Abstract
Hearing loss is the most common sensory disorder in the elderly population. Overall, 10% of the population has a hearing loss in the US, and this age-related hearing disorder is projected to afflict more than 28 million Americans by 2030. Age-related hearing loss is associated with loss of sensory hair cells (sensory hearing loss) and/or spiral ganglion neurons (neuronal hearing loss) in the cochlea of the inner ear. Many lines of evidence indicate that oxidative stress and associated mitochondrial dysfunction play a central role in age-related neurodegenerative diseases and are a cause of age-related neurosensory hearing loss. Yet, the molecular mechanisms of how oxidative stress and/or mitochondrial dysfunction lead to hearing loss during aging remain unclear, and currently there is no treatment for this age-dependent disorder. Several mouse models of aging and age-related diseases have been linked to age-related mitochondrial neurosensory hearing loss. Evaluation of these animal models has offered basic knowledge of the mechanism underlying hearing loss associated with oxidative stress, mitochondrial dysfunction, and aging. Here we review the evidence that specific mutations in the mitochondrial DNA or nuclear DNA that affect mitochondrial function result in increased oxidative damage and associated loss of sensory hair cells and/or spiral ganglion neurons in the cochlea during aging, thereby causing hearing loss in these mouse models. Future studies comparing these models will provide further insight into fundamental knowledge about the disordered process of hearing and treatments to improve the lives of individuals with communication disorders.
Keywords: Age-related hearing loss, oxidative stress, apoptosis, ROS
Introduction
A large body of evidence indicates that oxidative stress and associated mitochondrial dysfunction play a central role in aging and age-related diseases (Balaban et al., 2005; Lin and Beal, 2006; Wallace, 2005). It is now well established that mitochondria are a major source of reactive oxygen species (ROS) and a major site of ROS-induced oxidative damage, and that ROS production increases with age (Balaban et al., 2005; Lin and Beal, 2006; Wallace, 2005). Accordingly, ROS generated inside mitochondria are hypothesized to damage key mitochondrial components such as mitochondrial DNA (mtDNA), membranes, and respiratory chain proteins. This damage accumulates over time, leads to mitochondrial dysfunction and associated energy depletion, and results in tissue dysfunction. This theory is supported by the observations that overexpressing the mitochondrial antioxidant Sod2 (Sun et al., 2002) increases life span in Drosophila, while overexpressing the antioxidant catalase in the mitochondria increases lifespan in mice (Schriner et al., 2005).
Hearing loss is a common symptom of human mitochondrial diseases (Fischel-Ghodsian, 2003; Someya and Prolla, 2010). A role for mitochondrial dysfunction in hearing loss is supported by the findings that a number of genetic syndromes associated with hearing loss are due to defects in mitochondria (Fischel-Ghodsian, 2003; Someya and Prolla, 2010) and that specific mtDNA point mutations are known to contribute to mitochondrial disorders such as MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes) and MERRF (myoclonic epilepsy and ragged red fibers) that are associated with hearing loss (Fischel-Ghodsian, 2003). Several deletions of mtDNA have also been associated with mitochondrial disorders such as Kearns–Sayre syndrome whose symptoms include hearing loss (Fischel-Ghodsian, 2003). Furthermore, several mutations in the nuclear DNA POLG (mitochondrial DNA polymerase gamma) cause mitochondrial disorders such as Alpers syndrome whose symptoms also include hearing loss (Nguyen et al., 2005). These results strongly suggest that mitochondrial dysfunction plays a causal role in the development of hearing loss.
Age-related hearing loss (AHL) or presbycusis is a common feature of aging and is the most common sensory disorder in the elderly population (Gates and Mills, 2005; Liu and Yan, 2007; Someya and Prolla, 2010). AHL affects more than 40% of people over 65 years of age in the US and is projected to afflict 28 million Americans by 2030. Yet, currently there is no treatment for this age-related disorder. AHL is generally classified into three types based on the relationship between cochlear pathology and hearing levels: sensory hearing loss (loss of sensory hair cells), neuronal (loss of spiral ganglion neurons), and metabolic (strial atrophy) (Gates and Mills, 2005; Schuknecht, 1955), and it is now well established that most cases of AHL exhibit a mixture of these pathological changes (Gates and Mills, 2005). This idea is supported by the observation that the progressive loss of hair cells and spiral ganglion neurons leads to AHL because these two cell types do not regenerate in mammals (Gates and Mills, 2005).
There are several mouse models of aging and age-related diseases whose phenotypes are caused by the defects in mitochondria and symptoms include neurosensory hearing loss. Evaluation of these animal models has offered basic knowledge of the mechanism underlying neurosensory hearing loss associated with oxidative stress, mitochondrial dysfunction, and aging. In this review, we discuss the evidence that specific mutations in the mtDNA or nuclear DNA that affect mitochondrial function result in increased oxidative damage, and associated loss of sensory hair cells and/or spiral ganglion neurons in the cochlea during aging, thereby causing hearing loss in these animal models. The relatively early onset of hearing loss in mice, its slow progression, and our ability to monitor its progression non-invasively, make animal models of age-related mitochondrial neurosensory hearing loss an ideal system to study fundamental mechanisms of age-related mitochondrial diseases as well as neurodegenerative diseases in humans.
Polg knockin mice
Polg knockin mice were created by introducing a two base substitution, which results in a defect in mtDNA proof-reading ability (Kujoth et al., 2005; Trifunovic et al., 2004). Young Polg mutator mice are indistinguishable from wild-type (WT) littermates, but these mutator mice display a variety of premature aging phenotypes such as kyphosis and alopecia (Kujoth et al., 2005; Trifunovic et al., 2004) by 9 months of age. Importantly, Polg mutator mice display a reduced life span: the median survival of the Polg mutator mice was 416 days, while the median life span of WT mice was >850 days. Age-related weight loss, a common feature of elderly (Kalu and Masoro, 1988; Wallace, 2005; Weindruch, 1996), was noted from 24 weeks of age in these animals. A reduction in subcutaneous fat is also a common feature of aging skin (Kalu and Masoro, 1988; Wallace, 2005; Weindruch, 1996). Trifunovic et al (Trifunovic et al., 2004) performed quantitative assessments of body composition with X-ray densitometry of the whole mouse and found that the fat content was reduced in Polg mutator mice. The 40 weeks old mutator mice also displayed a reduced bone mineral density, a common feature of age-related osteoporosis. Furthermore, 9 months old Polg mutator mice displayed age-related loss of skeletal muscle, a common feature of sarcopenia (Lexell et al., 1988).
Auditory brainstem response (ABR) hearing tests were conducted in these animals: young Polg mutator mice displayed normal hearing compared to age-matched WT mice: however, by 9–10 months of age, Polg mutator mice displayed a significant elevation of ABR thresholds at 4, 8, and 16 kHz, indicating that mtDNA mutations play a causal role in AHL (Kujoth et al., 2005; Niu et al., 2007; Someya et al., 2008) (Table 1). Importantly, these results were consistent with the observation that mutations in the human POLG cause Alpers syndrome that is associated with both mitochondrial dysfunction and hearing loss (Nguyen et al., 2005). Moreover, examination of the histology of the basal cochlear region confirmed that 9–10 months old Polg mutator mice displayed severe loss of spiral ganglion neurons and hair cells, and these results were confirmed by cell counting (Niu et al., 2007; Someya et al., 2008). TUNEL staining and caspase-3 immunostaining also revealed that the levels of apoptosis markers were significantly elevated in the cochlea of Polg mutator mice.
Table 1.
Phenotypic comparisons of models of mitochondrial neurosensory hearing loss
| Mouse Model | mtDNA mutation PolgD257A/D257A |
oxidative stress MCAT TG |
oxidative stress Gpx1−/− |
apoptosis Bak−/− |
apoptosis Bcl-2 TG |
|---|---|---|---|---|---|
| Hearing | ↓ | ↑ | ↓ | ↑ | na |
| Sensory Hair cells | ↓ | ↑ | ↓ | ↑ | ↑ |
| SG neurons | ↓ | ↑ | na | ↑ | na |
| Life span | ↓ | ↑ | na | na | na |
| Oxidative Damage | na | ↓ | na | ↓ | na |
| Apoptosis | ↑ | na | na | ↓ | ↓ |
| Reference |
Kujoth et al., 2005 Niu et al., 2007 Someya et al., 2008 |
Schriner et al., 2005 Someya et al., 2009 |
Cheng et al., 1997 Ohlemiller et al., 2000 |
Lindsten et al., 2000 Someya et al., 2009 |
Martinou et al., 1994 Cunningham et al., 2004 |
na: no available data.
↑: indicates elevated levels compared to age-matched WT.
↓: indicates reduced levels compared to age-matched WT.
How does the Polg mutation lead to age-related neurosensory hearing loss in mice? As stated earlier, mitochondria are a major site of ROS-induced oxidative damage, and ROS generated inside mitochondria are thought to damage key mitochondrial components such as mtDNA (Balaban et al., 2005; Lin and Beal, 2006; Wallace, 2005). It is also thought that oxidative damage to mtDNA leads to further ROS production due to impaired electron transport that could result from mutations in the mitochondrial genome (Harman, 1972). Nucleus-encoded POLG is the only known DNA polymerase in animal cell mitochondria and has conserved polymerase and exonuclease domains, which repair mtDNA mutations (Kujoth et al., 2007). Because Polg mutator mice lack the ability to repair mtDNA mutations, mtDNA mutations accumulate beginning during development in these animals (Kujoth et al., 2005): the frequency of mtDNA mutations in the Polg mutant mice is 3 to 8 times that in wild-type mice in the heart, liver, and duodenum. This is consistent with the report that the frequency of mtDNA mutations in the temporal bone of patients with presbycusis is significantly higher compared to controls (Fischel-Ghodsian et al., 1997). Therefore, it is likely that age-related accumulation of mtDNA mutations results in increased ROS production due to impaired electron transport changes in the mitochondria, which in turn leads to further mitochondrial dysfunction, causing loss of hair cells and spiral ganglion neurons in the cochlea. The loss of these neurosensory cells then leads to the onset of hearing loss during aging.
MCAT transgenic mice
To determine the role of ROS in limiting mammalian life span, Schriner et al (Schriner et al., 2005) generated mice overexpressing human catalase, an antioxidant enzyme which removes hydrogen peroxide, in the peroxisome (PCAT), nucleus (NCAT), or mitochondria (MCAT). PCAT animals showed a slight extension of median life span, while NCAT mice did not show a significant extension of median and maximum life span. However, overexpression of catalase in the mitochondria (MCAT) extended both median and maximum life span, and the increased life span was confirmed in both males and females. MCAT mice also displayed increased catalase activities in the heart, skeletal muscle, and brain. Furthermore, oxidative DNA damage and hydrogen peroxide (H2O2) levels were significantly reduced in the heart from MCAT mice. These results were supported by the observation that catalase activity was significantly higher in the red blood cells of centenarians (Klapcinska et al., 2000).
ABR hearing tests were conducted in these transgenic animals. Young MCAT mice displayed normal hearing compared to age-matched WT mice: however, at 13 months of age, the mean ABR thresholds of MCAT mice were significantly lower than those of age-matched WT mice, indicating that catalase overexpression in the mitochondria slows the development of AHL (Someya et al., 2009) (Table 1). Consistent with these results, catalase overexpression in the mitochondria reduced age-related loss of spiral ganglion neurons and hair cells in the cochlea (Someya et al., 2009), and these results were confirmed by cell counting. Furthermore, oxidative DNA damage increased in the cochlea of WT mice during aging, while catalase overexpression in the mitochondria reduced oxidative DNA damage in the cochlea.
The Free radical theory of aging postulates that aging is the result of accumulated oxidative damage caused by ROS generated inside mitochondria (Beckman and Ames, 1998; Harman, 1956). In support of this hypothesis, ROS are generated in cochleae exposed to high intensity noise (Jacono et al., 1998; Ohlemiller et al., 1999), while age-related cochlear hair cell loss is enhanced in mice lacking the antioxidant enzyme Sod1 (McFadden et al., 1999b). Mice lacking the antioxidant enzymes Gpx1 or Sod1 also show enhanced susceptibility to noise-induced hearing loss (Ohlemiller et al., 2000). Moreover, oxidative protein damage increases with age in the cochleae of mice (Jiang et al., 2007; Someya et al., 2009; Staecker et al., 2001). Therefore, increased mitochondrial catalase activity in the cochlea may result in reduced levels of ROS, thereby protecting mitochondrial components and cochlear cells from oxidative stress, and slowing the development of AHL.
Gpx1 KO mice
The antioxidant enzyme Glutathione peroxidase 1 (Gpx1) plays an important role in mitochondrial antioxidant defense by reducing hydrogen peroxide (Halliwell and Gutteridge, 2007; Mari et al., 2009). Cheng et al (Cheng et al., 1997) generated mice deficient for Gpx1. Both male and female Gpx1−/− mice appear normal, are fertile, and do not show any physical abnormalities up to 20 months of age. Gpx1+/− mice show a 40–60% reduction in Gpx1 mRNA levels in the brain, heart, kidney, liver, and lung, while no Gpx1 mRNA was detected in these tissues of Gpx1−/− mice. Gpx1+/− mice also show a 40–60% reduction in Gpx1 activity in the same tissues, while no or very low Gpx1 activity was detected in these tissues from Gpx1−/− mice. Interestingly, no changes in the other antioxidant activities except glutathione reductase activity were found. Furthermore, no significant differences were found in oxidized protein and lipid levels in the brain, heart, kidney, liver, and lung between WT and Gpx1−/− mice, suggesting that changes in the Gpx1 antioxidant defense pathway are compensated by the other antioxidant defense pathways such as the catalase pathway, which also decomposes hydrogen peroxide into water (Schriner et al., 2005).
Ohlemiller et al (Ohlemiller et al., 2000) investigated whether Gpx1 deficiency increases noise-induced hearing loss in mice (Table 1) and found that Gpx1−/− mice showed significantly greater ABR threshold elevation after noise exposure compared to WT. Consistent with these hearing test results, noise-exposed Gpx1−/− mice showed significantly more sensory hair cell loss compared to WT mice. As stated earlier, ROS are generated in cochleae exposed to high intensity noise (Jacono et al., 1998; Ohlemiller et al., 1999), while age-related cochlear hair cell loss is enhanced in mice lacking the antioxidant enzyme Sod1 (Johnson et al., 2010; McFadden et al., 1999a; McFadden et al., 1999b). Therefore, the lack of mitochondrial Gpx1 activity in the cochlea may lead to increased levels of ROS and/or increased susceptibility to noise. This then leads to a decline in mitochondrial function, thereby promoting cochlear cell loss and the development of hearing loss during aging or noise exposure.
Bak knockout mice
In mitochondria, Bcl-2 family members play a major role in regulating apoptosis (Lindsten et al., 2000; Youle and Strasser, 2008). Of the Bcl-2 family members, the proapoptotic proteins Bak and Bax are hypothesized to play a central role in promoting mitochondria-mediated apoptosis (Lindsten et al., 2000; Youle and Strasser, 2008). These Bcl-2 proteins promote permeabilization of the outer mitochondrial membrane, leading to cytochrome c release, caspase activation, and cell death. Lindsten et al (Lindsten et al., 2000) generated Bak-deficient mice and found that Bak-deficient mice are fertile and do not display any developmental abnormalities, suggesting that the role of Bak may be redundant with that of other proapoptotic Bcl-2 family members such as Bax in development.
ABR hearing tests were conducted in these knockout animals. Young Bak−/− mice displayed normal hearing compared to age-matched WT mice: however, ABR hearing thresholds from middle-aged Bak−/− mice were found to be significantly lower than those of age-matched WT, indicating that Bak is required for the development of AHL (Someya et al., 2009) (Table 1). To investigate whether Bax and Bak may function in a redundant manner (Lindsten et al., 2000), hearing levels were also measured in Bax−/− mice. Interestingly, no significant differences were observed in ABR thresholds between middle-aged WT and Bax−/− mice, indicating that Bak and Bax do not function in a redundant manner in aged cochlear cells. Consistent with those hearing test results, middle-aged Bax−/− mice displayed only minor loss of SG neurons and hair cells in the cochlea compared to extensive cell loss in age-matched WT mice. Cell counting also demonstrated that Bak deficiency increased SG neuron survival as well as outer hair cell survival. Furthermore, TUNEL staining revealed that the levels of apoptosis markers were significantly reduced in the cochlea of middle-aged Bax−/− mice compared to age-matched WT mice.
How does Bak deficiency reduce loss of hair cells and spiral ganglion neurons and slow the development of AHL in mice? It is well well-established that the nuclear transcription factor p53 promotes apoptosis in response to DNA damage in neurons (Culmsee and Mattson, 2005). Previous studies have shown that, in response to cell stress, p53 rapidly translocates to mitochondria, directly binds to Bak, and leads to cytochrome c release and eventually to cell death (Leu et al., 2004; Mihara et al., 2003). Edwards et al (Edwards et al., 2007) have also reported that p53-induced apoptotic genes are induced in multiple tissues with aging. Consistent with the role for p53 in aging, p53 is activated in primary cultured hair cells from rats following exposure to the ototoxic drug cisplatin (Zhang et al., 2003), while deletion of p53 protects hair cells from cisplatin-induced cell death (Cheng et al., 2005). Therefore, cochlear cells lacking Bak may be more resistant to p53-mediated mitochondrial apoptosis in response to oxidative DNA damage, thereby protecting cochlear cells from cell death and slowing the development of AHL.
BCL-2 transgenic mice
As stated above, Bcl-2 family members play a major role in regulating apoptosis (Lindsten et al., 2000; Youle and Strasser, 2008). Of the Bcl-2 family members, the anti-apoptotic protein Bcl-2 is localized to the outer mitochondrial membrane where it functions to block cytochrome c release (Lindsten et al., 2000; Youle and Strasser, 2008): the presence of cytochrome c in the cytoplasm results in the activation of caspase-9 and cell death (11048732). Martinou et al (Martinou et al., 1994) generated transgenic mice in which neurons overexpress human BCL-2 using the neuron-specific enolase promoter (Forss-Petter et al., 1990). BCL-2 mRNA was detected in the uterus, kidney, testis, heart, and brain of these transgenic animals, but not in the non-neuronal tissues such as vagina and liver. Interestingly, the weight of the brain from BCL-2 Tg mice was increased by 12%, indicating hypertrophy of the brain. Furthermore, BCL-2 transgenic mice displayed a 40% increase in facial motoneuron number compared to WT mice. Ganglion neurons were also counted in the central retina, and the transgenic animals displayed a 50% increase in the number of ganglion neurons
Cunningham et al (Cunningham et al., 2004) first confirmed that BCL-2 proteins were detected in the hair cells of the utricle in the vestibular system of the inner ear from the same transgenic mice (Table 1). Overexpression of BCL-2 increased hair cell survival in the cultured utricle organs treated with neomycin, which causes hair cell death (Cunningham et al., 2002), although untreated WT and BCL-2 overexpressing utricle organs contained similar numbers of hair cells. Furthermore, Bcl-2 overexpression reduced active caspase-9 positive hair cells in the utricle compared to WT cells. As stated earlier, the anti-apoptotic protein Bcl-2 is localized to the outer mitochondrial membrane where it functions to block mitochondrial apoptosis by inhibiting cytochrome c release (Lindsten et al., 2000; Youle and Strasser, 2008). Therefore, cochlear cells overexpressing Bcl-2 may be more resistant to mitochondrial apoptosis, thereby protecting cochlear cells from cell death and slowing the development of AHL.
Conclusions
Most inbred mouse strains display some degree of AHL, and the age of onset of AHL is known to vary from 3 months in DBA/2J mice to over 20 months in CBA/CaJ mice (Zheng et al., 1999). The background strain of the Polg mutator, MCAT transgenic, Gpx1 KO, Bak KO, and BCL-2 transgenic mice is the C57BL/6J strain, which displays the classic pattern of AHL by 12–15 months of age (Hunter and Willott, 1987; Zheng et al., 1999), a pattern similar to that reported in humans (Gates and Mills, 2005). However, this mouse strain is also known to carry a specific mutation in the cadherin 23 gene (Cdh23753A), which encodes a component of the hair cell tip link (Keithley et al., 2004; Noben-Trauth et al., 2003). Because this mutation promotes early onset of AHL in C57BL/6J mice (Keithley et al., 2004; Noben-Trauth et al., 2003; Zheng et al., 1999), hair cells in these mouse models carrying the Cdh23753A mutation may be more susceptible to oxidative stress and oxidative stress-induced apoptosis during aging, thereby limiting the general implication of the findings from these models. However, in both C57BL/6J strain and CBA/CaJ strain, which does not possess the Cdh23753A mutation and displays late onset of AHL by 18–20 months of age (Ohlemiller, 2006; Zheng et al., 1999), the onset of AHL begins in the high-frequency region and spreads toward the low frequencies with age, and the loss of hair cells and spiral ganglion neurons begins in the base and spreads toward the apex of the cochlea with age (Ohlemiller, 2006; Someya and Prolla, 2010; Zheng et al., 1999), which are the classic patterns of AHL reported in humans (Gates and Mills, 2005; Zheng et al., 1999). Moreover, oxidative damage increases with age in the cochlea of both the C57BL/6J and CBA/J strains (Jiang et al., 2007; Someya et al., 2009; Staecker et al., 2001), which also does not carry the Cdh23753A mutation (Ohlemiller, 2006; Zheng et al., 1999). Therefore, we speculate that the Cdh23753A allele affects age of onset of AHL, but the basic mechanisms of cochlear aging are likely to be similar in C57BL/6J, CBA/CaJ and CBA/J strains, and the presence of the Cdh23753A mutation in the mouse models is less likely to limit the general implication of the findings from these models, as far as the basic roles of oxidative stress and mitochondrial dysfunction in AHL.
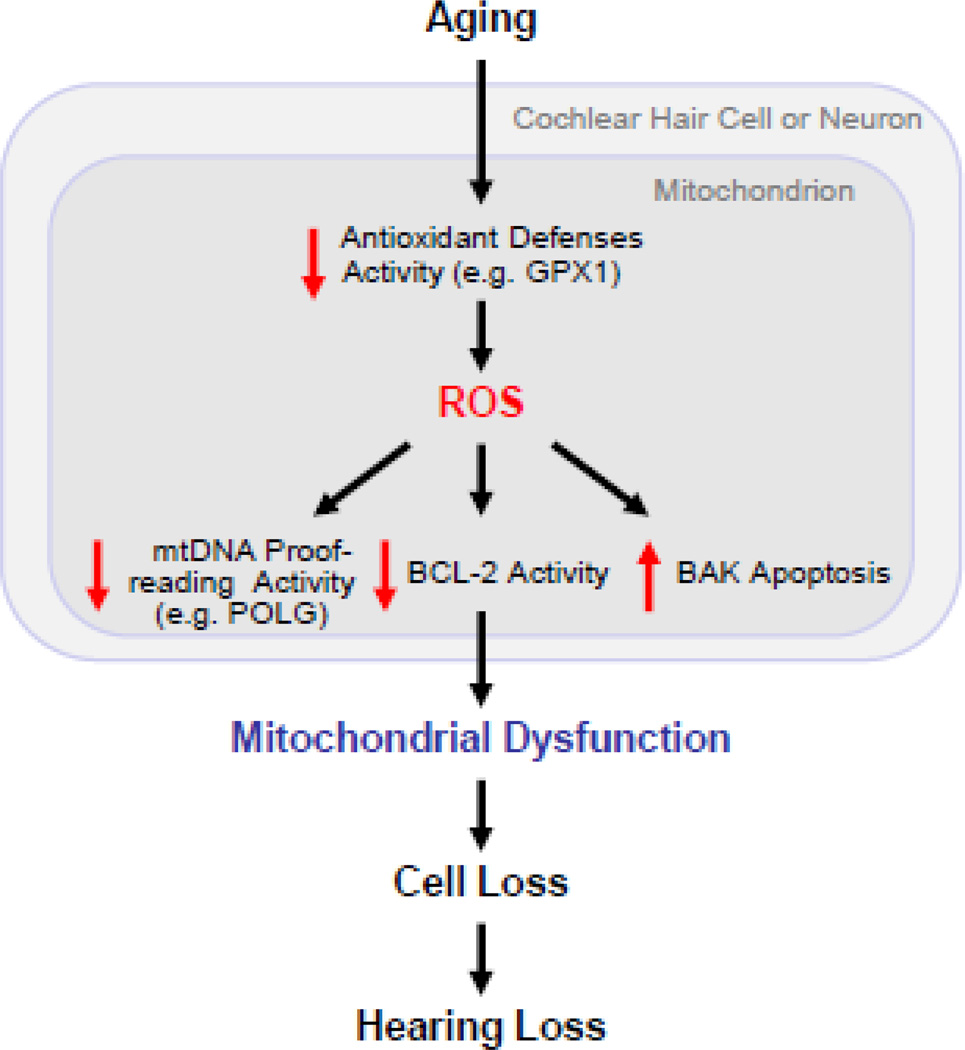
Although AHL is likely a multifactorial process, there is now a growing body of evidence implicating oxidative stress and associated mitochondrial dysfunction as key factors in the development of AHL, and the findings from the five mouse model studies discussed above significantly strengthen the mechanistic connections between oxidative stress, mitochondrial dysfunction, apoptosis, loss of neurosensory cochlear cells, and hearing loss during aging. What are the common age-related mechanisms of AHL across all the mouse models discussed? We postulate that ROS generated inside mitochondria play a central role in AHL in all these models (Fig. 1). Direct evidence for ROS in AHL comes from the observations that overexpression of the antioxidant enzyme catalase in the mitochondria reduces oxidative DNA damage and loss of sensory hair cells and spiral ganglion neurons in the cochlea, and slows the development of AHL in mice (Someya et al., 2009), and that the antioxidant enzyme Gpx1 deficiency significantly increases sensory hair cell loss and ABR thresholds after noise exposure compared to WT (Ohlemiller et al., 2000) (Fig. 1). These findings provide strong evidence that ROS generated inside mitochondria play a causal role in the loss of sensory hair cells, spiral ganglion neurons, and hearing during aging. Another evidence for ROS in AHL comes from the observation that mice engineered to carry a mutation that disrupts the exonuclease domain of the mitochondrial Polg show early onset of age-related loss of sensory hair cell, spiral ganglion neuron, and hearing (Kujoth et al., 2005; Niu et al., 2007; Someya et al., 2008). Because ROS generated inside mitochondria are known to damage key mitochondrial components such as mtDNA (Balaban et al., 2005; Lin and Beal, 2006; Wallace, 2005) and presbycusis patients display a higher frequency of mtDNA mutations in the temporal bone (Fischel-Ghodsian et al., 1997), the findings from the Polg mutator mouse study also support our hypothesis that ROS play a central role in AHL. The Free Radical Theory Aging postulates that ROS damage key cell components such as nuclear DNA and mtDNA (Beckman and Ames, 1998; Harman, 1956). Such damage accumulates over time and drives the aging process. It is also well established that oxidative DNA damage can initiate an apoptosis program and that p53 promotes Bak-mediated mitochondrial apoptosis in response to DNA damage (Culmsee and Mattson, 2005; Erster et al., 2004; Leu et al., 2004; Mihara et al., 2003). Hence, the findings from the Bak KO mouse model study provide a link between ROS, Bak-dependent mitochondrial apoptosis, and age-related loss of sensory hair cells, spiral ganglion neurons, and hearing. This idea is also supported by the observations that overexpression of the anti-apoptotic gene Bcl-2 increases hair cell survival (Cunningham et al., 2002), and that Bcl-2 functions to block mitochondrial apoptosis by inhibiting cytochrome c release (Lindsten et al., 2000; Youle and Strasser, 2008). In summary, the findings from those mouse models undoubtedly strengthen the mechanistic connections between oxidative stress, mitochondrial dysfunction, age-related hearing loss in mammals, and future studies comparing these models will provide further insight into fundamental knowledge about the disordered process of hearing loss during aging. Yet, many questions remain, including whether the same results as those from the five mouse models will be observed in the CBA/CaJ mouse background, and importantly, how applicable these findings are to human presbycusis as well as other human age-related neurodegenerative diseases. Therefore, it is our hope that the findings from these models will be validated in the CBA/CaJ background as well as other mammalian species, including humans.
Fig. 1.
A model for mitochondrial neurosensory hearing loss. In this model, ROS play a central role in AHL.
Acknowledgments
Work in the Someya laboratory is supported by NIH/NIDCD (1R03DC011840-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Current opinion in otolaryngology & head and neck surgery. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Ho YS, Ross DA, Valentine BA, Combs GF, Lei XG. Cellular glutathione peroxidase knockout mice express normal levels of selenium-dependent plasma and phospholipid hydroperoxide glutathione peroxidases in various tissues. The Journal of nutrition. 1997;127:1445–1450. doi: 10.1093/jn/127.8.1445. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochemical and biophysical research communications. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. Journal of neurobiology. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Molecular and cellular biology. 2004;24:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Mitochondrial deafness. Ear and hearing. 2003;24:303–313. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N, Bykhovskaya Y, Taylor K, Kahen T, Cantor R, Ehrenman K, Smith R, Keithley E. Temporal bone analysis of patients with presbycusis reveals high frequency of mitochondrial mutations. Hear Res. 1997;110:147–154. doi: 10.1016/s0378-5955(97)00077-4. [DOI] [PubMed] [Google Scholar]
- Forss-Petter S, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe JG. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford ; New York: Oxford University Press; 2007. [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? Journal of the American Geriatrics Society. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Yu H, Ding D, Jiang H, Gagnon LH, Salvi RJ. Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hear Res. 2010;268:85–92. doi: 10.1016/j.heares.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalu DN, Masoro EJ. The biology of aging, with particular reference to the musculoskeletal system. Clinics in geriatric medicine. 1988;4:257–267. [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapcinska B, Derejczyk J, Wieczorowska-Tobis K, Sobczak A, Sadowska-Krepa E, Danch A. Antioxidant defense in centenarians (a preliminary study) Acta biochimica Polonica. 2000;47:281–292. [PubMed] [Google Scholar]
- Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS genetics. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nature cell biology. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999a;413:101–112. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999b;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Molecular cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Nguyen KV, Ostergaard E, Ravn SH, Balslev T, Danielsen ER, Vardag A, McKiernan PJ, Gray G, Naviaux RK. POLG mutations in Alpers syndrome. Neurology. 2005;65:1493–1495. doi: 10.1212/01.wnl.0000182814.55361.70. [DOI] [PubMed] [Google Scholar]
- Niu X, Trifunovic A, Larsson NG, Canlon B. Somatic mtDNA mutations cause progressive hearing loss in the mouse. Experimental cell research. 2007;313:3924–3934. doi: 10.1016/j.yexcr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Presbycusis. The Laryngoscope. 1955;65:402–419. doi: 10.1097/00005537-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mechanisms of ageing and development. 2010;131:480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, Prolla TA. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiology of aging. 2008;29:1080–1092. doi: 10.1016/j.neurobiolaging.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121:666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicologic pathology. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular cell biology. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]