Abstract
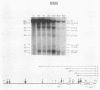
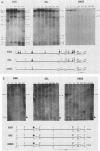
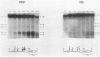
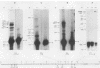
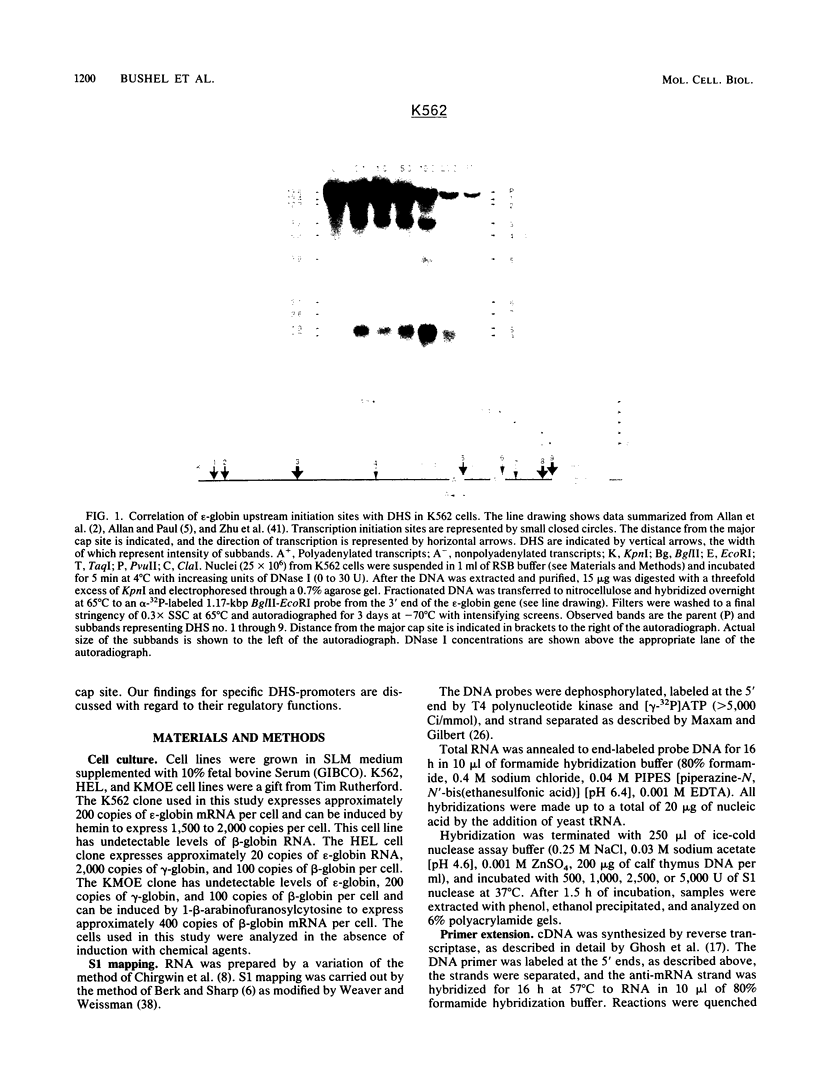
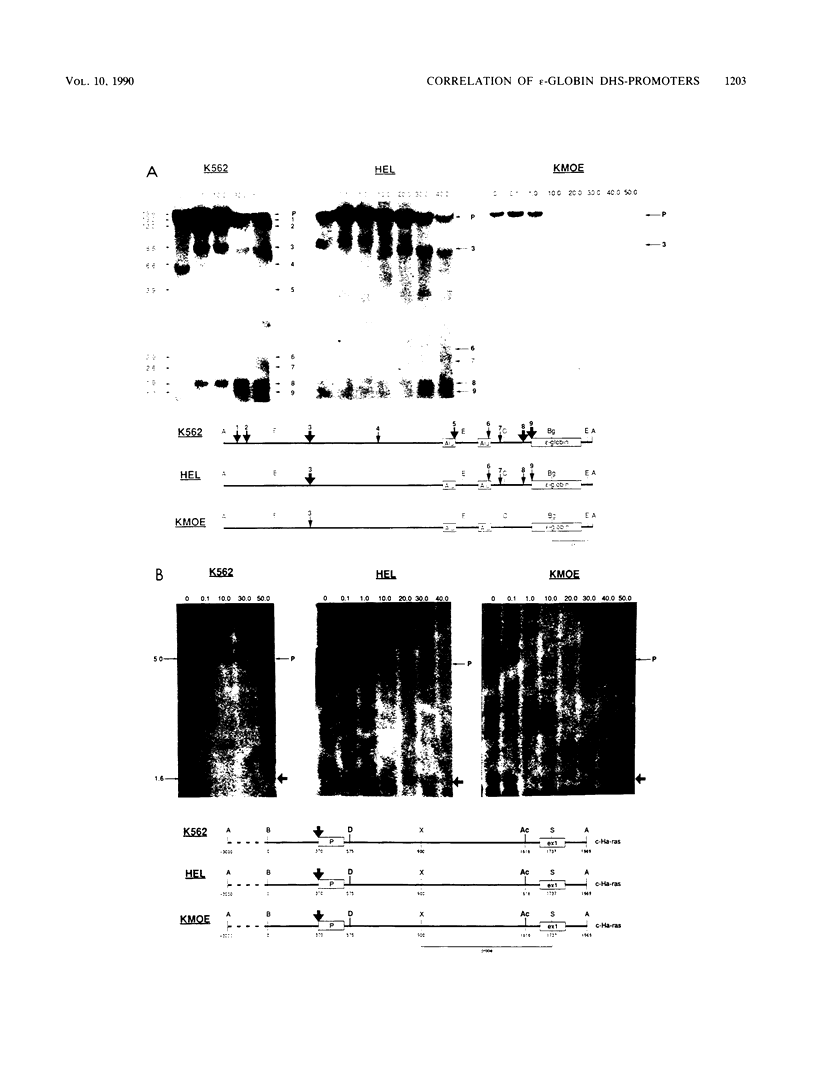
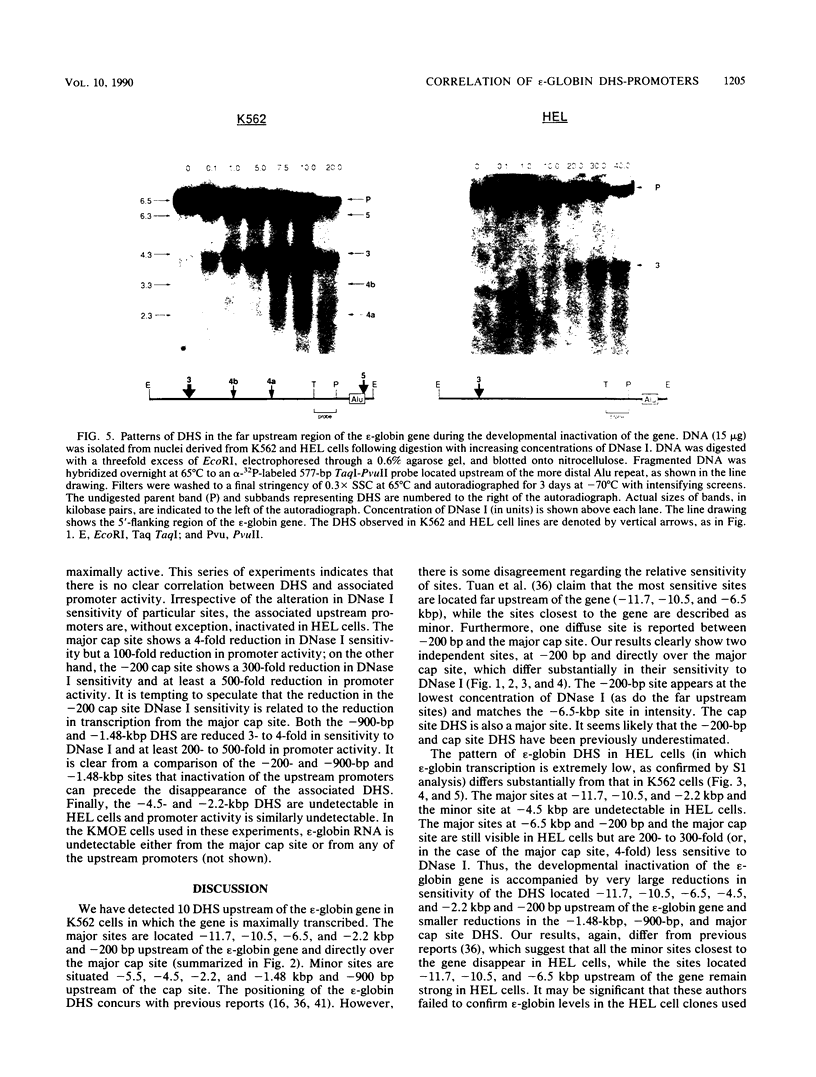
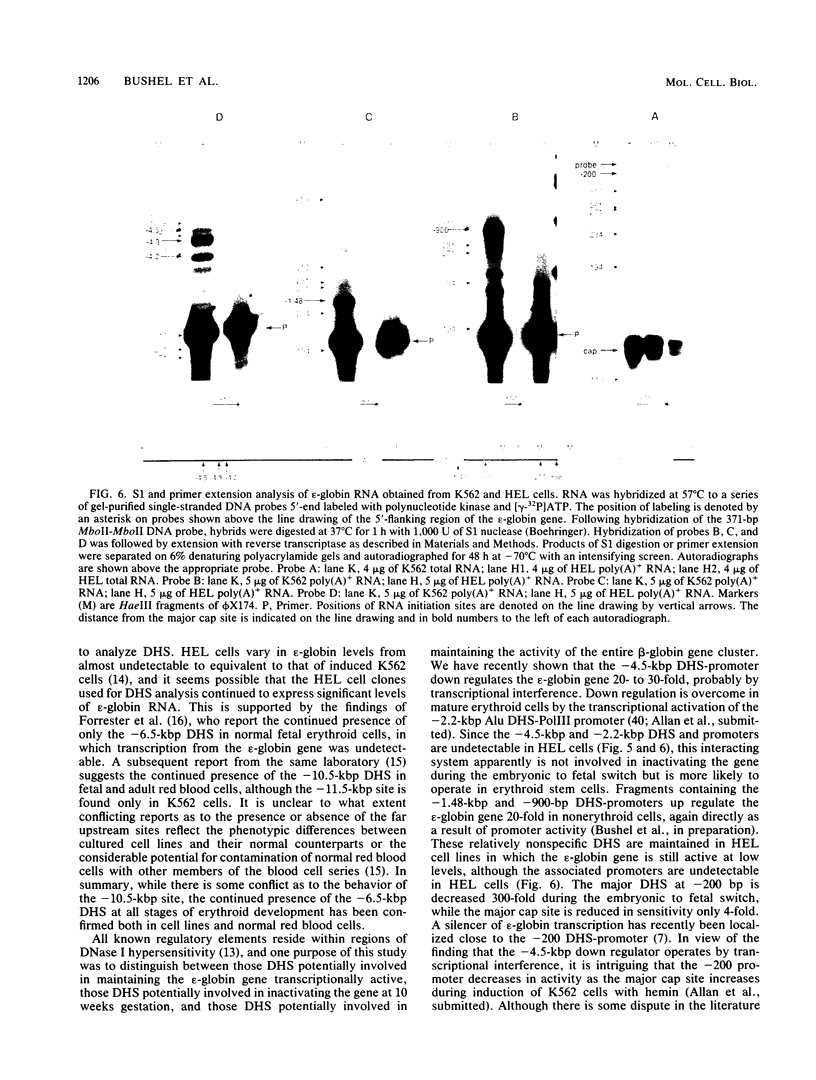
DNA 5' to the human epsilon-globin gene exhibits unique patterns of DNase I-hypersensitive sites (DHS) in three human erythroleukemic cell lines which represent the embryonic (K562), fetal (HEL), and adult (KMOE) stages of erythroid development. We have mapped 10 epsilon-globin DHS in K562 cells, in which the epsilon-globin gene is maximally active. Major sites are located -11.7, -10.5, -6.5, -2.2 kilobase pairs (kbp) and -200 base pairs (bp) upstream of the gene and directly over the major cap site. Minor sites are located -5.5, -4.5, and -1.48 kbp and -900 bp upstream of the cap site. In HEL cells, in which the epsilon-globin gene is expressed at extremely low levels, the -11.7-, -10.5-, -5.5-, -4.5-, and -2.2-kbp DHS are no longer detectable; the -200-bp site is approximately 300-fold less sensitive to DNase I; and the -1.48-kbp, -900-bp, and major cap site DHS are 3- to 4-fold less sensitive. Only the DHS located -6.5 kbp relative to the major cap site is detectable at all three stages of erythroid development, including KMOE cells in which epsilon-globin synthesis is undetectable. We suggest that this site may be implicated in maintaining the entire beta-globin cluster in an active chromatin conformation. The five DHS downstream of the -6.5-kbp element possess associated promoters. Thus two distinct types of DHS exist--promoter positive and promoter negative. In HEL cells, all the upstream promoters are inactivated, although the -1.48-kbp and -900- and -200-bp DHS are still present. This suggests that the maintenance of DHS and regulation of their associated promoters occur by independent mechanisms. The inactivation of the upstream promoters in HEL cells while the major cap site remains active represents a unique pattern of expression and suggests that HEL cells possess regulatory factors which specifically down regulate the epsilon-globin upstream promoters.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alan M., Grindlay G. J., Stefani L., Paul J. Epsilon globin gene transcripts originating upstream of the mRNA cap site in K562 cells and normal human embryos. Nucleic Acids Res. 1982 Sep 11;10(17):5133–5147. doi: 10.1093/nar/10.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Allan M., Montague P., Grindlay G. J., Sibbet G., Donovan-Peluso M., Bank A., Paul J. Tissue specific transcription of the human epsilon-globin gene following transfection into the embryonic erythroid cell line K562. Nucleic Acids Res. 1985 Sep 11;13(17):6125–6136. doi: 10.1093/nar/13.17.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Paul J. Transcription in vivo of an Alu family member upstream from the human epsilon-globin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1193–1200. doi: 10.1093/nar/12.2.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Zhu J. D., Montague P., Paul J. Differential response of multiple epsilon-globin cap sites to cis- and trans-acting controls. Cell. 1984 Sep;38(2):399–407. doi: 10.1016/0092-8674(84)90495-1. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Cowie A., Tyndall C., Kamen R. Sequences at the capped 5'-ends of polyoma virus late region mRNAs: an example of extreme terminal heterogeneity. Nucleic Acids Res. 1981 Dec 11;9(23):6305–6322. doi: 10.1093/nar/9.23.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Di Segni G., Carrara G., Tocchini-Valentini G. R., Shoulders C. C., Baralle F. E. Selective in vitro transcription of one of the two Alu family repeats present in the 5' flanking region of the human epsilon-globin gene. Nucleic Acids Res. 1981 Dec 21;9(24):6709–6722. doi: 10.1093/nar/9.24.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. Anatomy of hypersensitive sites. Nature. 1984 May 17;309(5965):213–214. doi: 10.1038/309213a0. [DOI] [PubMed] [Google Scholar]
- Enver T., Zhang J. W., Anagnou N. P., Stamatoyannopoulos G., Papayannopoulou T. Developmental programs of human erythroleukemia cells: globin gene expression and methylation. Mol Cell Biol. 1988 Nov;8(11):4917–4926. doi: 10.1128/mcb.8.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Grindlay G. J., Lanyon W. G., Allan M., Paul J. Alternative sites of transcription initiation upstream of the canonical cap site in human gamma-globin and beta-globin genes. Nucleic Acids Res. 1984 Feb 24;12(4):1811–1820. doi: 10.1093/nar/12.4.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hess J., Perez-Stable C., Deisseroth A., Shen C. K. Characterization of an unique RNA initiated immediately upstream from human alpha 1 globin gene in vivo and in vitro: polymerase II-dependence, tissue specificity, and subcellular location. Nucleic Acids Res. 1985 Sep 11;13(17):6059–6074. doi: 10.1093/nar/13.17.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M., Yagawa K., Nakamura K., Okano H. Synthesis of adult-type hemoglobin in human erythremia cell line. Blood. 1984 Jul;64(1):314–317. [PubMed] [Google Scholar]
- Lachman H. M., Mears J. G. DNase I hypersensitivity in the gamma globin gene locus of K562 cells. Nucleic Acids Res. 1983 Sep 10;11(17):6065–6077. doi: 10.1093/nar/11.17.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes N. F., Paul J., Wu J., Allan M. c-Ha-ras gene bidirectional promoter expressed in vitro: location and regulation. Mol Cell Biol. 1989 Sep;9(9):3758–3770. doi: 10.1128/mcb.9.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Berk A. J. Far upstream initiation sites for adenovirus early region 1A transcription are utilized after the onset of viral DNA replication. J Virol. 1983 Feb;45(2):594–599. doi: 10.1128/jvi.45.2.594-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Structure of a nuclease-sensitive region inside the immunoglobin kappa gene: evidence for a role in gene regulation. Nucleic Acids Res. 1983 Jul 25;11(14):4775–4792. doi: 10.1093/nar/11.14.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Allan M., Paul J. The chromatin structure of the human epsilon globin gene: nuclease hypersensitive sites correlate with multiple initiation sites of transcription. Nucleic Acids Res. 1984 Dec 11;12(23):9191–9204. doi: 10.1093/nar/12.23.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]