Abstract
Background
Identifying variables that predict drug use in treatment-seeking drug addicted individuals is a crucial research and therapeutic goal. This study tested the hypothesis that choice to view cocaine images is associated with concurrent and prospective drug use in cocaine addiction.
Methods
To establish choice-concurrent drug use associations, 71 cocaine addicted subjects (43 current users and 28 treatment seekers) provided data on (A) choice to view cocaine images and affectively pleasant, unpleasant, and neutral images [collected under explicit contingencies (when choice was made between two fully visible side-by-side images) and under more probabilistic contingencies (when choice was made between pictures hidden under flipped-over cards)]; and (B) past-month cocaine and other drug use. To establish choice-prospective drug use associations, 20 of these treatment-seeking subjects were followed over the next six months.
Results
Baseline cocaine-related picture choice as measured by both tasks positively correlated with subjects’ concurrent cocaine and other drug use as driven by the actively-using subjects. In a subsequent multiple regression analysis, choice to view cocaine images as compared with affectively pleasant images (under probabilistic contingencies) was the only predictor that continued to be significantly associated with drug use. Importantly, this same baseline cocaine>pleasant probabilistic choice also predicted the number of days drugs were used (cocaine, alcohol, and marijuana) over the next six months.
Conclusions
Simulated cocaine choice – especially when probabilistic and when compared with other positive reinforcers – may provide a valid laboratory marker of current and future drug use in cocaine addiction.
Keywords: cocaine addiction, abstinence, cocaine choice behavior, relapse, treatment outcome, IAPS, reinforcement
1. INTRODUCTION
While many individuals who develop maladaptive patterns of drug use are able to successfully recover (Heyman, 2009), an important minority develops chronic, compulsive patterns of substance use characterized by a high risk for relapse even after long-term treatment and abstinence. Identifying variables that predict relapse in treatment-seeking drug addicted individuals is, therefore, a crucial research and therapeutic goal, as select individuals could be targeted for tailored interventions to reduce risk of relapse. Similarly to animal studies where stress, drug cues, or a priming dose of the drug trigger reinstatement (Sinha et al., 2011), variables used to predict relapse in human cocaine addiction have included response to stress (Sinha et al., 2006; but see Preston and Epstein, 2011), craving (Rohsenow et al., 2007), and recent cocaine use including positive urine screens (Ahmadi et al., 2009; García-Fernández et al., 2011; Poling et al., 2007). Other variables have included neuropsychological functioning encompassing the presence of comorbid psychiatric disorders (Poling et al., 2007), poorer commitment to behavioral change (Aharonovich et al., 2008), lower general cognitive and executive functioning (Aharonovich et al., 2008, 2006, 2003; Verdejo-Garcia et al., 2012) and drug-related attention bias (Carpenter et al., 2012, 2006; Marissen et al., 2006).
Our current goal was to identify whether simulated cocaine choice is an additional predictor of relapse. In particular, we aimed to compare the choice to view drug-associated stimuli with the choice to view other pleasant stimuli. This approach parallels human laboratory studies where the availability of alternative reinforcers such as money curbs cocaine self-administration (Donny et al., 2004; Hart et al., 2000; Stoops et al., 2010), especially when such alternative reinforcers are easier to obtain (Stoops et al., 2012) or more likely to be available (Vosburg et al., 2010). The availability of money in such studies may improve general decision-making (Vadhan et al., 2009) or incentivize abstinence (e.g., through contingency management) (Higgins et al., 2004). Most relevant to our study goals, money may constitute a suitable alternative reinforcer to drugs, which has been argued as an essential component of choice paradigms (i.e., to differentiate addicted individuals from those who self-administer drugs because of a lack of other viable options; Ahmed, 2010). To model simulated drug-related choice in abstaining individuals (in whom direct drug administration is unethical), we recently developed two neuropsychological drug choice tasks: one with explicit task contingencies and one with more probabilistic task contingencies. On these tasks, individuals with cocaine use disorder (CUD) indicated choice for viewing cocaine images vis-à-vis standardized pleasant, unpleasant, and neutral images; higher choice to view cocaine images, particularly versus choice to view pleasant images (cocaine>pleasant), correlated with more frequent cocaine use in the past month (Moeller et al., 2010, 2009). In the present study, leaning heavily on the research reviewed above that collectively highlights the importance of suitable alternative reinforcers in drug choice paradigms, we hypothesized that higher cocaine>pleasant choice would prospectively predict more severe drug use over the next six months. In addition to this central goal of predicting relapse, we tested associations between baseline choice and concurrent drug use in a larger (updated) sample of CUD, while also exploring the influence of individual differences in treatment-seeking status.
2. METHODS
2.1 Subjects
Seventy-one CUD (43 active cocaine users and 28 individuals who were seeking treatment for cocaine dependence at study time) were recruited from newspaper advertisements, word-of-mouth, and treatment facilities located in the New York Tri-State Area (see Table 1 for demographic and drug use information). Of these, 39 CUD have been included in prior reports; 32 were studied here for the first time. Moreover, the current study is entirely novel as prior studies did not include prediction of relapse. For predicting relapse, we followed for an additional six months after baseline 20 of these treatment-seekers, a CUD subgroup representing the severe end of the addiction spectrum (i.e., as these individuals were currently seeking treatment for their maladaptive and intransigent drug use) and therefore representing an appropriate and important sample in whom to predict relapse. All subjects provided written informed consent to participate in accordance with Stony Brook University’s Institutional Review Board. Subjects underwent a comprehensive diagnostic interview (see Supplementary Material for interview components). This interview identified the following cocaine-related diagnoses in the current sample: current cocaine dependence (N=55), cocaine dependence in early partial remission (N=8), cocaine dependence in early full remission (N=5), cocaine dependence in sustained partial remission (N=1), and cocaine dependence in fully sustained remission (N=2) (see Supplementary Material for subjects’ comorbidities).
Table 1.
Baseline demographics and drug use of all study subjects as a function of treatment status and abstinence.
| All Subjects: Between- group test (N=71) |
In Treatment (N=28) |
Not in Treatment (N=43) |
Follow-up Subjects: Between- group Test (N=20) |
Abstinent (N=11) |
Relapsed (N=9) |
|
|---|---|---|---|---|---|---|
| Gender (male/female) | χ2= 3.2 | 21/7 | 39/4 | χ2= 0.6 | 9/2 | 6/3 |
| Ethnicity (African-American/Caucasian/Other) | χ 2= 3.6 | 15/12/1 | 31/12/0 | χ2= 4.2 | 4/6/1 | 7/1/1 |
| History of cigarette smoking (current or past/never) | χ2= 6.1* | 25/3 | 36/7 | χ2= .02 | 10/1 | 8/1 |
| Daily frequency of smoking (for current smokers) | Z = −2.2*a | 4.9 ± 4.0 | 7.9 ± 5.4 | Z = −1.7b | 7.9 ± 4.3 | 3.8 ± 3.1 |
| Education (years) | t = 0.5 | 12.6 ± 2.7 | 12.8 ± 1.6 | t = 0.8 | 13.5 ± 4.0 | 12.4 ± 0.9 |
| Age (years) | t = 1.9 | 41.8 ± 8.4 | 45.1 ± 6.2 | t = −0.9 | 40.7 ± 10.3 | 44.0 ± 4.9 |
| Socio-economic status | t = 2.4* | 29.4 ± 9.9 | 35.2 ± 10.1 | t = 1.0 | 33.7 ± 9.9 | 29.1 ± 11.5 |
| Non-verbal intelligence: Wechsler Abbreviated Scale of Intelligence: Matrix Reasoning scaled score |
t = −1.0 | 10.3 ± 3.3 | 9.4 ± 3.1 | t = −0.6 | 10.0 ± 2.9 | 10.8 ± 3.2 |
| Verbal IQ: Wide Range Achievement Test III: grade equivalent score |
t = 0.2 | 11.0 ± 3.3 | 11.2 ± 2.7 | t = 2.0 | 12.7 ± 0.1 | 9.9 ± 1.4 |
| Self-reported state depression | Z = −2.0* | 6.4 ± 7.8 | 8.9 ± 7.1 | t = −1.6 | 4.3 ± 5.3 | 10.3 ± 11.3 |
| Presence of current comorbidities (yes/no) | χ2= 3.37 | 9/19 | 6/37 | χ2=0.1 | 4/7 | 4/5 |
| Age at onset of cocaine use | Z = −1.8 | 23.6 ± 8.3 | 26.0 ± 7.0 | Z = −0.3 | 24.8 ± 9.4 | 24.9 ± 8.0 |
| Duration of use (years) | Z = −1.0 | 14.5 ± 9.0 | 16.4 ± 7.4 | Z = −1.2 | 12.4 ± 8.8 | 16.9 ± 7.5 |
| Frequency of use (days/week): last 30 days | Z = −4.9*** | 0.7 ± 1.9 | 3.0 ± 2.6 | Z = −0.7 | 0.2 ± 0.5 | 0.1 ± 0.3 |
| Current use in $ per use (min – max, median): last 30 days |
Z = −5.2*** | 0 – 150, 0 | 0 – 600, 50 | Z = −0.1 | 0 – 150, 0 | 0 – 80, 0 |
| Duration of current abstinence (days) (min – max, median) |
Z = −5.8*** | 3 – 927, 97.5 | 0 – 1825, 3 | Z = −3.1** | 23 – 700, 165 | 24 – 48, 30 |
| Number of instances of cocaine, marijuana, or alcohol use to intoxication in the last 30 days |
Z = −5.7*** | 1.3 ± 3.8 | 14.3 ± 11.5 | Z = −1.2 | 1.4 ± 4.2 | 1.4 ± 0.5 |
| Total score on the Cocaine Selective Severity Assessment Scale (measure of withdrawal symptoms) (0–126) |
Z = −2.7** | 12.0 ± 9.9 | 17.0 ± 9.6 | Z = −1.8 | 10.0 ± 8.6 | 18.8 ± 12.9 |
| Severity of Dependence Scale (0–15) | Z = −1.3 | 8.9 ± 3.9 | 7.8 ± 3.5 | Z = −0.3 | 8.3 ± 4.3 | 8.8 ± 4.5 |
| Cocaine Craving Questionnaire (0–45) | Z = −3.6*** | 8.9 ± 8.9 | 20.1 ± 12.6 | Z = −0.6 | 8.6 ± 5.4 | 9.4 ± 12.3 |
| Urine Status (Positive/Negative)c | χ2= 28.4*** | 0/28 | 27/16 | χ2= 0.0 | 0/11 | 0/9 |
Note.
N=48;
N=13;
ascertained with a triage urine panel for abused drugs (Biopsych), objectively confirming use of cocaine within 72 hours of study time;
p<0.05,
p<0.01,
p<0.001.
2.2 Procedures
2.2.1 Drug Choice Tasks (All Subjects)
Completed at baseline, these two previously validated drug choice tasks have been extensively described elsewhere (Moeller et al., in press, 2010, 2009). They use standardized pleasant (e.g., babies), unpleasant (e.g., disfigurement), and neutral (e.g., household objects) images selected from the International Affective Picture System (IAPS; Lang et al., 2008); and matched cocaine images (on size and ratio of human to non-human content) depicting cocaine and individuals preparing, using, or simulating use of cocaine. These choice tasks assess subjects’ objective preference for viewing these images, therefore distinct from tasks of attention bias (Carpenter et al., 2012, 2006; Hester et al., 2006; Marissen et al., 2006). In particular, attention is captured by emotional stimuli of both pleasant and unpleasant valence (Hajcak et al., 2010), whereas choice entails computing the value of the given options, involving other considerations such as commodity, cost, risk, and motivation (Padoa-Schioppa, 2011).
2.2.1.1 Explicit Choice Task
Subjects chose via continued button pressing between two fully-visible side-by-side images from different picture categories. Choice for a desired image enlarged this chosen image to fully cover the screen, which subjects could view for the trial duration of 5000 ms by continued button pressing; 500 ms of non-response, however, returned the side-by-side image display. After each trial, a new trial with new images ensued. Button pressing (i.e., “working”) for images was an important design feature of this task, meant to echo classical animal studies where subjects work for drug infusions as related to the severity of addiction. More proximally, it was modeled after a prior functional magnetic resonance imaging study where human subjects button pressed to keep beautiful faces (compared with less attractive faces) on the screen (Aharon et al., 2001). Data processing for this explicit task occurred as follows: during each trial, we calculated which of the two pictures had the higher number of button presses, and then indicated that picture category as the choice for that trial. For example, if during a trial there were 10 button presses for a cocaine image and 8 button presses for a pleasant image, then the score for the trial was scored as ‘’ for cocaine. We then summed the total number of these ‘choices’ separately for each picture category (pleasant, unpleasant, neutral, cocaine) across the 70 trials that comprised the task (note that for trials where equal number of presses occurred for both available picture types, the trial was scored as 0.5 for both picture categories).
2.2.1.2 Probabilistic Learning Choice Task
On each trial, subjects chose via a single button press to view pictures hidden under flipped-over cards, arranged in four decks. Immediately after choosing from a particular “deck,” an image was revealed that covered the entire screen for 2000 ms of passive viewing. The images were arranged probabilistically: each deck contained 26 (out of a total of 30) pictures from a particular category (e.g., pleasant), allowing pictures from other categories to be interspersed within each deck (two pictures from a secondary category, e.g., cocaine; and one picture from each of the two remaining categories, e.g., unpleasant and neutral). Such incomplete certainty of the choice outcome enabled insight assessment (Moeller et al., in press, 2010; see Supplementary Material for description and analyses of this variable vis-à-vis drug use severity 1 ). After subjects selected from a particular deck eight total times (corresponding to one task run), deck location of the four picture categories shifted (note that the task was designed so that at least one other picture category would be interspersed within the first eight card draws of each deck, such that each run always included presentation of at least two picture types). Data processing for this probabilistic task occurred as follows: during each trial, the chosen picture category was recorded (e.g., selection of a cocaine deck). Then, across the four total task runs, we summed the total number of deck selections, separately for each of the four picture categories (pleasant, unpleasant, neutral, cocaine). Unlike the “working” component of the explicit task, the probabilistic task was meant instead to tap into more standard notions of choice. For example, from a behavioral economic perspective, probabilistic choice could be thought to reflect intensity (preference for cocaine images over the alternative images when there is no cost associated with the choice), whereas explicit choice could be thought to reflect breakpoint (Omax: preference for cocaine images over alternative images when there is a cost of effort expenditure; e.g., MacKillop et al., 2010). Thus, these complementary task approaches can be viewed as strengths that could possibly increase the variance accounted for in prospective drug use.
2.2.2 Relapse Assessment (N=20 Baseline Treatment Seekers)
Relapse and associated drug use were determined using two core strategies. First, after completing the baseline study session, subjects’ abstinence was tracked and verified through monthly follow-up phone calls to the subjects (and to designated collaterals, such as a sibling or treatment counselor). Second, because not all subjects provided complete data for the monthly phone calls (either because subjects could not be reached or because these study procedures were not yet in place for several early subjects; 5/20), we also consulted data that were collected when subjects returned to the laboratory for an in-depth, 6-month follow-up interview. As part of this second visit, subjects completed: (A) an extensive in-house drug history questionnaire, which ascertained whether, when, and how much subjects used a particular drug; (B) the Timeline Follow-Back Calendar (Miller and Del Boca, 1994), which uses a retrospective calendar to prompt subjects to remember drug use (including number of days and amount used) in the past 90 days; (C) the Addiction Severity Index (McLellan et al., 1992), completed for a second time at this follow-up session; and (D) a triage urine panel for drugs of abuse. All measures were pooled to provide the following data about 6-month drug use after baseline: (i) whether the subject relapsed (yes/no) and (ii) the number of days the subject used cocaine (and other drugs/alcohol). Subjects were informed about the importance of reliable self-reporting, and they had no incentive for denial or biased reporting since neither inclusion into the study nor compensation depended on abstinence.
2.3 Statistical Analyses
Prior to our main analyses, we created the following choice difference scores, for both the explicit and probabilistic tasks: cocaine minus pleasant (cocaine>pleasant); cocaine minus neutral (cocaine>neutral); cocaine minus unpleasant (cocaine>unpleasant); and cocaine minus the average of pleasant, unpleasant, and neutral (cocaine>average). Although we had a priori hypotheses about cocaine>pleasant choice (Moeller et al., in press, 2010, 2009), we also inspected the other difference scores. We then used these newly created choice variables in correlational analyses, testing their associations with concurrent and prospective drug use.
For concurrent drug use, variables included the average number of days per week of cocaine use (from our in-house drug history questionnaire) and the number of days of any drug use (encompassing cocaine, marijuana, or alcohol) over the last month (from the Addiction Severity Index). We first performed these correlations across all CUD, and then separately for treatment-seekers and non-treatment-seekers; in addition, exploratory correlations were conducted separately as a function of cocaine urine status (the latter performed in the non-treatment-seekers only, as no treatment-seekers tested positive for cocaine in urine; see Supplementary Material for these exploratory analyses 2 ). Choice task variables that were significantly associated with both drug use measures, indicating convergent validity of those particular choice variables, were then entered as simultaneous predictors in two separate multiple regression analyses (with the two drug use variables serving as the dependent variables) to determine which choice variable(s) were the most robust correlates of concurrent drug use. These multiple regression analyses also guided our decision of which variables to include in the critical longitudinal analyses; the variables that reached significance in these multiple regressions were subsequently used to predict prospective drug use.
For prospective drug use, variables included the total number of days of cocaine use, and the total number of days of any drug use (again encompassing cocaine, marijuana, or alcohol) over the next six months. These variables were ascertained mainly using the Timeline Follow-Back Calendar, but were cross-checked with monthly phone call data, the drug history questionnaire, and the Addiction Severity Index. Because of the small sample size (N=20), these correlations with prospective drug use were only performed across all treatment-seeking subjects. Partial correlations were also conducted to ensure that our results were not attributable to other common predictors of relapse (that were available for inspection in this study), or to demographic variables or comorbidities that differed between treatment-seekers and non-treatment seekers or between relapsers and abstainers (Table 1).
Criteria for significance were as follows. For concurrent associations using the full sample, significance was set at p<0.01 to minimize Type I error. Other analyses, including the multiple regression analyses in the full sample and the prospective associations in the reduced sample, were considered significant at the more common p<0.05 threshold, as the variables included in these latter analyses first needed to be significant at p<0.01 in the full-sample correlation analyses, still providing protection against Type I error.
3. RESULTS
3.1 Choice Associations with Concurrent Drug Use (Full Sample)
Table 2 presents correlations between the choice variables and concurrent drug use. Across all subjects, positive correlations emerged between cocaine-related choice on both tasks with days per week of cocaine use and drug use (encompassing cocaine, marijuana, and alcohol) in the past month, with 11/16 correlations reaching significance. Likely because 23/28 treatment-seekers reported no drug use in the past month, these correlations were driven by non-treatment-seeking subjects (Table 2). This interpretation is also quantitatively supported by direct comparisons between the magnitudes of these groups’ correlations (using standard formulas for contrasting r values using Fisher’s r to z transformation), where treatment-seekers and non-treatment-seekers differed on all correlations except one (Table 2). Demographic variables that differed between treatment-seekers and non-treatment-seekers (i.e., cigarette smoking history, socioeconomic status, self-reported state depression; Table 1) did not explain these associations, as tested with partial correlations controlling for these variables (Supplementary Material provides additional description3).
Table 2.
Correlations between choice variables and concurrent drug use in the previous month among all subjects and split by treatment-seeking status.
| Correlation Difference |
All Subjects |
Non-Treatment Seeking Subjects |
Treatment-Seeking Subjects |
|||||
|---|---|---|---|---|---|---|---|---|
| Choice Variable | za | zb | Current Cocaine Use: Days Per Week |
Total Drugc Use Days |
Current Cocaine Use: Days Per Week |
Total Drugc Use Days |
Current Cocaine Use: Days Per Week |
Total Drugc Use Days |
| Cocaine>Pleasant Explicit |
1.9 | 2.2* | .27 | .31* | .40* | .45* | −.06 | −.08 |
| Cocaine>Neutral Explicit |
2.0* | 2.5* | .34* | .38* | .47* | .53** | .00 | −.07 |
| Cocaine>Unpleasant Explicit |
2.2* | 2.7* | .39* | .44** | .47* | .51* | −.07 | −.14 |
| Cocaine>Average Explicit |
2.2* | 2.7* | .36* | .41* | .48* | .53** | −.04 | −.10 |
| Cocaine>Pleasant Probabilistic |
3.3* | 3.4* | .32* | .38* | .53** | .56** | −.24 | −.22 |
| Cocaine>Neutral Probabilistic |
3.1* | 3.4* | .17 | .22 | .42* | .45* | −.33 | −.36 |
| Cocaine>Unpleasant Probabilistic |
2.9* | 3.4* | .28 | .39* | .38 | .41* | −.33 | −.40 |
| Cocaine>Average Probabilistic |
3.4* | 3.6* | .28 | .35* | .48* | .51** | −.33 | −.35 |
Note.
Correlation difference between treatment-seekers and non-treatment-seekers on ‘Current ocaine Use: Days Per Week’;
correlation difference between treatment-seekers and nontreatment-seekers on ‘Total Drug Use Days: Last 30 Days’;
includes cocaine, marijuana, and alcohol;
p<0.01 (or p<0.05 for z values indicating correlation differences between the groups),
p<0.001.
When we simultaneously entered into multiple regressions the four variables that significantly correlated with both concurrent drug use variables (cocaine>pleasant probabilistic, cocaine>neutral explicit, cocaine>unpleasant explicit, and cocaine>average explicit choice), only cocaine>pleasant probabilistic choice was significantly associated with days of drug use (cocaine, alcohol, marijuana) in the last 30 days (β=0.24, p<0.05; for all other predictors and for prediction of cocaine use in the last 30 days, p>0.06). These findings collectively highlight the potential importance of cocaine>pleasant probabilistic choice (see Figures 1A-B for scatterplots of this variable’s association with the two drug use variables); we therefore used this baseline variable to predict drug use over the next six months.
Figure 1.
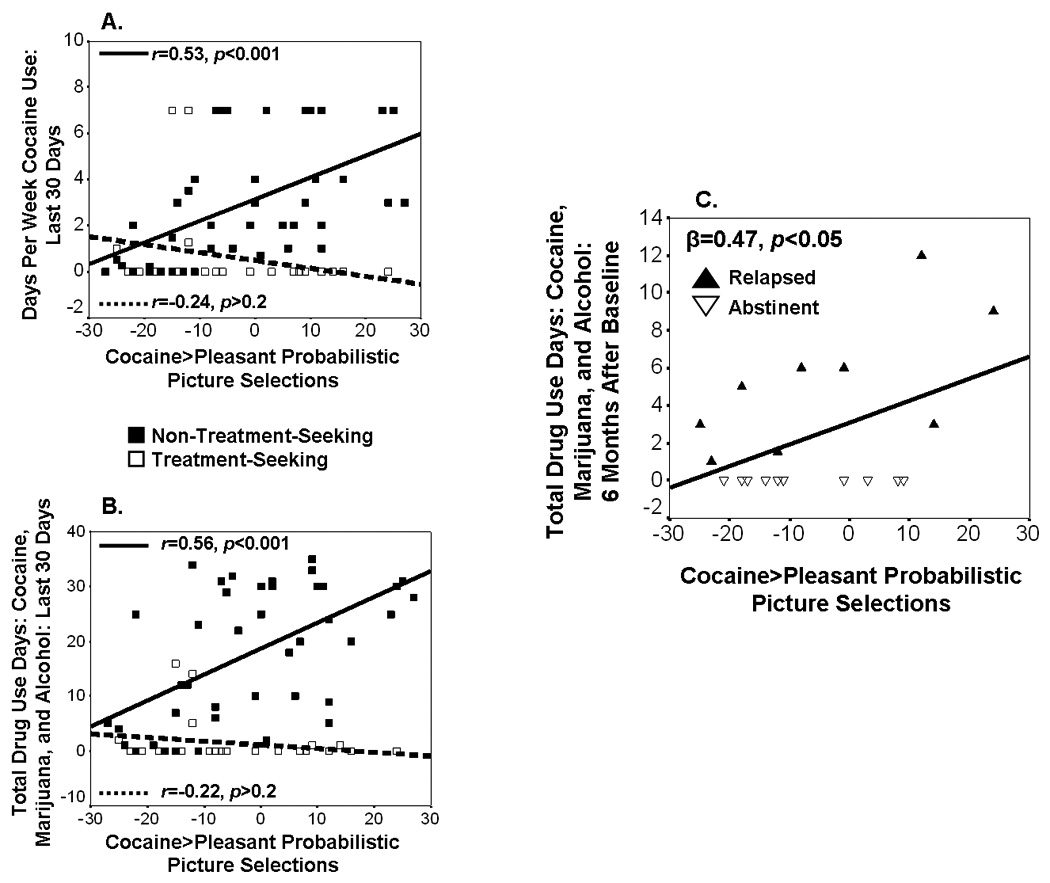
Concurrent and prospective associations between cocaine>pleasant probabilistic selections and drug use. Scatterplots display this choice variable’s associations with (A) concurrent cocaine use in the last 30 days and (B) concurrent drug use (cocaine, alcohol, and marijuana) in the last 30 days, both separately for treatment-seekers and non-treatment-seekers; and (C) prospective drug use (cocaine, alcohol, and marijuana) over the next 6 months in 20 treatment-seekers, separately for relapsers and abstainers.
3.2 Choice Prediction of Prospective Drug Use (N=20)
Cocaine>pleasant probabilistic choice predicted more drug use days over the next six months in the 20 initially treatment-seeking subjects (Figure 1C). This association, although slightly attenuated likely because of reduced power, was still detected when controlling for length of abstinence at baseline (β=0.41, p=0.05), which differed between relapsers and abstainers in the expected direction (relapsers<abstinent; Table 1). Moreover, cocaine>pleasant selections predicted drug use days above and beyond other common predictors of relapse including the presence of current comorbidity (β=0.46, p<0.05), IQ (matrix reasoning: β=0.47, p<0.05), cocaine craving (β=0.44, p=0.05), baseline frequency of cocaine use (past month) (β=0.46, p<0.05), and baseline drug use days (cocaine, alcohol, and marijuana, past month) (β=0.46, p<0.05).
4. DISCUSSION
The current study found that simulated baseline choice to view images of cocaine, when compared with choice to view affectively pleasant pictures and when under probabilistic contingencies, prospectively predicts drug use over the next six months in initially treatment-seeking CUD. This association was not attributable to other factors that commonly predict relapse (e.g., presence of current comorbidity, IQ, cocaine craving, baseline frequency of cocaine use, or baseline drug use days). Because probabilistic cocaine choice was most robust (compared with explicit cocaine choice), from a behavioral economic perspective current data suggest a role of cocaine choice intensity (compared with cocaine choice breakpoint) in predicting such prospective drug use. This finding that drug-related choice behavior predicted future drug use is consistent with a prior study showing that baseline nicotine choice (self-administration of placebo versus nicotine spray) predicted greater withdrawal and faster relapse over the next year (Perkins et al., 2002); Moreover, this finding that was observed using the cocaine>pleasant contrast in particular supports the idea that approach motivation for positive non-drug reinforcers may protect against drug use, consistent with studies where CUD self-administered less cocaine when alternative rewards (e.g., money) were available (Donny et al., 2004; Hart et al., 2000; Stoops et al., 2012, 2010; Vosburg et al., 2010) and where CUD resumed drug responding when alternative reinforcers (e.g., food) were taken away (Quick et al., 2011). Current results using the cocaine>pleasant contrast also support theory suggesting that the availability of other reinforcers is an important, if not critical component of studying drug-seeking behavior in addiction (Ahmed, 2010). For example, one relevant study showed that the choice of tobacco over another attractive reinforcer (chocolate) was positively associated with individual differences in nicotine dependence severity (Hogarth and Chase, 2011). Accordingly, these cocaine>pleasant results could inform treatment approaches that employ contingency management (Higgins et al., 2004), insofar as inappropriate responding to positive reinforcers, here assessed as pleasant picture choice, may portend worse clinical outcomes (Heinz et al., 2007; Lubman et al., 2009). Future studies could aim to uncover the neural mechanisms of how cocaine choice translates into actual cocaine seeking (and associated clinical outcome). This process could involve dysregulated striatal dopamine signaling (Martinez et al., 2011) and/or midbrain responsiveness to salient stimuli during cognitive control (Moeller et al., 2012). Supporting this view, blunted striatal dopaminergic release to a stimulant challenge and lower striatal dopamine D1 receptor availability in CUD have been associated with choosing cocaine over money (Martinez et al., 2007, 2009). Other potential mechanisms could include brain regions/networks underlying salience or executive control (Seeley et al., 2007), habit formation (Ashby et al., 2010; Graybiel, 2008), or decision-making (Paulus et al., 2005).
In addition to this central finding of predicting prospective drug use, the current study extended our previous results (Moeller et al., 2010, 2009), revealing that cocaine-related choice was correlated with concurrent drug use in a larger sample of CUD. These results contribute to long-standing efforts that have investigated preferential choice of rewarding stimuli in health [e.g., food, attractive faces (Armel et al., 2008; Krajbich et al., 2010; Shimojo et al., 2003)], here extending such efforts into reinforcing drug stimuli in addiction. This larger sample also enabled us for the first time to inspect differential associations between choice and drug use as a function of individual differences in treatment-seeking status (and further modulation of this variable by recency of use, a variable previously shown to impact neuropsychological functioning (Woicik et al., 2009), electrocortical cue-reactivity (Dunning et al., 2011), and response to money (Parvaz et al., 2012) in CUD; see Supplementary Material4). In particular, although concurrent choice-drug use associations were driven by the non-treatment-seekers (active users), supplementary, exploratory analyses as a function of cocaine urine status revealed that the higher the cocaine-related choice, the higher was the total number of drug use days (during the 30 days preceding testing) especially in the non-treatment-seekers with at least three days of cocaine abstinence (negative urine). These results attest to the potential utility of cocaine-related choice in a recently abstinent population, bolstering our main longitudinal analyses.
A limitation of this study is missing data related to monthly subject phone calls, which could be confounded with drug use/relapse. Because we therefore relied on retrospective data that were collected during a second laboratory session six months after baseline, some instances of drug use, or amounts of drug use, may have been forgotten or inaccurately recalled. Future studies could follow inpatients from the beginning of treatment, enabling more reliable tracking of subject drug use; with additional subjects, such studies could also employ multiple regression approaches similar to that conducted for the full sample that could account for other common predictors of relapse simultaneously (note that the current sample size of the follow-up subjects was insufficient for such an analysis). A second limitation is that we are unable to conclusively determine whether cocaine choice is a proximal or distal predictor of drug use. Although we statistically accounted for several common predictors of relapse (increasing confidence in choice as a proximal predictor), other potentially mediating or moderating factors such as motivation (e.g., for treatment) remain to be accounted for in future studies. A proximal influence of probabilistic cocaine choice on drug use could also be tested through laboratory human self-administration studies, during which extraneous factors could be controlled. A third limitation is that we cannot determine the precise mechanisms governing choice on these tasks, and how such mechanisms relate to relapse. Learning theory posits at least three different possible processes (Hogarth, 2012; Hogarth and Chase, 2011; Tricomi et al., 2009): (A) explicit knowledge of the response-outcome contingency, such that cocaine choice is mediated by subjects’ beliefs regarding the value of cocaine versus pleasant pictures; (B) stimulus-response/reinforcement (habit) learning, such that the strength of such stimulus-response associations is determined by the reinforcement value of cocaine versus pleasant pictures (and with little forethought for the consequences, possibly tapping into more implicit/automatic associations); and (C) ideomotor learning, such that the outcome (picture) elicits the response through a backward outcome-response association. Because the explicit task but not probabilistic task may have tapped into such ideomotor learning but was not the most robust correlate of drug use in this study, our data do not appear to favor the interpretation that ideomotor learning contributes to the prediction of relapse. This perspective also agrees with previous studies where Pavlovian instrumental transfer of control over drug choice, which relies on the ideomotor process, was not a marker for dependence (Hogarth, 2012; Hogarth and Chase, 2011, 2012). Instead, because poor explicit knowledge (impaired insight) of choice on the probabilistic task, possibly reflecting a contribution of implicit processes, was also associated with increased severity of cocaine use (see Supplementary Material 5 ), current results suggest a role of stimulus-response-guided choice behavior in predicting prospective drug use. Nevertheless, future task refinements are needed to disentangle these competing perspectives (particularly stimulus-response reinforcement versus response-outcome contingency). Future task refinements can also help clarify (A) which facets of choice may best model cocaine addiction, and (B) the relationship between choice and attention bias (e.g., via eye-tracking).
In conclusion, our results suggest that choice for viewing cocaine pictures can be added to an already impressive repertoire of clinical predictors that forecast drug-relevant outcomes in cocaine addiction (Poling et al., 2007). Because our results showed that cocaine-related choice predicted prospective drug independently of other common predictors of relapse, it is possible that this variable could account for drug use variance not captured by other available measures. Our simulated cocaine choice paradigms, ideal for use in treatment-seeking subjects, lean heavily on the perspectives that disadvantageous decision-making forms a core deficit in neuropsychiatric disorders including addiction (Paulus, 2007; Redish et al., 2008), and that drugs and drug-related stimuli assume heightened motivational significance at the expense of other positive reinforcers (Goldstein and Volkow, 2011). As such, our results offer the intriguing possibility that simulated cocaine choice (perhaps especially during a probabilistic context) could be used to objectively, yet noninvasively help to identify which individuals might require additional therapeutic resources for achieving longer-term abstinence.
Supplementary Material
Acknowledgements
We thank Muhammad A. Parvaz for additional help with data collection, and Nelly Alia-Klein and Erin Little for conducting psychiatric interviews. We also thank staff at Phoenix House, Samaritan Village, and Yale for subject referrals and help with subject follow-ups. Acknowledgements Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Role of Funding Source
This study was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579; to SJM: 1F32DA030017-01). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
S.J.M., P.A.W., and R.Z.G. designed the research; P.A.W. and A.B.K. coordinated and conducted the research; S.J.M., N.B.-W., P.A.W., and T.M. analyzed data; S.J.M., N.B.-W., and R.Z.G. wrote the paper. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Pychol. Addict. Behav. 2008;22:557–562. doi: 10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi J, Kampman KM, Oslin DM, Pettinati HM, Dackis C, Sparkman T. Predictors of treatment outcome in outpatient cocaine and alcohol dependence treatment. AmJAddict. 2009;18:81–86. doi: 10.1080/10550490802545174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci. Biobehav. Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Armel KC, Beaumel A, Rangel A. Biasing simple choices by manipulating relative visual attention. Judgm. Decis. Mak. 2008;3:396–403. [Google Scholar]
- Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn. Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Martinez D, Vadhan NP, Barnes-Holmes D, Nunes EV. Measures of attentional bias and relational responding are associated with behavioral treatment outcome for cocaine dependence. Am. J. Drug Alcohol Abuse. 2012;38:146–154. doi: 10.3109/00952990.2011.643986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict. Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine selfadministration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology (Berl.) 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users – an ERP study. EurJNeurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fernández G, Secades-Villa R, García-Rodríguez O, Alvarez-López H, Sánchez- Hervás E, Fernández-Hermida JR, Fernández-Artamendi S. Individual characteristics and response to contingency management treatment for cocaine addiction. Psicothema. 2011;23:114–118. [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav. Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol. Clin. Exp. Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction: A Disorder of Choice. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu. Rev. Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: Evidence for independent additive controllers. J. Exp. Psychol. Anim. Behav. Process. 2012;38:266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drugseeking: implications for dependence vulnerability. Exp. Psychol. Anim. Behav. Process. 2011;37:261–276. doi: 10.1037/a0022913. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Evaluating psychological markers for human nicotine dependence: tobacco choice, extinction, and Pavlovian-to-instrumental transfer. Exp. Clin. Psychopharmacol. 2012;20:213–224. doi: 10.1037/a0027203. [DOI] [PubMed] [Google Scholar]
- Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nat. Neurosci. 2010;13:1292–1298. doi: 10.1038/nn.2635. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainsville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch. Gen. Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Jr, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J. Abnorm. Psychol. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang YY, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to selfadminister cocaine. Am. J. Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi- Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse. Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J. Stud. Alcohol. Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. doi: 10.1093/brain/aws252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol. Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J, Telang F, Wang GJ, Wang R, Sinha R, Carise D, Astone-Twerell J, Bolger J, Volkow ND, Goldstein RZ. Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict. Biol. 2012;17:1013–1025. doi: 10.1111/j.1369-1600.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annu. Rev. Neurosci. 2011;34:333–359. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Maloney T, Moeller SJ, Woicik PA, Alia-Klein N, Telang F, Wang GJ, Squires NK, Volkow ND, Goldstein RZ. Sensitivity to monetary reward is most severely compromised in recently abstaining cocaine addicted individuals: a cross-sectional ERP study. Psychiatry Res. 2012;203:75–82. doi: 10.1016/j.pscychresns.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch. Gen. Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C, Wilson AS. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol. 2002;21:332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am. J. Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl.) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: role of dopamine D(1) receptors. Neuropsychopharmacology. 2011;36:1015–1020. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav. Brain Sci. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J. Stud. Alcohol Drugs. 2007;68:641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo S, Simion C, Shimojo E, Scheier C. Gaze bias both reflects and influences preference. Nat. Neurosci. 2003;6:1317–1322. doi: 10.1038/nn1150. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl.) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Alternative reinforcer response cost impacts cocaine choice in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:189–193. doi: 10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Rush CR. Monetary alternative reinforcers more effectively decrease intranasal cocaine choice than food alternative reinforcers. Pharmacol. Biochem. Behav. 2010;95:187–191. doi: 10.1016/j.pbb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur. J. Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: effects of a cash monetary contingency on Gambling task performance. Drug Alcohol Depend. 2009;102:95–101. doi: 10.1016/j.drugalcdep.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, Gonzalez- Saiz F, Fernandez-Calderon F, Bilbao-Acedos I, Perez-Garcia M. Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug Alcohol Depend. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Haney M, Rubin E, Foltin RW. Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend. 2010;110:144–150. doi: 10.1016/j.drugalcdep.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


