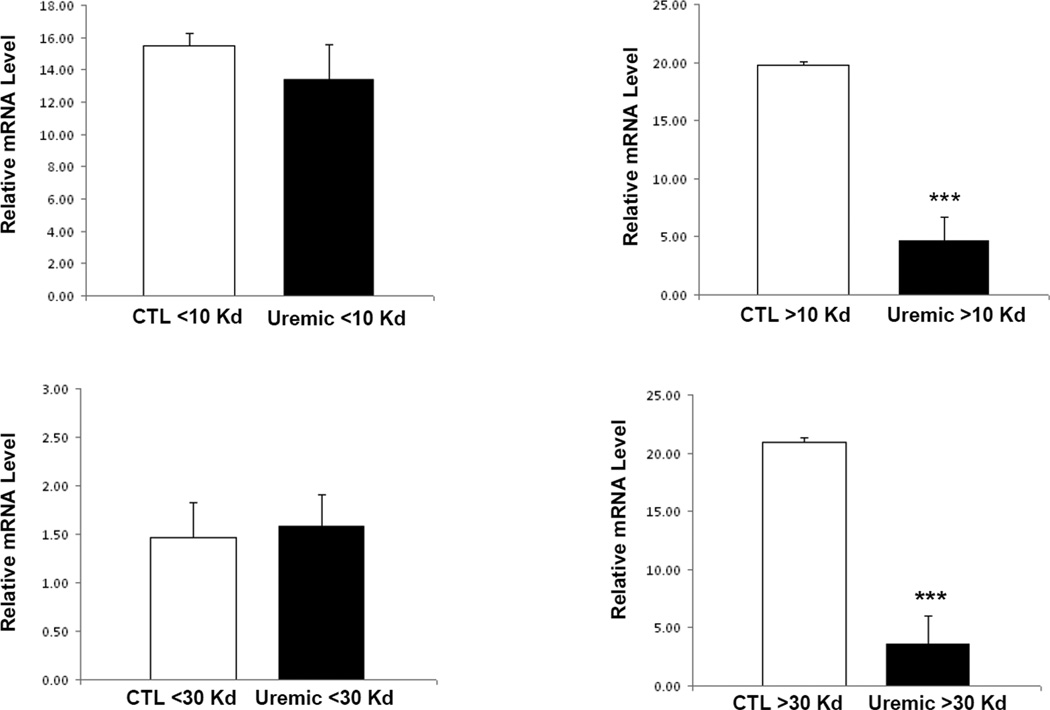
Effect of uremic plasma subfractions on ApoA-I expression
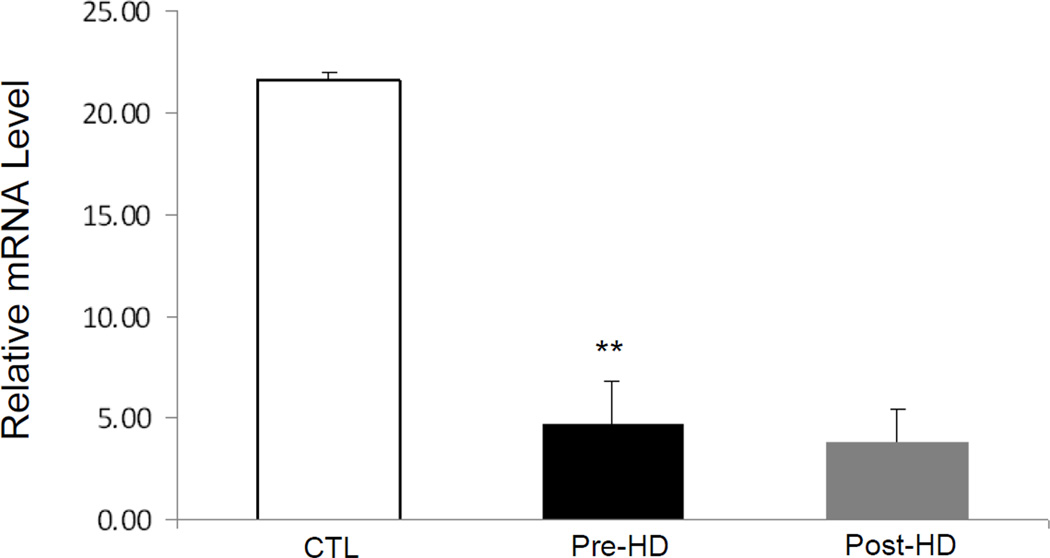
To determine the size of the molecule(s) in the uremic plasma which mediate down-regulation of ApoA-I expression, Amicon filters were used to fractionate uremic and control plasma. The effect of the given plasma sub-fractions was subsequently tested ApoA-I mRNA expression in HepG2 cells. The uremic plasma fractions containing molecules with MW equal or greater than 30 Kd caused a significant decrease in ApoA-I mRNA expression when compared to the corresponding normal plasma fractions. However no significant difference was found in ApoA-I expression in cells exposed to plasma fractions containing molecules with MW less than 30Kd between the uremic and control samples (Figure 4). To determine whether hemodialysis can remove the uremic toxin/s responsible for inhibition of ApoA-I expression, cells were exposed to pooled pre- and post-dialysis plasma samples and the results were compared with those obtained with normal plasma. Compared with the normal plasma both pre-dialysis and post-dialysis plasma lowered ApoA-I mRNA abundance in HepG2 cells to the same degree (Figure 5). These findings suggest that the toxin(s) responsible for inhibition of ApoA-I expression is/are not removed by hemodialysis. The latter finding is consistent with the results of the sub-fractionation studies noted above.
Figure 4.
ApoA-I mRNA expression in HepG2 cells exposed to 10% fractionated uremic or control plasma. Amicon filters with nominal molecular weight limit 10Kd and 30Kd were used to fractionate the plasma. Cells were exposed to media for 48 hours. Data represent means ± SEM of at least 3 independent experiments. ***p<0.001
Figure 5.
ApoA-I mRNA expression in HepG2 cells exposed to 10% control, predialysis or postdialysis plasma. Cells were exposed to media for 48 hours. Data represent means ± SEM of at least 3 independent experiments. CTL vs. pre-HD plasma**p<0.01, pre-HD vs. post-HD no significant difference detected.
Effect of uremic plasma on ApoA-I promoter activity
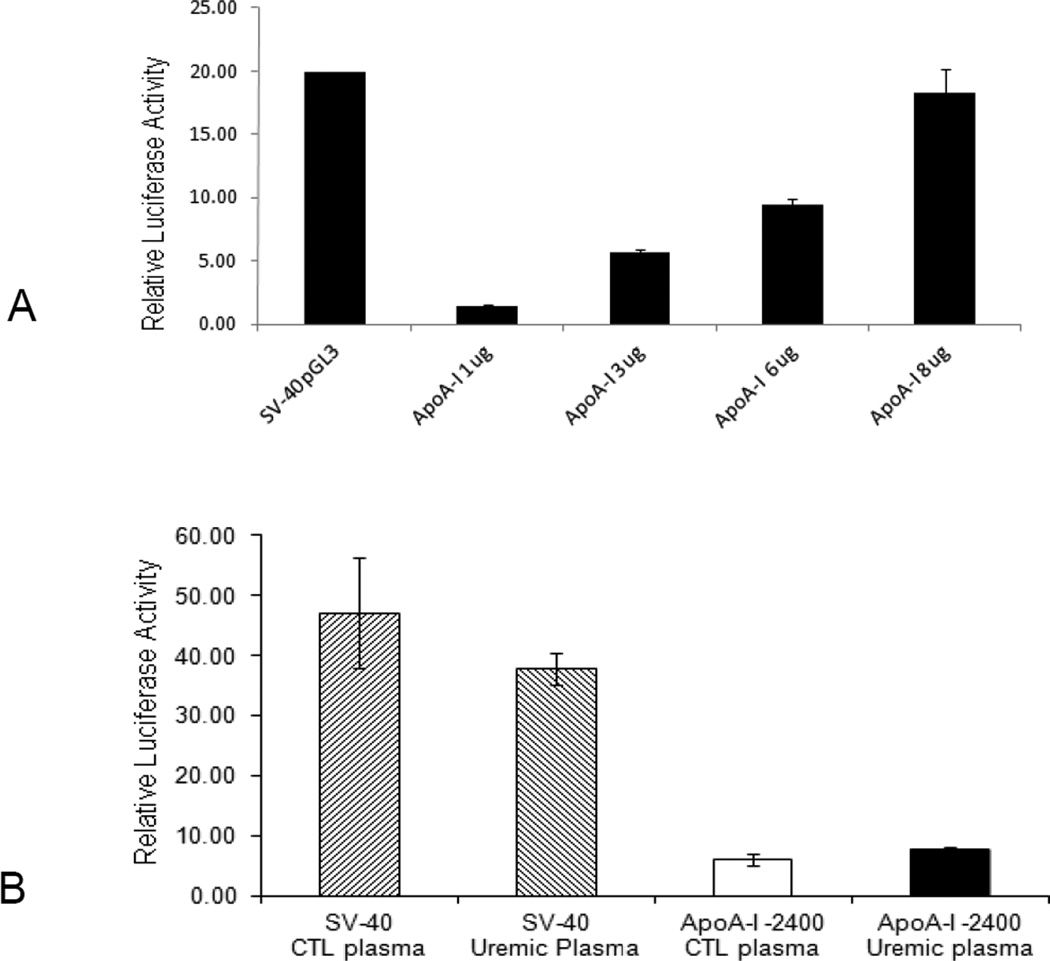
In order to determine whether uremia-induced down-regulation of ApoA-I expression is due to the reduction of the promoter activity, the −2096 to +293 segment of this gene was cloned into a pGL3-luciferase reporter plasmid (39). Increasing concentrations of the plasmid were transfected into HepG2 cells and this resulted in increasing luciferase activity which confirmed the functionality of this promoter region (Figure 6A). HepG2 cells were then transfected with the plasmid containing the ApoA-I promoter and subsequently exposed to media containing 10% uremic or control plasma. The results showed that uremic plasma had no effect on ApoA-I promoter activity (Figure 6B). Based on these findings down-regulation of ApoA-I expression does not appear to be mediated at the promoter level.
Figure 6.
A. Transfected ApoA-I promoter activity in HepG2 cells. The cell line was transfected with the promoter-luciferase construct for ApoA-I, with the results of a luciferase assay for each transfection shown. Firefly luciferase activity was normalized relative to the activity of simultaneously expressed Renilla luciferase. The results are expressed relative to the pGL3-basic vector. B. Effect of uremic and control plasma on ApoA-I promoter activity. The Hep G2 cells were transfected with the promoter-luciferase construct for ApoA-I, subsequently the cells were exposed to uremic or control plasma. Cells transfected with SV-40 pGL3 were used as control to rule out a general effect. The results are expressed relative to the pGL3-basic vector set at 1 and represent means ± SEM of at least 3 independent experiments.
Effect of uremic plasma on ApoA-I RNA stability
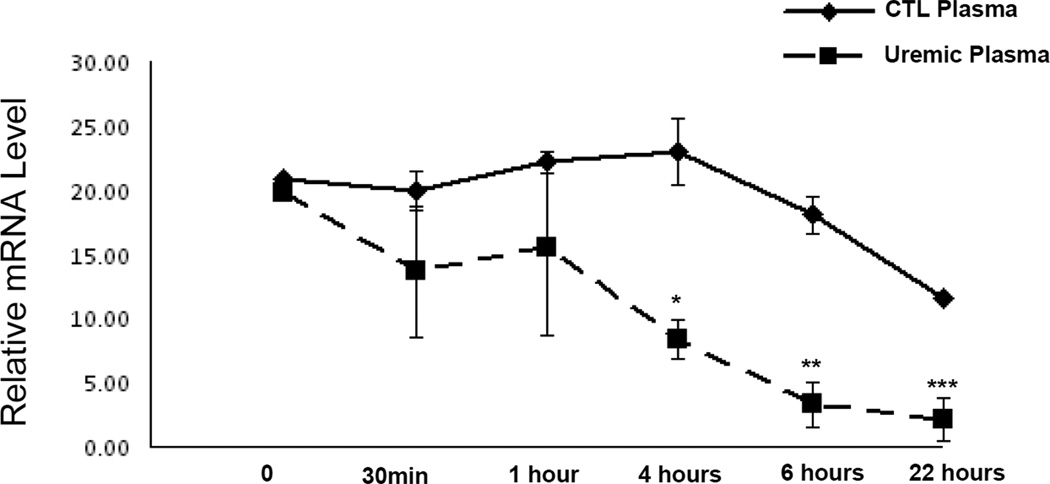
To determine whether the observed reduction of ApoA-I mRNA abundance in HepG2 cells following exposure to uremic plasma was, in part, due to altered RNA stability, cells were serum-starved and subsequently incubated with 1 µM actinomycin D and 10% uremic or control plasma. We found an accelerated time-dependent reduction in ApoA-I mRNA level in the cells exposed to uremic plasma when compared with that seen in cells exposed to normal control plasma (Figure 7). These findings illustrate that uremia-induced down-regulation of ApoA-I is at least partly due to its reduced RNA stability.
Figure 7.
RNA stability of ApoA-I mRNA in HepG2 cells treated with uremic or control plasma. HepG2 cells maintained in media containing 10% control or uremic media were analyzed for RNA stability by the addition of actinomycin D (1 µM, a potent transcription inhibitor) to the growth medium with specific time point isolation of total RNA followed by reverse transcription with oligo-dT and real-time quantitative PCR analysis with specific primers to ApoA-I or β-actin. Solid line represents control conditions, whereas dashed line shows uremic conditions.
Discussion
Patients with CKD and end stage renal disease commonly exhibit ApoA-I deficiency (34–35). ApoA1 deficiency in this population has been variably shown to be due to its increased catabolism (42–43), and decreased production (44). In addition, exposure to serum from hemodialysis patients has been shown decrease ApoA-I expression, synthesis and secretion in cultured hepatoma cell line (HepG2) (45–46). The reduction in plasma ApoA1 concentration in rats with CKD induced by 5/6 nephrectomy is accompanied by marked reduction of its hepatic mRNA abundance (47). Taken together the available in vivo and in vitro studies point to the inhibitory effect of uremic milieu on hepatic ApoA-I gene expression resulting in decreased production and secretion of this molecule. However, the nature and the mechanism(s) of action of the uremic products which cause down regulation of ApoA-I gene expression have not been deciphered. In the present study, we confirmed the previously published findings that exposure to uremic plasma reduces ApoA-I production and expression in a human hepatic cell line (HepG2). We further showed that the molecules responsible for this effect are larger than 30Kd and cannot be removed by hemodialysis. In addition, the study revealed that the inhibitory effect of uremic milieu on ApoA-I expression is reversible and ApoA-I expression returns to control levels once uremic plasma is replaced with the normal plasma.
Earlier studies have shown that inflammation suppresses ApoA-I expression at the transcriptional level (48). These findings may partially explain the association of inflammatory states with the reduction of plasma ApoA-I level as observed in patients with rheumatoid arthritis (49) and acute infections (50). Given the fact that CKD is a pro-oxidative/pro-inflammatory state as documented extensively by numerous investigators (51–55), we hypothesized that down-regulation of ApoA-I in CKD is caused by an inhibitory effect at the promoter level. We tested this hypothesis using an ApoA-I promoter reporter plasmid construct. Contrary to our expectation, uremic plasma did not alter ApoA-I promoter activity in our cell culture model. This observation suggests that, down-regulation of ApoA-I by uremic plasma does not seem to be mediated at the promoter level.
An alternative mechanism for the ApoA-I mRNA depletion by exposure to uremic milieu may be reduction of RNA stability. The importance of this concept was illustrated by Mooradian et al, who demonstrated that the glucosamine-induced reduction of ApoA-I mRNA level in HepG2 cells was secondary to decreased mRNA stability (56). Similarly we found that uremic plasma had a profoundly negative effect on ApoA-I RNA stability. Based on these findings down-regulation of ApoA-I expression by uremic milieu is at least partly mediated via decreased RNA stability.
While these findings further advance our understanding of the mechanisms responsible for CKD induced ApoA-I deficiency, several limitations will need to be mentioned. The current study was performed in an in-vitro model of simulated uremia and further in-vivo studies will be necessary to strengthen these findings. Furthermore, a general destabilizing effect of the uremic milieu on total mRNA cannot be excluded although the expression of ApoA-I mRNA in the present and previous studies were adjusted against the expression of a house-keeping gene. It should be noted that plasma glucose level in the pooled sample from CKD patients was about 30% higher than that in the pooled plasma from the healthy controls. This is due to the inclusion of patients with diabetes mellitus which is the most common cause of CKD. In addition the potential mixture of samples from fed/fasting states could have contributed to the higher glucose level observed in the pooled CKD plasma. By modifying the plasma levels of free fatty acids and/or insulin which can influence lipid processing, signaling and RNA stability these factors could have contributed to the negative effect of pooled plasma from CKD patients on ApoA-1 RNA stability.
In conclusion, exposure to the uremic milieu causes down-regulation of ApoA-I mRNA expression and ApoA-I protein production in cultured hepatocytes. This effect is due to post-transcriptional dysregulation (decreased RNA stability) of ApoA-I mRNA rather than depressed promoter activity. Furthermore, experiments employing plasma sub-fractions and pre- and post dialysis plasma revealed that the product(s) or toxins responsible for this effect are not removed by hemodialysis.
Acknowledgments
This work was supported in part by a grant from the NIH DK082130 (H.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal's policy on conflicts of interest and have none to declare.
References
- 1.K/DOQI clinical practice for chronickidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S7–S266. [PubMed] [Google Scholar]
- 2.Excerpts from the United States Renal Data system 2005 Annual Data Report. Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2006;47:S1–S286. [Google Scholar]
- 3.Excerpts from the United States Renal Data system 2004 Annual Data Report. Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2005;45:S1–S280. doi: 10.1053/j.ajkd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri ND. Dyslipidemia of chronic renal failure: The nature, mechanisms and potential consequences. Am J Physiol, Renal Physiol. 2006;290:262–272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri ND, Moradi H. Mechanism of dyslipidemia of chronic renal failure. Hemodialysis Int. 2006;10:1–7. doi: 10.1111/j.1542-4758.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 7.Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nature, Reviews Nephrology. 2010;6:287–296. doi: 10.1038/nrneph.2010.36. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76:437–444. doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi H, Yuan J, Ni Z, Norris K, Vaziri ND. Reverse cholesterol transport pathway in experimental chronic kidney disease. Am J Nephrol. 2009;30:147–154. doi: 10.1159/000210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moradi H, Pahl MV, Elahimehr R, Vaziri ND. Impaired Antioxidant Activity of HDL in Chronic Kidney Disease. Translational Research. 2009;153:77–85. doi: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND, Liang K, Parks JS. Downregulation of hepatic lecithin:cholesterol acyltransferase (LCAT) gene expression in chronic renal failure. Kidney Int. 2001;59:2192–2196. doi: 10.1046/j.1523-1755.2001.00734.x. [DOI] [PubMed] [Google Scholar]
- 12.Liang K, Vaziri ND. Upregulation of Acyl-CoA: Cholesterol acyltransferase (ACAT) in chronic renal failure. Am J Physiol: Endocrine and Metab. 2002;283:E676–E681. doi: 10.1152/ajpendo.00364.2001. [DOI] [PubMed] [Google Scholar]
- 13.Pahl MV, Ni Z, Sepassi L, Vaziri ND. Plasma Phosphlipid Transfer Protein, Cholesteryl Ester Transfer Protein and Lecithin: Cholesterol Aacyltransferase in End-Stage Renal Disease. Nephrol Dial Transplant. 2009;24:2541–2546. doi: 10.1093/ndt/gfp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Moradi H, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol, Renal Physiol. 2009;296:F1297–F1306. doi: 10.1152/ajprenal.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moradi H, Ganji S, Kamanna Vj, Pahl MV, Vaziri ND. Increased Monocyte Adhesion-Promoting Capacity of Plasma in End-Stage Renal Disease - Response to Antioxidant Therapy. Clinical Nephrology. 2010;74:273–281. doi: 10.5414/cnp74273. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri ND. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr. 2010;20:S35–S43. doi: 10.1053/j.jrn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purification. 2011;31:189–196. doi: 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- 18.Epstein M, Vaziri ND. Role of statins in the management of dyslipidemia of chronic kidney disease; Current concepts and emerging treatment paradigms. Nature Reviews, Nephrol. 2012;8:214–223. doi: 10.1038/nrneph.2012.33. [DOI] [PubMed] [Google Scholar]
- 19.Kaysen GA. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr. 2009;19:73–77. doi: 10.1053/j.jrn.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Kaysen GA. New insights into lipid metabolism in chronic kidney disease. J Ren Nutr. 2011;21:120–123. doi: 10.1053/j.jrn.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 22.Casteli WP. Cholesterol and lipids in the risk of coronary artery disease- the Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 23.Goldbourt U, Yaari JS, Medalie JH. Isolated low HDL cholesterol as a risk for coronary artery disease mortality: a 21-year follow-up of 8,000 men. Arterioscler Thromb Vasc Biol. 1997;17:107–113. doi: 10.1161/01.atv.17.1.107. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Sacks FM, Salvin S, Willet WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 25.Shai I, Rimm EB, Hankinson SE, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–2830. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 26.Walldius G, Junger I. Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr Opin Cardiol. 2007;22:359–367. doi: 10.1097/HCO.0b013e3281bd8849. [DOI] [PubMed] [Google Scholar]
- 27.Rader D. Molecular regulation of HDL metabolism and function: implications for novel therapies. J clin invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellani LW, Lusis AJ. ApoA-II versus ApoA-I: two for one is not always a good deal. Arterioscler Thromb Vasc Biol. 2001;21:1870–1872. [PubMed] [Google Scholar]
- 29.Williamson R, Lee D, Hagaman J, Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7134–7138. doi: 10.1073/pnas.89.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer EJ, Heaton WH, Wetzel MG, Brewer HB. Plasma apolipoprotein A-1 absence associated with a marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis. 1982;2:16–26. doi: 10.1161/01.atv.2.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Rubin E, Krauss R, Spangler E, Verstuyft J, Clift S. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein A-I. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 32.Plump A, Scott C, Breslow J. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Puré E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 34.Attman PO, Alaupovic P, Gustafson A. Serum apolipoprotein profile of patients with chronic renal failure. Kidney Int. 198;32:368–375. doi: 10.1038/ki.1987.219. [DOI] [PubMed] [Google Scholar]
- 35.Attman PO, Alaupovic P. Lipid and apolipoprotein profiles of uremic dyslipoproteinemia--relation to renal function and dialysis. Nephron. 1991;57:401–410. doi: 10.1159/000186303. [DOI] [PubMed] [Google Scholar]
- 36.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 37.Parolini C, Chiesa G, Zhu Y, et al. Targeted replacement of mouse apolipoprotein A-I with human ApoA-I or the mutant ApoA-IMilano. Evidence of APOA-IM impaired hepatic secretion. J Biol Chem. 2003;278:4740–4746. doi: 10.1074/jbc.M207335200. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD, et al. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Yuen YM, Leung LK. Genistein and daidzein induced apoA-1 transactivation in hepG2 cells expressing oestrogen receptor-α. Br J Nutr. 2008;99:1007–1012. doi: 10.1017/S0007114507853426. [DOI] [PubMed] [Google Scholar]
- 40.Gibson UEM, Heid CA, Williams PM. A novel method for real-time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 41.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol. 2007;292:G275–G281. doi: 10.1152/ajpgi.00327.2006. [DOI] [PubMed] [Google Scholar]
- 42.Batista MC, Welty FK, Diffenderfer MR, et al. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism. 2004;53:1255–1261. doi: 10.1016/j.metabol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Okubo K, Ikewaki K, Sakai S, Tada N, Kawaguchi Y, Mochizuki S. Abnormal HDL apolipoprotein A-I and A-II kinetics in hemodialysis patients: a stable isotope study. J Am Soc Nephrol. 2004;15:1008–1015. doi: 10.1097/01.asn.0000117286.85443.7d. [DOI] [PubMed] [Google Scholar]
- 44.Fuh MM, Lee CM, Jeng CY, et al. Effect of chronic renal failure on high-density lipoprotein kinetics. Kidney Int. 1990;37:1295–1300. doi: 10.1038/ki.1990.114. [DOI] [PubMed] [Google Scholar]
- 45.Kamanna VS, Kashyap ML, Pai R, et al. Uremic serum subfraction inhibits apolipoprotein A-I production by a human hepatoma cell line. J Am Soc Nephrol. 1994;5:193–200. doi: 10.1681/ASN.V52193. [DOI] [PubMed] [Google Scholar]
- 46.Shah GM, Lin ZL, Kamanna VS, et al. Effect of serum subfractions from peritoneal dialysis patients on Hep-G2 cell apolipoprotein A-I and B metabolism. Kidney Int. 1996;50:2079–2087. doi: 10.1038/ki.1996.532. [DOI] [PubMed] [Google Scholar]
- 47.Vaziri ND, Deng G, Liang K. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol Dial Transplant. 1999;14:1462–1466. doi: 10.1093/ndt/14.6.1462. [DOI] [PubMed] [Google Scholar]
- 48.Haas MJ, Horani M, Mreyoud A, Plummer B, Wong NC, Mooradian AD. Suppression of apolipoprotein AI gene expression in HepG2 cells by TNF alpha and IL-1beta. Biochim Biophys Acta. 2003;1623:120–128. doi: 10.1016/j.bbagen.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Park YB, Lee SK, Lee WK, et al. Lipid profiles in untreated patients with rheumatoid arthritis. J. Rheumatol. 1999;26:1701–1704. [PubMed] [Google Scholar]
- 50.Gidding SS, Stone NJ, Bookstein LC, Laskarzewski PM, Stein EA. Month-to-month variability of lipids, lipoproteins, and apolipoproteins and the impact of acute infection in adolescents. J. Pediatr. 1998;133:242–246. doi: 10.1016/s0022-3476(98)70227-6. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri ND. Oxidative stress in chronic renal failure: The nature, mechanism and consequences. Semin Nephrol. 2004;24:469–473. doi: 10.1016/j.semnephrol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Vaziri ND, Ni Z, Wang XQ, Oveisi F, Zhou XJ. Downregulation of nitric oxide synthase in chronic renal insufficiency: Role of excess PTH. Am J Physiol: Renal Physiol. 1998;274:F642–F649. doi: 10.1152/ajprenal.1998.274.4.F642. [DOI] [PubMed] [Google Scholar]
- 53.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 54.McCullough PA. Why is chronic kidney disease the ‘‘spoiler’’ for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725–728. doi: 10.1016/s0735-1097(02)02955-8. [DOI] [PubMed] [Google Scholar]
- 55.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 56.Haas MJ, Wong NC, Mooradian AD. Effect of glucosamine on apolipoprotein AI mRNA stabilization and expression in HepG2 cells. Metabolism. 2004;53:766–771. doi: 10.1016/j.metabol.2003.11.027. [DOI] [PubMed] [Google Scholar]