Abstract
The current study examined age differences in the association between daily negative affect, average negative affect, and diurnal cortisol among participants from the National Study of Daily Experiences (N = 1423; age range: 33–84). Across four consecutive days, participants reported the negative emotions they experienced and provided four saliva samples per day, from which cortisol was assayed. Results revealed that higher levels of average negative affect were associated with greater daily cortisol output (area-under-the-curve, with respect to ground), but only among the older participants in our sample. Higher levels of daily negative affect were also associated with elevated levels of bedtime cortisol, but only among older adults who, on average, reported lower levels of average negative affect. Findings support the theory of Strength and Vulnerability Integration (SAVI) and underscore the importance of age when examining associations between negative affective states and diurnal cortisol.
Keywords: cortisol, HPA-axis, affect, emotion, age, aging, older adults, multilevel modeling
Research attests to age-related strengths in affective experience. Negative affect (NA) decreases across the life-span, whereas positive affect (PA) remains relatively stable, showing declines only in very late adulthood (see reviews by Charles & Carstensen, 2007; Consedine & Magai, 2006). The theory of Strength and Vulnerability Integration (SAVI; Charles, 2010; Charles & Piazza, 2009) posits that these empirical findings reflect increases in older adults’ motivation to maintain affective well-being, as well as age-related improvements in the ability to effectively employ emotion regulation strategies. According to SAVI, age-related strengths in emotion regulation are adaptive because sustained physiological arousal from NA becomes more costly with increasing age (Charles, 2010). This increased cost is due to the body becoming less resilient over time, which makes it difficult for people to adjust to the sustained physiological arousal caused by NA. In the current study, we test aspects of SAVI by examining whether sustained levels of NA (as indicated by high mean levels of daily NA) are related to a worse physiological outcome among older adults compared to their younger counterparts. We use the hormone cortisol as a biomarker with which to test this hypothesis.
Age and Cortisol
Cortisol, the end-product of the HPA-axis, is vital for many physiological processes, such as immune system modulation, blood pressure regulation, and glucose metabolism (Lovallo & Tomas, 2000). Cortisol follows the same diurnal pattern across age groups (Van Cauter, Leproult, & Kupfer, 1996), sharply increasing within one hour after waking and steadily declining thereafter, until reaching a nadir in the late evening hours (Lovallo & Thomas, 2000). With age, however, the decline in cortisol observed across the course of the day is attenuated, resulting in a higher end-of-day nadir (for review, see Piazza, Almeida, Dmitrieva, & Klein, 2010; Raff et al., 1999; Yen & Laughlin, 1998). Older age is also related to higher basal levels throughout the day (Chahal & Drake, 2007; for review, see Epel, Burke, & Wolkowitz, 2009), such that mean cortisol levels are estimated to increase between 20% and 50% from the age of 20 to the age of 80 (Van Cauter, et al., 1996).
Although research attests to age-related changes in diurnal cortisol, considerable heterogeneity exists within age groups, some of which may be attributable to indices of emotional well-being. For example, although mean levels of cortisol increase with age, older adults who engage in effective coping strategies have lower total daily cortisol output than older adults who do not engage in effective coping strategies (O’Donnell, Badrick, Kumari, & Steptoe, 2008). Similarly, the cortisol awakening response is elevated among lonelier older adults (e.g., Adam, Hawkley, Kudielka, & Cacioppo, 2006) and among those who report having low social status (Wright & Steptoe, 2005) compared to their more socially connected peers. To the extent that more effective coping strategies, decreased loneliness, and higher social status are associated with lower levels of sustained distress, these findings point to the role of better HPA-functioning among older adults who experience less NA compared to those who experience more NA. No study, however, has explicitly examined whether sustained levels of NA may create within-group differences that are larger among increasingly older adults. In other words, it is unclear if same-aged individuals who experience different levels of average NA (e.g., low versus high) show different cortisol profiles, and--if so--whether this association becomes stronger with increasing age.
Affect and Cortisol
Studies examining the association between affect and cortisol indicate that sustained negative mood states are linked to higher levels of cortisol and cortisol dysregulation. At the extreme, clinical affective disorders such as depression are associated with dysregulated patterns of cortisol (Bremmer et al., 2007; Burke, Davis, Otte, & Mohr, 2005; Mantella et al., 2008). At non-clinical levels of distress and with more transient measures of NA, findings are mixed. A number of studies have revealed an association between elevated levels of daily NA and increased diurnal cortisol (e.g., Adam, et al., 2006; Smyth, et al., 1998). For example, in their ecological momentary sampling study, Smyth and colleagues found that higher levels of NA were associated with higher levels of cortisol across the day (Smyth et al., 1998). Other studies, however, have failed to detect associations between daily NA and cortisol (Ice, 2006; Polk, Cohen, Doyle, & Skoner, & Kirschbaum, 2005; Simpson et al., 2008). One possible reason for these discrepant findings is that minor fluctuations in NA may not be strong enough to consistently elicit changes in levels of cortisol. Alternatively, the effects of these minor fluctuations may vary according to how much NA an individual experiences on average. Adjusting to elevated daily NA may be particularly difficult for people who, in general, experience high levels of NA. For these individuals, the wear-and-tear of additional NA may be exacerbated, particularly among older adults who also face the physiological vulnerabilities associated with aging. It is also possible, however, that people who experience high levels of average NA reach a ceiling effect of sorts, whereby additional NA may not elicit as large of a cortisol response as it does in people who experience low levels of average NA. Moreover, these individuals may show evidence of physiological inflexibility, such that even on days when they do not experience high daily NA, their levels of cortisol may be heightened.
Of the previous studies that have explored associations between naturally-occurring affective states and cortisol, many have done so with age ranges spanning fewer than 20 years (e.g., Adam et al., 2006; Steptoe, Wardle, & Marmot, 2005). Other studies have examined wider age ranges, but have focused either on individuals younger than approximately 60 years of age (e.g., Jacobs et al., 2007; Polk et al., 2005; van Eck, Berkhof, Nicolson, & Sulon, 1996) or on more transient assessments of NA among those older than approximately 60 years of age (e.g., Evans et al., 2007). Moreover, many studies have focused on laboratory-based experiments that examine acute response to NA, rather than naturally-occurring mood states (e.g., e.g., Hatzinger, Brand, Herzig, & Holsboer-Trachsler, 2011; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). These studies have greatly informed the literature; yet, to truly understand the associations between daily NA, average NA, and age, it is necessary to use a large data set that includes multiple measures of NA and participants spanning across a wide age range. The current study utilizes such a data set to examine whether there are associations between daily NA and average (or sustained) NA, and whether this association changes as a function of age.
The Current Study
We examine the links between diurnal cortisol and negative affective states in individuals spanning five decades of life (ages 33–84), who completed a series of daily diary interviews. Given that our measure of NA assessed affective experience across the day and was usually reported at the end of the day, we examined cortisol measures across similar timeframes - total cortisol output across the course of the day (area-under-the-curve with respect to ground, AUCg) and bedtime cortisol - as opposed to assessments prior to the affective experience (i.e. upon awakening). We did, however, also explore the association between affect and cortisol at these earlier time points. Based on SAVI, we hypothesized the following:
-
Hypothesis 1
High levels of NA will be associated with higher levels of total daily cortisol output (AUCg), but these associations will increase in magnitude with age
-
Hypothesis 2
High levels of NA will be associated with elevated levels of bedtime cortisol, but these associations will increase in magnitude with age.
We also examined the interaction between daily and average NA for both AUCg and bedtime cortisol to see if the interaction between these two experiences becomes more costly (e.g., results in higher levels of cortisol) among successively older adults.
Method
Participants
Participants (N = 2,022) completed the second wave of the National Study of Daily Experiences (NSDE), which is the daily diary portion of the Midlife Development in the United States (MIDUS) survey (Almeida, McGonagle, & King, 2009). Of the 2,022 NSDE participants, which included 128 participants from the Milwaukee subsample (for details, see Love, Seeman, Weinstein, & Ryff, 2010), 1730 (752 male; 978 female) provided cortisol. Participants with cortisol data ranged in age from 33–84 (Mean = 56.4, SD = 12.1) and were primarily European-American (86.2%). Participants were fairly well-educated, with approximately 40% having graduated from college. Those who provided saliva samples and those who did not were similar with respect to age, t(2001) = .82, p = .41 and education, t(1819) = 1.27, p = .21. However, ethnic minorities were significantly less likely to complete the cortisol protocol than were whites (χ2(1, N = 2003) = 33.78 p < .001), with ethnic minorities comprising 13.8% of participants who completed the cortisol protocol versus 27% of those who did not complete the protocol. In addition, 5% of men did not completed the cortisol protocol versus 8.4% of women, a difference that trended toward significance (χ2(1, N = 2003) = 3.96, p = .054).
Procedure
Across eight consecutive evenings, participants completed brief telephone interviews, during which they were asked about the events they experienced during the previous 24 hours. This interview included questions regarding participants’ affective state, their physical health status, and the stressors they encountered (Almeida, Wethington, & Kessler, 2002). Interviews were staggered across day of the week and participants were compensated $45.00. On four of the interview days (days 2–5) participants also provided saliva samples.
Measures
Negative affect (NA) was assessed using combined items from the Non-Specific Psychological Distress Scale (Kessler et al., 2002; Mroczek & Kolarz, 1998) and a modified version of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1998). Participants rated, on a scale of 0 (not at all) to 4 (all of the time), how often they experienced each of the following 14 emotions or emotion states during the previous 24 hours: restless or fidgety, nervous, worthless, so sad that nothing could cheer them up, that everything was an effort, hopeless, lonely, afraid, jittery, irritable, ashamed, upset, angry, and frustrated. Mean scores across all items were calculated for each participant, on each interview day (α = .85). We calculated average NA scores for each participant by taking the mean of NA scores reported across all eight interview days. Daily NA was assessed by examining an individual’s NA score on any one given day. This method of NA categorization is similar to that used by other researchers (e.g., Polk, et al., 2005). Average NA scores were grand-mean centered; daily NA scores were person-centered, such that an individual’s score reflected deviation from his or her own average NA (Hoffman & Stawski, 2009).
Positive Affect (PA) was assessed by having participants rate on a scale of 0 (not at all) to 4 (all of the time), how often they experienced each of the following 13 emotions or emotion states during the previous 24 hours: in good spirits, cheerful, extremely happy, calm and peaceful, satisfied, full of life, close to others, like you belong, enthusiastic, attentive, proud, active, and confident. Mean scores across all items were calculated for each participant on each interview day (α = .94). Calculation of average PA and daily PA was identical to the technique described for NA. Average PA scores were grand-mean centered, whereas daily PA scores were person-centered.
Collection and assessment of salivary cortisol
Prior to their initial NSDE interview, participants received a Home Saliva Collection Kit, which included a detailed instruction sheet, and sixteen numbered and color-coded salivette collection devices (Sarstedt, Nümbrecht, Germany). Interviewers reviewed collection procedures with participants during the first interview, and saliva collection began the next day. Participants provided saliva samples four times per day on four consecutive interview days: immediately after waking, 30 minutes after waking, before lunch, and before bed. Participants were instructed not to eat, brush their teeth, or consume caffeine for 30 minutes prior to the collection of each sample.
The saliva collection kit included a paper-pen log to record the sample collection time. Following the four collection days, participants were instructed to mail their saliva kit to the laboratory in a pre-paid, addressed box (see Almeida, Piazza, & Stawski, 2009 for a detailed description of the reliability and validity of this procedure). Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL, Hamburg, Germany), with intra-assay and inter-assay coefficient of variations below 5% (Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992).
Analytic Strategy
We used multilevel models (Snidjers & Bosker, 1999) to model and predict variability in cortisol levels across days and persons. This framework makes it possible to examine both between- and within-person differences through a two-level hierarchical model (Radenbusch & Bryk, 2002), where Level 1 represents within-person variability and Level 2 allows for the inclusion of between-person variables. We examined mean daily cortisol output, using area-under-the-curve with respect to ground (AUCg; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). We also explored the association between affect and cortisol at each time point (waking (T1), 30 minutes after waking (T2), and before lunch (T3), in addition to testing the hypotheses regarding levels of cortisol before bed (T4)). The following general model was used for each analysis; however, the outcome variable depended upon which cortisol formulation we used (i.e., AUCg, cortisol at each individual time point).
The Level 1 outcome variable, cortisolit, refers to the value of cortisol for person i on day t, and is a function of a person-specific intercept (π0it), an individual’s daily NA score, calculated by subtracting his/her average NA score from his/her daily NA score (π1it), and within-person error (eit). The intercept and slope of the Level 1 model are the outcome variables of the Level 2 model, where between-person variables were examined. These factors included the participants’ age, average level of NA, and an average NA by age interaction. Level 1 covariates included interview day and, for analyses examining individual time points, person-mean centered cortisol collection time, calculated by subtracting an individual’s average cortisol collection time from his/her daily cortisol collection time. We did not include cortisol collection time in analyses examining AUCg because time is accounted for in the formula used for calculating this variable; see Pruessner et al., 2003). Level 2 covariates included gender (coded yes/no), race (coded white/other, due to the small number of ethnic minorities), education (assessed via an ordinal scale representing: less than a high school degree; a high school degree or general equivalency diploma; some college; a college degree; or at least some graduate school), smoking status (coded yes/no), medication use (coded yes/no), number of chronic health conditions, and for cortisol at individual time points, average cortisol collection time. Cross-level interactions included: daily NA by age for both models. We also included an average NA by daily NA interaction, as well as an age by average NA by daily NA interaction for all models, but significant findings only emerged for bedtime cortisol. Analyses were conducted using SAS PROC MIXED (SAS Institute, 2001) and estimated from unstructured covariance matrices by means of full maximum likelihood.
Preparing cortisol for analyses
For each of the four time points, cortisol data were excluded if values were greater than three standard deviations above the mean. For waking, values above 203.59 were excluded (n = 16 samples). For 30 minutes post-waking, values above 148.07 were excluded (n = 37 samples). For before lunch, values above 68.54 were excluded (n = 54 samples). For before bed, values above 173.29 were excluded (n = 30 samples). These new values were used to calculate AUCg. Cortisol samples were also excluded if there were no corresponding times for when the sample was taken (n = 306 or 1.1% of samples), or if the participants providing the sample were shift workers (n = 656 or 2.4% of samples). To correct for positive skewness, cortisol was transformed prior to analyses using natural log transformation.
Analyses were conducted only on people who had complete data on all variables of interest, as well as on any variable that could potentially affect cortisol levels (e.g., medication use). A total of 53 participants were excluded because they did not provide cortisol collection time and/or had invalid days due to issues such as night-shift work. Of the remaining 1677 participants, 254 were missing information on one or more of the variables of interest, resulting in a final sample of 1423 participants.
Results
Separate models examined each type of cortisol assessment (i.e., AUCg, individual time points). In all analyses, both continuous and quadratic terms for age were examined. Because the quadratic terms were not significant, age was modeled as a continuous, centered variable. In all model iterations, daily NA was included as a random slope. For all significant interactions, simple slopes and regions of significance were derived using the method outlined by Preacher and colleagues (Preacher, Curran, Bauer, 2006). Calculators used in the current study can be found on their website: http://www.quantpsy.org/interact/hlm3.htm.
Age, NA, and AUCg
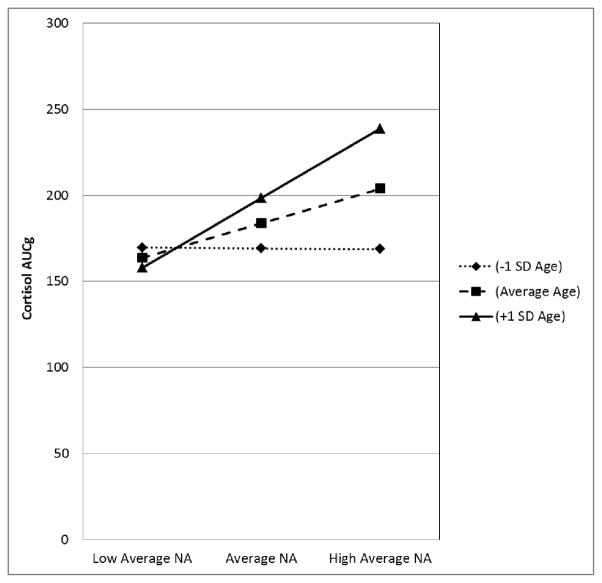
Our first hypothesis was that high levels of daily NA and average NA would be associated with higher levels of total daily cortisol output (i.e., AUCg), and that this association would increase in magnitude with age. To determine if there was enough within-person variability in AUCg to warrant the use of multi-level modeling, we conducted an unconditional means model and calculated an intra-class correlation (ICC; the amount of between-person versus within-person variance in AUCg). The Level 1 variance was significant (p < .001), indicating that it was permissible to proceed. Results of the analyses testing our hypotheses are displayed in Table 1. The main effects model (Model 1) revealed no significant effects of daily or average NA on AUCg. However, the interaction model (Model 2) revealed a significant age by average NA interaction. To decompose this interaction we used Preacher’s calculators to estimate the predicted values of the simple slopes for the effect of average NA on AUCg at −/+1 standard deviation (SD) for age and −/+1 SD for average NA. This analysis indicated that the difference in AUCg between people reporting low (−1SD) versus high (+1SD) average NA was not significant for participants at −1SD age or approximately 44.3 years (Slope Estimate = −.43, SE = 13.31; p = .97.), but was significant for participants at the mean sample age or approximately 56.4 years (Slope Estimate = 20.04, SE = 9.95, p = .044), as well as those at +1SD age or approximately 68.5 years (Slope Estimate = 40.51, SE = 14.32, p = .005; see Figure 1). Regions of significance testing revealed that the association between AUCg, average NA, and age were significant only for those participants 53 years of age and over. For people of average sample age, AUCg was 11% higher among those reporting elevated levels of NA (i.e., +1SD) compared to those reporting average levels of NA. A similar pattern was found for older adults (i.e., those +1SD mean age): in this group, AUCg was 20.4% higher among those reporting higher levels of NA (+1SD) compared to their same-aged peers reporting average levels of NA (−1SD). No significant interactions emerged between daily NA and age, indicating that the association between age and cortisol was only apparent when examining NA as a trait-level variable. Thus, our first hypothesis was partially supported. The final model also revealed that older participants, males, and smokers had higher AUCg than did younger participants, females, and non-smokers.
Table 1.
Age, NA, and Cortisol AUCg
| Fixed Effects | Model 1 | Model 2 |
|---|---|---|
|
| ||
| Estimate (SE) | Estimate(SE) | |
| Intercept | 182.26 (8.11)*** | 183.30 (8.13)*** |
| Day | −2.13 (.81)** | −2.13 (.81)** |
| Age | 1.15 (.17)*** | 1.20 (.18)*** |
| Sex (1 = Male) | −12.42 (4.06)** | −12.61 (4.05)** |
| Education | .12 (1.73) | −.03 (1.73) |
| Chronic illnesses | −.59 (1.15) | −.58 (1.15) |
| Smoking status (1 = Yes) | −25.61 (6.40)*** | −25.88 (6.39)*** |
| Race (1 = White) | −7.07 (5.09) | 7.14 (6.08) |
| Medication Use (1 = Yes) | −1.19 (4.18) | −1.23 (4.17) |
| Negative Affect (WP) | 10.77 (5.66) | 10.97 (5.72) |
| Negative Affect (BP) | 14.76 (9.64) | 20.04 (9.95)* |
| Age x Negative Affect (WP) | −.02 (.49) | |
| Age x Negative Affect (BP) | 1.69 (.79)* | |
| Random Effects | ||
| Intercept | 3979.46 (215.03)*** | 3966.67 (214.30)*** |
| Negative Affect (WP) | 1669.16 (972.11)* | 1640.38 (961.40)* |
| Residual | 3253.25 (91.13)*** | 3523.00 (91.07)*** |
| Model Fit | ||
| −2 Log Likelihood | 49129.7 | 49125.1 |
Note:
p<.05,
p<.01,
p<.001.
WP = Within-Person, BP = Between-Person
Figure 1.
Age differences in the association between average NA and cortisol AUCg among adults one SD below mean sample age, mean sample age, and one SD above mean sample age. Note: Figures are based on estimates from adjusted models; NA = Negative Affect; SD = Standard Deviation
Age, daily NA, and cortisol at individual time points
Our next set of analyses examined the associations between NA, age and cortisol at various time points throughout the day (i.e., upon waking, 30 minutes after waking, before lunch, and before bed). We hypothesized that high levels of daily NA and average NA would be associated with elevated levels of bedtime cortisol, but that this association would increase in magnitude with age. We also examined the association between age and NA for the first three time points (i.e., waking, 30 minutes after waking, and before lunch), but no significant associations were detected.
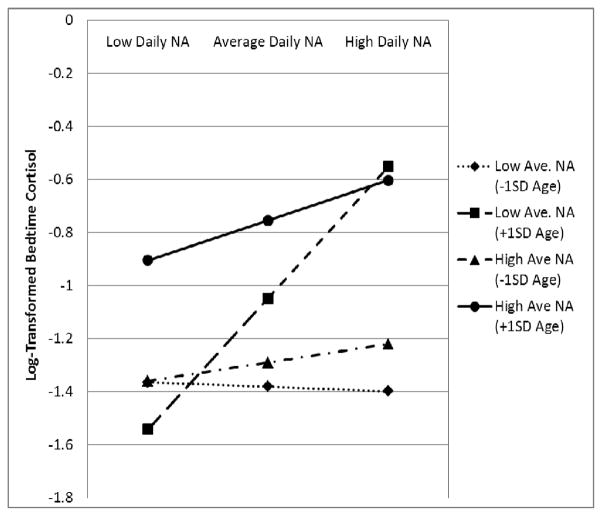
For bedtime cortisol, the Level 1 variance was significant (p < .001), indicating that there was enough variability to warrant the use of multi-level modeling. T able 2 displays the results. At the main effects level (Model 1), bedtime cortisol was positively associated with both average NA and daily NA. In Model 2, a significant age by average NA interaction emerged, but this effect was subsumed by a three-way average NA x daily NA x age interaction that was revealed in our final model (Model 4). To decompose this 3-way interaction, we estimated the predicted values of the simple slopes for the effect of daily NA on bedtime cortisol at −/+1 SD for age and −/+1 SD for average NA.
The slope estimate of within-person daily NA at −1SD age and −1SD average NA was −.02 (SE =.14, p = .9), at −1SD age and +1SD average NA was .07 (SE =.08, p = .4), at +1SD age and −1SD average NA was .49 (SE =.16, p = .003), and at +1SD age and +1SD average NA was .15 (SE =.1, p = .13). Thus, the simple slopes indicate that the within-person association between daily NA and bedtime cortisol was not statistically significant at −1SD age at any level of average NA. For participants at +1 SD age, the association between daily NA and bedtime cortisol was positive and statistically significant, but only among older adults reporting lower levels of average NA. For these individuals, moving from a day when they experienced average levels of NA to a day when they experienced a 1 SD increase in NA resulted in a 47.3% increase in their bedtime cortisol. In contrast, participants at +1 SD age who reported high levels of average NA, had higher levels of bedtime cortisol than their less negative peers, but appeared to be less reactive to changes in daily NA (see Figure 2). Regions of significance testing indicated that for people reporting low average NA, only those aged 55 and older showed increased reactivity on days they experienced elevated daily NA. However, for people reporting high average NA, no regions of significance could be derived. Thus, the oldest adults in the sample with high average NA had bedtime cortisol levels that were not associated with their daily levels of NA. The final model (Model 4) also revealed that levels of bedtime cortisol were higher among participants who, on average, collected their saliva samples earlier in the evening (BP collection time). However, on days participants provided saliva samples later than usual, their cortisol levels were also higher than usual (WP collection time). Smoking, age, and ethnic minority status were also associated with higher levels of bedtime cortisol.
Figure 2.
Age, NA, and bedtime cortisol among adults one SD below mean sample age, mean sample age, and one SD above mean sample age. Note: Figures are based on estimates from adjusted models; NA = Negative Affect; SD = Standard Deviation
Is it negative emotion-specific?
One question with these findings is whether the significant associations are due to negative emotions specifically, or are simply due to greater frequency of being in an emotional state. The current study assessed the frequency of emotions experienced throughout the day (from not at all to all of the time), and perhaps experiencing emotions for a longer amount of time – and not NA, per se – is driving the findings. If this alternative hypothesis is true, then high frequency of positive emotions should produce similar findings. To address this question, we conducted an additional set of analyses examining the associations between cortisol, age and positive affect (PA). All variables were identical to those included in models examining NA; however, instead of including NA in these models, we included PA, and all applicable interactions.
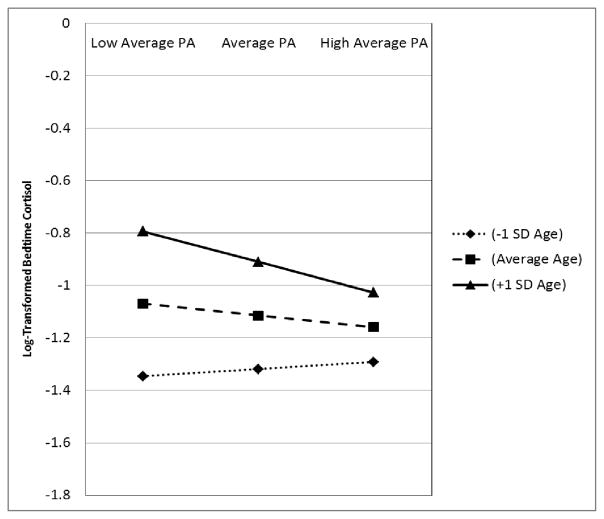
We first examined the associations between AUCg, age, and PA, but no significant effects emerged. We therefore proceeded to examine the associations between bedtime cortisol, age and PA. Results are presented in Table 3. As Model 2 indicates, a significant interaction between average PA and age emerged. To decompose this interaction we once again used Preacher’s calculators to estimate the predicted values of the simple slopes for the effect of average PA on bedtime cortisol at −/+1 SD for age and −/+1 SD for average PA. This analysis indicated that the difference in bedtime cortisol between people reporting low (−1SD) versus high (+1SD) average PA was not significant for participants at −1SD age (Slope Estimate = .03, SE = .04, p = .54), or the sample average age (Slope Estimate = −1.11, SE = .38, p = .17), but was significant for participants at +1SD age (Slope Estimate = −.12, SE = .05, p = .013). Participants 1SD above mean age, who reported high levels of PA (+1SD) had levels of bedtime cortisol that were 12.9% lower than their same-aged peers reporting average levels of PA (−1SD). Regions of significance testing revealed that the interaction between PA and bedtime cortisol was significant only among people aged 60 years and older. Figure 3 presents this interaction, which reveals the opposite of what was found with NA.
Table 3.
Age, PA, and Bedtime Cortisol
| Fixed Effects | Model 1 | Model 2 |
|---|---|---|
|
| ||
| Estimate (SE) | Estimate(SE) | |
| Intercept | −1.103 (.374)** | −1.114 (.374)** |
| Sample Time (WP) | .094 (.016)*** | .095 (.016)*** |
| Sample Time (BP) | −.081 (.025)** | −.081 (.025)** |
| Day | .002 (.009) | .002 (.009) |
| Age | .017 (.002)*** | .017 (.002)*** |
| Sex (1 = Male) | −.045 (.045) | −.044 (.045) |
| Education | −.014 (.019) | −.013 (.019) |
| Chronic illnesses | .013 (.012) | .013 (.012) |
| Smoking status (1 = Yes) | −.361 (.070)*** | −.359 (.070)*** |
| Race (1 = White) | .367 (.064)*** | .368 (.064)*** |
| Medication Use (1 = Yes) | .019 (.046) | .019 (.046) |
| Positive Affect (WP) | −.045 (.032) | −.043 (.033) |
| Positive Affect (BP) | −.045 (.033) | −.045 (.033) |
| Age x Negative Affect (WP) | −.001 (.003) | −.000 (.003) |
| Age x Negative Affect (BP) | −.006 (.003)* | −.006 (.003)* |
| Random Effects (WPNA) | ||
| Intercept | .492 (.025)*** | .492 (.025)*** |
| Negative Affect (WP) | .041 (.034) | |
| Covariance | .002 (.026) | |
| Residual | .525 (.012)*** | .520 (.013)*** |
| Model Fit | ||
| −2 Log Likelihood | 13267.3 | 13265.4 |
Note:
p<.05,
p<.01,
p<.001.
WP = Within-Person, BP = Between-Person
Figure 3.
Age differences in the association between average PA and bedtime cortisol among adults one SD below mean sample age, mean sample age, and one SD above mean sample age. Note: Figures are based on estimates from adjusted models; NA = Negative Affect; SD = Standard Deviation
Measures of effect size
Finally, we wanted to determine the amount of variance explained by our variables of interest. The main effects of age, average NA, and daily NA explained 7.3% of the variance in bedtime cortisol. With the addition of the two- and three-way interactions, total variance explained increased to 7.7%. For AUCg, the main effects of age, average NA, and daily NA explained 3.8% of the variance. With the addition of the two-way interactions, total variance explained increased to 4.1%.
Discussion
Emotions are adaptive and functional: They influence the people we see, the activities we engage in, and the choices we make. They are indicative of our needs and are critical for our survival. Yet, their occurrence also leads to increased physiological arousal, which, over time, can lead to wear-and-tear on bodily systems (McEwen, 1998). Adding to the literature on the effects of emotion on physical health biomarkers (for reviews, see Cohen & Pressman, 2006; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002), the current study revealed that the negative emotions people experience are not without consequence. Yet, the association between negative affective states and cortisol was significant only for the older adults in our sample, a finding that supports the theory of Strength and Vulnerability Integration (SAVI). SAVI posits that adverse emotional states pose an increased risk to physiological functioning later in life because the body becomes less resilient with age, making it difficult to modulate the increased physiological arousal caused by adverse emotional states.
Affective states and cortisol
Previous research examining the association between affective states and cortisol has yielded mixed findings (e.g., Polk et al., 2005; VanEck et al., 1998), and the current study indicates that disparate reports may reflect the different aspects of NA assessed (more sustained as opposed to more transient), as well as the ages of the participants examined. Across all analyses, the association between affect and cortisol was only significant among participants in their 50’s and older, as indicated by the regions of significance tests. In terms of AUCg, for example, high levels of average NA were associated with increased levels of cortisol only among people who were age 53 and older. If participants in this study had only included individuals younger than age 53, no significant effects would have emerged, leading us to conclude that there are no associations between average NA and cortisol. The large age-range in the current study, however, allowed us to comprehensively examine the effects of affect and cortisol, and add a unique contribution to the growing literature on age, affective states and cortisol.
Findings from the current study differ from those indicating that negative emotional experiences during laboratory tasks reliably elicit a cortisol response among younger adults (e.g. Dickerson & Kemeny, 2004). One possible explanation that reconciles findings from the current study to those from acute laboratory studies is that younger adults experience increases in cortisol at the time negative emotions occur, but recover from this departure from stasis by the time they collect their cortisol samples. This finding is similar to a study that examined cortisol levels and blood pressure during high and low stress periods at work among younger and older teachers (Ritvanen, Louhevaara, Helin, Väisänen, & Hänninen, 2006). During high stress periods, older and younger teachers had similar levels of reactivity, but only younger teachers had levels that were lower during the less stressful periods of their workday, indicating some degree of recovery from these daily stressors (Ritvanen et al., 2006). Our results are also consistent with studies that find greater cardiovascular reactivity with age in response to a psychological stressor (Ong, Rothstein, & Uchino, 2012; Wirtz et al., 2008), particularly among lonely older adults (Ong et al., 2010). Together, these studies suggest age-related declines in people’s ability to modulate their physiological response to negative emotional experiences, a conclusion supported by the current study.
Bedtime cortisol, NA, and age
The current study also revealed that the association between daily NA and bedtime cortisol is informed by taking into account the average NA people experience. By separating average NA from daily NA, we were able to separate the effect of NA experienced in any one given day from the effect of NA experienced in general (for discussion, see Hoffman & Stawski, 2009). Using this methodology, the current study revealed that examining NA over multiple days may be more informative in elucidating the association between affect and cortisol than examining NA across the course of one day only.
Our findings indicate that on days people experience low to moderate levels of daily NA, bedtime cortisol is highest among older adults who, on average, experience high levels of average NA. This finding supports SAVI’s tenet that sustained physiological arousal from NA becomes more costly with increasing age (Charles, 2010). It is also consistent with studies showing that bedtime cortisol is elevated among people faced with adverse situations, such as financial strain (Grossi, et al., 2001), low socioeconomic status (e.g., Cohen et al., 2006), and high levels of perceived stress (Powell et al., 2002).
In terms of reactivity, only older adults who reported low average NA showed a significant change in their bedtime cortisol from a low NA day to a high NA day. At first glimpse, this result appears to counter SAVI’s tenet that living under conditions of sustained NA makes increased emotional arousal more difficult to modulate with increasing age. However, this interpretation does not explain why older adults with high average NA have consistently high levels of cortisol regardless of their daily NA. Findings from the current study can also be interpreted as the result of high levels of sustained NA over time reducing physiological flexibility--that is, the inability to respond to additional negative stimuli. Indeed, the oldest adults in the sample with high average NA are no longer benefiting from a day that is lower in NA than usual – they are always responding as if their day is high in NA. Perhaps, ongoing high levels of NA may have altered the set-point of cortisol for these older adults. This explanation is similar to suggestions made by Hawkley and Cacioppo (2007), who emphasized the problem of accumulated changes caused by psychological stressors. They concluded that experiencing such adverse states such as chronic feelings of loneliness may actually accelerate the aging process in later life.
AUCg versus bedtime cortisol
For both bedtime cortisol and AUCg, significant associations emerged for average NA and age. However, not all of our findings were consistent across the two aspects of cortisol assessed. For example, although a three-way interaction emerged for bedtime cortisol, no similar interaction emerged for AUCg. Moreover, at the main effects level, NA was associated with bedtime cortisol, but not with AUCg. We believe that these disparate findings are due to AUCg being highly influenced by morning baseline level and the morning rise--two important components of the diurnal cycle that occur prior to the experience of the daily reported negative emotions. Bedtime cortisol, in contrast, may be a more sensitive marker of NA experienced that day, as it may be more highly driven by the accumulation of NA across the day than the measure of AUCg. Indeed, in their study examining the association between socioeconomic status (SES) and cortisol, Cohen and colleagues found that lower SES was associated with a flatter diurnal decline, as a result of a higher evening nadir, and concluded that bedtime cortisol may be particularly sensitive to stress exposure (Cohen et al., 2006). Future research may want to further explicate the situations and emotions that drive various aspects of the diurnal cycle and determine whether age may play a role in these associations.
Arousal versus valence
SAVI focuses on the benefits of avoiding NA and the costs of exposure to negative emotions. However, other researchers have found that higher levels of affective arousal—regardless of valence—may have negative consequences for older adults. For example, high arousal adversely affects the cognitive performance of older adults relative to younger adults regardless of valence (Wurm, Labouvie-Vief, Aycock, Rebucal, & Koch, 2004). In the health literature, the presence of high levels of PA has also been associated with health outcomes. Indeed, other researchers have examined the importance of PA when examining cortisol levels and overall physical health (e.g., Pressman & Cohen, 2006). To determine whether PA is protective, or if high levels of exposure to any emotional state—regardless of valence—is positively associated with cortisol, we conducted an additional analysis with PA as the main variable predicting bedtime cortisol. If it is any emotional state that influences levels of cortisol, our analyses examining PA as the main predictor variable should have emulated those found with NA—that is, higher levels of any affective state should have been associated with elevated cortisol among successively older adults. Instead, we found the opposite pattern—that higher levels of PA are associated with lower levels of bedtime cortisol among successively older adults. This indicates that it is NA, as opposed to PA that is associated with higher cortisol levels in older adults.
Future research
SAVI posits that in response to affective distress, older adults will have a more difficult time modulating their arousal than will their younger counterparts. The current study examined a very narrow view of physiological arousal, focusing solely on the hormone cortisol. This is an important biomarker to examine, as it is strongly related to health outcomes. For example, consistently high levels of basal cortisol are associated with memory impairment (Sapolsky, 1996), progression of chronic disease, and a diminished immune system response (Lovallo & Thomas, 2000; McEwen, 1998). Additionally, a higher end-of-day nadir is associated with loss of bone mineral density (Raff, et al., 1999), increased frailty (Varadhan et al., 2008), and memory impairments (Seeman, McEwen, Singer, Albert & Rowe, 1997). When NA is taken into account, higher daily cortisol levels are also associated with an increased likelihood of reporting physical symptoms over a two-year period (Wrosch, Miller, Lupien, & Pruessner, 2008). As these studies attest, understanding conditions that elicit cortisol is an important research topic. Because physiological arousal incorporates more than one biological system, however, future work should take a multi-system approach to testing SAVI, which would allow for further refinement of the theory.
Limitations
There are some limitations to the current study. First, because saliva collection time was indicated through self-report, there was no objective verification of actual collection time. Future research may want to use additional objective verification of recording time—such as smart caps and actigraphs—to ensure that samples are collected at specified times (for discussion, see Almeida, Piazza, & Stawski, 2009).
An additional limitation was not including participants younger than age 33. Although the current study had an impressive age span, from 33 to 84, the addition of younger participants would have allowed us to examine the association between NA and cortisol among adults of all ages. Given the physiological flexibility associated with youth, we believe our results would have remained unchanged, but we cannot say for certain because our hypotheses were not tested in this age group. This is a particularly important research endeavor, as the literature indicates that the highest levels of NA are reported in early adulthood, which is also the time when rates of many mental illnesses peak (for review, see Piazza & Charles, 2006). In addition, we make assumptions as to why we see no changes associated with NA among the youngest participants in the sample, but further studies will need to examine the exact mechanisms responsible for our pattern of findings. Finally, our conclusions are based upon cross-sectional data, and it is necessary to confirm our findings using longitudinal data.
Conclusion
Despite the limitations of the current study, the strengths in our design include its ability to distinguish both between- and within-person differences in a large national sample spanning 50 years of adulthood. Our results indicate that the cost of adverse daily affective states increases with age. Health researchers have attempted to describe how psychological experiences influence physiological health. The current study indicates that the association between these two components of well-being may vary both by age and by the negative affective experiences people report.
Table 2.
Age, NA and Bedtime Cortisol
| Fixed Effects | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
|
| ||||
| Estimate (SE) | Estimate(SE) | Estimate(SE) | Estimate (SE) | |
| Intercept | −1.163 (.374)** | −1.147 (.374)** | −1.141 (.374)** | −1.118 (.374)** |
| Sample Time (WP) | .096 (.164)*** | .095 (.016)*** | .095 (.016)*** | .094 (.016)*** |
| Sample Time (BP) | −.082 (.025)*** | −.082 (.025)*** | −.082 (.025)*** | −.081 (.025)*** |
| Day | .003 (.009) | .003 (.009) | .004 (.009) | .004 (.009) |
| Age | .017 (.002)*** | .018 (.002)*** | .018 (.002)*** | .018 (.002)*** |
| Sex (1 = Male) | −.049 (.044) | −.050 (.044) | −.051 (.044) | −.051 (.044) |
| Race (1 = White) | .355 (.064)*** | .354 (.064)*** | .354 (.064)*** | .354 (.064)*** |
| Education | −.013 (.019) | −.015 (.019) | −.015 (.018) | −.015 (.019) |
| Chronic illnesses | .006 (.012) | .005 (.012) | .005 (.012) | .005 (.012) |
| Smoking status (1 = Yes) | −.337 (.070)*** | −.342 (.070)*** | −.342 (.070)*** | −.342 (.070)*** |
| Medication Use (1 = Yes) | .025 (.046) | .025 (.046) | .025 (.046) | .025 (.046) |
| Negative Affect (WP) | .135 (.060)* | .149 (.061)* | .165 (.073)* | .175 (.072)* |
| Negative Affect (BP) | .345 (.102)*** | .399 (.105)*** | .398 (.105)*** | .386 (.105)*** |
| Age x Negative Affect (WP) | .007 (.005) | .006 (.005) | .012 (.006)* | |
| Age x Negative Affect (BP) | .017 (.008)* | .017 (.008)* | .016 (.008) | |
| NA (WP) x NA (BP) | −.072 (.185) | .012 (.006) | ||
| Age x NA (WP) x NA (BP) | −.035 (.017)* | |||
| Random Effects | ||||
| Intercept | .491 (.025)*** | .489 (.025)*** | .489 (.025)*** | .489 (.025)*** |
| Negative Affect (WP) | .128 (.084) | .135 (.085) | .132 (.085) | .104 (.081) |
| Covariance | −.014 (.049) | −.009 (.049) | −.011 (.049) | −.021 (.048) |
| Residual | .519 (.013)*** | .518 (.013)*** | .519 (.013)*** | .519 (.013)*** |
| Model Fit | ||||
| −2 Log Likelihood | 13338.1 | 13250.6 | 13250.5 | 13246.4 |
Note:
p<.05,
p<.01,
p<.001.
WP = Within-Person, BP = Between-Person
Acknowledgments
This work was supported by National Institute on Aging Grants awarded to David M. Almeida (P01AG020166 and R01AG019239).
Contributor Information
Jennifer R. Piazza, Department of Human Development and Family Studies, Pennsylvania State University, University Park
Susan T. Charles, Department of Psychology and Social Behavior, University of California, Irvine
Robert S. Stawski, Institute for Social Research, University of Michigan
David M. Almeida, Department of Human Development and Family Studies, Pennsylvania State University, University Park
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski R. Inter-individual differences and intra-individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Deeg DJH, Beekman ATF, Penninx BWJH, Lips P, Hoogendijk WJG. Major depression in late life is associated with both hypo-and hypercortisolemia. Biological Psychiatry. 2007;62:479–486. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. The Journal of Pathology. 2007;211:179–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Charles ST. Strength and Vulnerability Integration (SAVI): A Model of Emotional Well-Being in Later Adulthood. Psychological Bulletin. 2010;136:1068–1091. doi: 10.1037/a0021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Carstensen LL. Emotion regulation and aging. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 307–320. [Google Scholar]
- Charles ST, Piazza JR. Age differences in affective well-being: Context matters. Social and Personality Psychology Compass. 2009;3:711–734. doi: 10.1111/j.1751-9004.2009.00202.x. [DOI] [Google Scholar]
- Cohen S, Pressman SD. Positive affect and health. Current Directions in Psychological Science. 2006;15:122–125. doi: 10.1037/0033-2909.131.6.925. [DOI] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Consedine NS, Magai C. Emotion development in adulthood: A developmental functionalist review and critique. In: Hoare C, editor. The Oxford Handbook of Adult Development and Learning. New York: Oxford University Press; 2006. pp. 209–244. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Epel ES, Burke HM, Wolkowitz OM. The psychoneuroendocrinology of aging: Anabolic and catabolic hormones. In: Aldwin CM, Park CL, Spiro AI, editors. Handbook of health psychology and aging. New York, NY: Guilford Press; 2009. pp. 119–141. [Google Scholar]
- Evans P, Forte D, Jacobs C, Fredhoi C, Aitchison E, Hucklebridge F, Clow A. Cortisol secretory activity in older people in relation to positive and negative well-being. Psychoneuroendocrinology. 2007;32:322–930. doi: 10.1016/j.psyneuen.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Grossi G, Perski A, Lundberg U, Soares J. Associations between financial strain and the diurnal salivary cortisol secretion of long-term unemployed individuals. Integrative Physiological and Behavioral Science. 2001;36:205–219. doi: 10.1007/BF02734094. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Herzig N, Holsboer-Trachsler E. In healthy young and elderly adults, hypothalamic-pituitary-adrenocortical axis reactivity (HPA AR) varies with increasing pharmacological challenge and with age, but not with gender. Journal of Psychiatric Research. 2011;45:1373–1380. doi: 10.1016/j.jpsychires.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Aging and loneliness: Downhill quickly? Current Directions in Psychological Science. 2007;16:187–191. doi: 10.1111/j.1467-8721.2007.00501.x. [DOI] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. doi: 10.1146/annurev.psych.093008.100356. [DOI] [Google Scholar]
- Ice GH. Factors influencing cortisol level and slope among community dwelling older adults in Minnesota. Journal of Cross-Cultural Gerontology. 2006;20:91–108. doi: 10.1007/s10823-005-9085-5. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe L, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in nonspecific psychological distress. Psychological Medicine. 2002;32:959–976. doi: 10.1017/S0033291702006074. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: Back to the future. Psychosomatic Medicine. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. doi: 0033-3174/02/6401-0015. [DOI] [PubMed] [Google Scholar]
- Kudlieka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Thomas TL. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. New York: Cambridge University Press; 2000. pp. 342–367. [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037/0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–611. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Ong AD, Rothstein JD, Uchino BN. Loneliness accentuates age differences in cardiovascular responses to social evaluative threat. Psychology and Aging. 2012;27:190–198. doi: 10.1037/a0025570. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. Journals of Gerontology: Psychological Sciences. 2010;65:513–252. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza JR, Charles ST. Mental health of the Baby Boomers. In: Krauss-Whitbourne S, Willis S, editors. The Baby Boomers grow up: Contemporary perspectives on midlife. Hillsdale, NJ: Erlbaum; 2006. pp. 111–146. [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Powell LH, Lovallo WR, Matthews KA, Meyer P, Midgley AR, Baum A, et al. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosomatic Medicine. 2002;64:502–509. doi: 10.1097/00006842-200205000-00015. doi: 0033-3174/02/6403-0502. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2006;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for the computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radenbusch SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Raff H, Raff EH, Duthie EH, Wilson CR, Sasse EA, Rudman I, Mattson D. Elevated salivary cortisol in the evening in healthy elderly men and women: Correlation with bone mineral density. Journal of Gerontology: Medical Sciences. 1999;54:479–483. doi: 10.1093/gerona/54.9.M479. [DOI] [PubMed] [Google Scholar]
- Ritvanen T, Louhevaara V, Helin P, Väisänen S, Hänninen O. Responses of the autonomic nervous system during periods of perceived high and low work stress in younger and older female teachers. Applied Ergonomics. 2006;37:311–318. doi: 10.1016/j.apergo.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT software: Changes and enhancements through release 6.12. Cary, NC: SAS Institute Inc; 2001. [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82:2458–2465. doi: 10.1210/jc.82.8.2458. [DOI] [PubMed] [Google Scholar]
- Simpson EEA, McConville C, Rae G, O’Connor JM, Stewart-Knox BJ, Coudray C, Strain JJ. Salivary cortisol, stress and mood in healthy older adults: The Zenith study. Biological Psychology. 2008;78:1–9. doi: 10.1016/j.biopsycho.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/S0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Snidjers TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London, England: Sage; 1999. [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. The Journal of Clinical Endocrinology & Metabolism. 1996;81(7):2468–2473. doi: 10.1210/jc.81.7.2468. [DOI] [PubMed] [Google Scholar]
- van Eck MM, Berkhof H, Nicolson N, Sulon J. The effects of perceive distress, traits, mood states, and stressful daily events on salivary cortisol levels. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. Retrieved from http://www.psychosomaticmedicine.org/content/58/5/447.long. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190. Retrieved from http://biomedgerontology.oxfordjournals.org.ezaccess.libraries.psu.edu/content/63/2/190.full. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1998;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, Redwine LS, Baertschi C, Spillmann M, Ehert U, von Kanel R. Coagulation activity before and after acute psychosocial stress increases with age. Psychosomatic Medicine. 2008;70:476–481. doi: 10.1097/PSY.0b013e31816e03a5. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Lupien S, Pruessner JC. Diurnal cortisol secretion and 2-year changes in older adults’ acute physical symptoms: The moderating roles of negative affect and sleep. Health Psychology. 2008;27:685–693. doi: 10.1037/0278-6133.27.6.685. [DOI] [PubMed] [Google Scholar]
- Wurm LH, Labouvie-Vief G, Aycock J, Rebucal KA, Koch HE. Performance in auditory and visual emotional stroop tasks: A comparison of older and younger adults. Psychology and Aging. 2004;19:523–535. doi: 10.1037/0882-7974.19.3.523. [DOI] [PubMed] [Google Scholar]
- Yen SS, Laughlin GA. Aging and the adrenal cortex. Experimental Gerontology. 1998;33:897–910. doi: 10.1016/S0531-5565(98)00046-1. [DOI] [PubMed] [Google Scholar]