Abstract
Pigmented villonodular synovitis (PVNS) is a rare, benign proliferative disease of the synovial tissue that affects a single joint or a tendon sheath. Data from the literature present only a few cases of multifocal PVNS. This paper presents multifocal PVNS in the adult. This disease can affect bilateral shoulders, hips and knees. The diagnosis may be delayed by the slow evolution of the disease (up to ten years); some patients may be seen with late-stage degenerative joints, serious complications, painful and functionally uncompensated, with significant locomotion deficit. PVNS requires a radical treatment with prosthetic arthroplasty associated with synovectomy. Complex imaging (X-Rays, magnetic resonance imaging (MRI), ultrasound) and macroscopic appearance of the lesions during surgery confirms the clinical diagnosis of multifocal PVNS with secondary bone lesions. Histology marks the final diagnosis of multifocal PVNS. The postoperative results are good, with recovery in functional parameters of the joints with endoprosthesis.
Introduction
Villonodular synovitis is a rare disease of the synovial tissue that affects a single joint or a tendon sheath. Villonodular synovitis of the tendon sheath was first described by Chassaignac in 1852 [1]. In 1865, Simon described the intra-articular nodular form of the disease, while in 1909, Mosser described the diffuse form [2], using a different terminology. Nevertheless, it was Jaffe that in 1941 reunited all the forms of the disease under the same name—pigmented villonodular synovitis (PVNS)—and classified it into diffuse and focal (nodular), articular and extra-articular [3]. The annual global incidence of this pathology was evaluated in 1980 by Myers [4] as 1.8 new cases per one million people. It seems to be more likely to occur during the fourth decade of life, but cases have been documented in children (under ten years) and elderly (over 70 years). Most studies show equal occurrence in males and females. The localization of the disease varies, with the knee being the most commonly affected joint, followed by the hip, elbow, shoulder, fingers, toes and tarsal joints. The hip is involved in 15 % of the cases, and this seems to be the reason why very few studies have been published about PVNS of the hip.
Clinical expression
PVNS is generally a monoarticular disease; Nilsonne and Moberger declared in 1969 that “the lesion is always monoarticular” [5]. Only few cases have been described to involve multifocal localizations in children and adults. The first report was made in 1949 by Kelikian and Lewis, and described three members of the same family having knees affected by the disease. However, they did not mention whether the diagnosis was made histologically, or merely by arthrography. Greenfield and Wallace reported a case of bilateral knee involvement that is the first description of the diffuse form [6], while Byers describes a case of bilateral ankle disease [1]. In 1969, Gehweiler and Wilson showed the presence of multifocal villonodular synovitis in two patients, aged 56 and 60 years, involving both knees and respectively, knee and hip [6]. Other cases of the polyarticular form of the disease were reported, the most frequent being the symmetrical bifocal form affecting the knee, hip, shoulder, wrists, ankle and foot [7–17]. In 2006, Mukkopadhyay presented a case that he had been following for the past 25 years. The first symptoms occurred when the patient was five years old, and —both knees were affected; later on, when aged 22, the patient presented PVNS in the knee, ankle, hip, both elbows, right shoulder and wrists [18]. The first case of poliarticular PVNS in children was published in 1975 by Leszczynski et al., and it presented a seven year-old girl with PVNS involving the right knee and both ankles [19]. Other authors presenting pediatric forms were Walls and Nogi [20], Martin et al. [21], Pavlica et al. [22], and Kay et al. [23]. Vedantam et al. described in 1998 a PVNS patient with congenital abnormalities of the genitourinary tract [24]. Chandan et al. [25], and Tavangar [26] described similar cases in five year-old girls. Clinical manifestation of the disease are not specific, and are mostly dominated by episodes of mechanical pain, swollenness, limitation of mobility and sometimes articular blockage, depending on the joint affected by the condition [27]. When the hip is affected, the clinical signs are more likely to be related to the degradation of the joint than to the proliferation of synovial membrane, whereas the destructive articular lesions are well tolerated for a long period of time. This slow evolution, in most cases, explains the late diagnosis; usually there are five to ten years between the beginning of the disease and the treatment. Some patients may be diagnosed with PVNS and treated ten years after the first symptoms, and only in the presence of secondary destructive articular lesions.
Radiology and biology
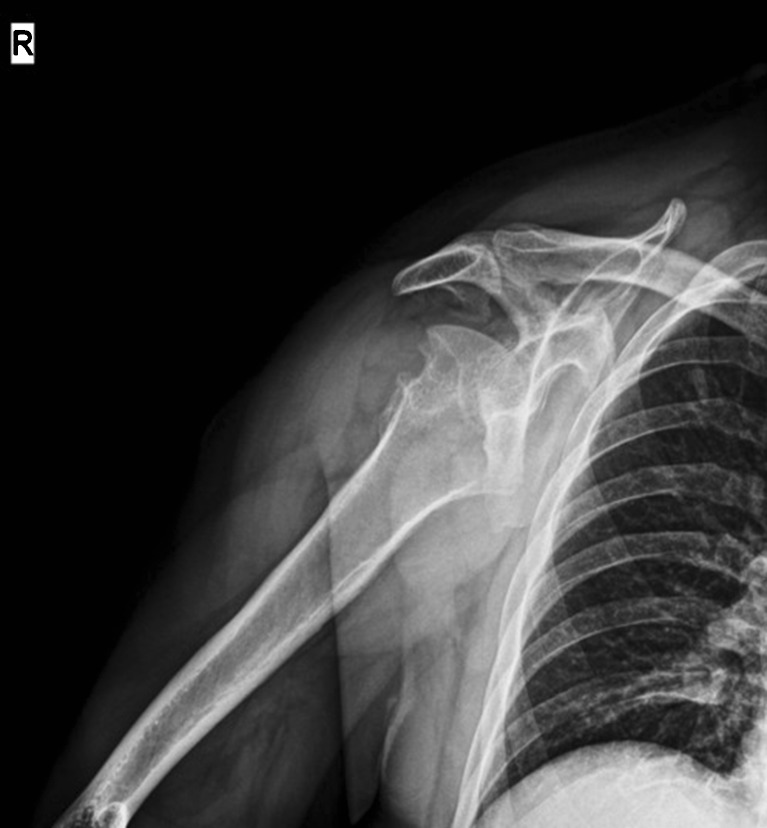
Clinical examination of the PVMS is followed and completed by plain X-Rays of the affected joints. A shoulder case revealed large erosions of the posterior and superior aspect of the humeral head with cortical erosion, head destruction and large cephalic geodes, as well as old glenoid erosions with narrowed articular space (Fig. 1). Radiography imaging of the opposite shoulder revealed the same changes, although more discrete and without the articular interline being affected. The radiography of a hip revealed “mirror” pseudocystic images of the neck and acetabulum outside the bearing area and narrowed articular space (Fig. 2). Radiological examination can be normal or it can reveal intra-articular effusion, periarticular soft tissues more opaque than those around because of hemosiderin deposits, and juxta-articular bone erosion. The hip radiography can be rather suggestive when it shows pseudocystic formations on the acetabulum and femoral neck, particular, because they develop outside the support area (as compared to such conditions as coxarthrosis and coxitis), and suggest the proliferation of the synovial membrane into areas of low articular pressure and areas of capsular ligaments inserts [10, 32]. The radiography of a knee revealed pseudocystic intercondylar imagines.
Fig. 1.
Radiography of the shoulder, showing large cortical erosions and deformity, large cephalic geodes, glenoid erosion, narrowed articular space
Fig. 2.
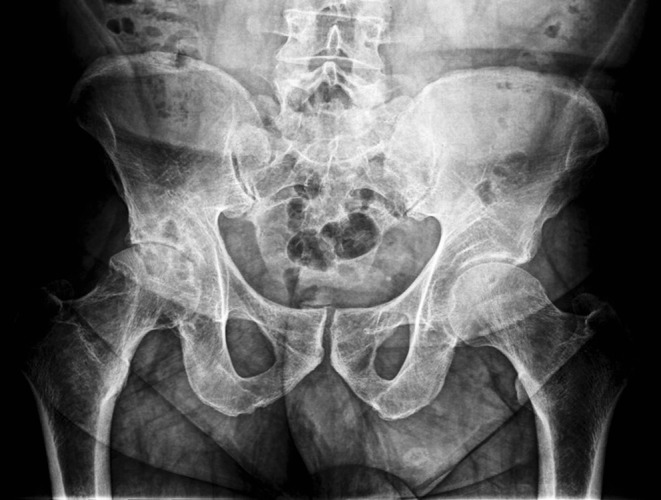
Radiography of the pelvis, showing pseudocystic images on the acetabulum and right femoral neck outside the bearing area, narrowed articular space
An ultrasound of the shoulder may reveal the involvement of the long head of the biceps, with severe tenosynovitis and the presence of a massive collection in the subdeltoid bursa.
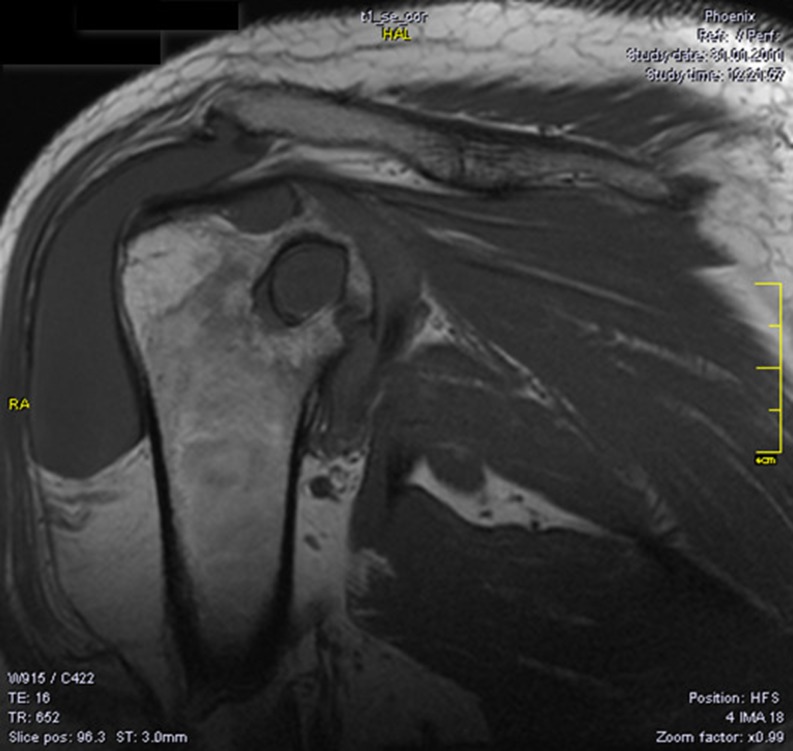
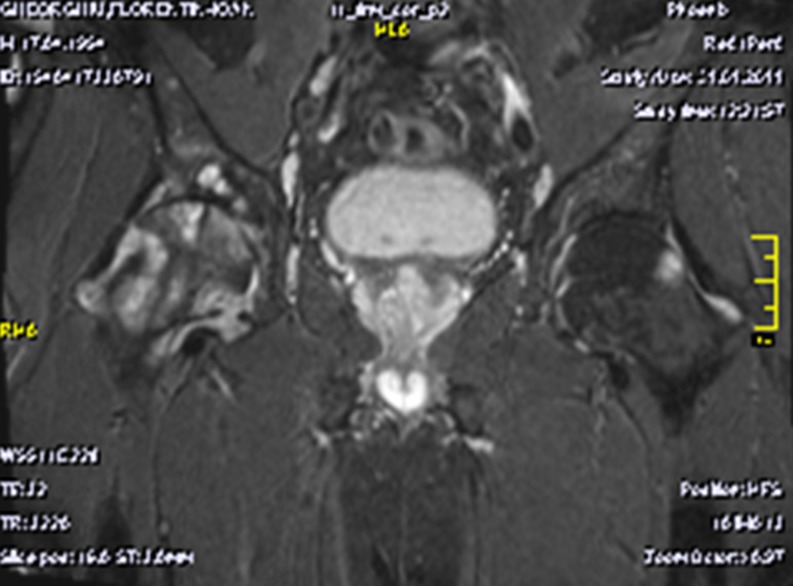
Nowadays, magnetic resonance imaging (MRI) examination is essential in the PVNS diagnosis. The investigation shows images of articular effusion, and the hyperplasia of the synovial membrane can include nodular images with weak T1 and T2 signal because of the hemosiderin deposits (also responsible for the microscopic image of the synovial membrane with ochre-brownish appearance). The MRI may reveal significant inflammatory changes with destructive potential at the level of the concerned joints: marked hypertrophy of the synovial and diffuse oedematous changes in the humeral head and neck and in the glenoid, small marginal erosions and large subchondral cysts on both articular surfaces, larger on the upper part of the humeral head, and thick fluid accumulation in the subacromial-subdeltoid bursa (Fig. 3). The hip MRI could reveal diffuse oedematous changes on the femoral head and neck and on the acetabulum, as well as small marginal erosions and large subchondral cysts on both articular surfaces, larger on the femoral head and neck, and hypertrophy of the articular synovitis with T2 hypersignal (Fig. 4).
Fig. 3.
Magnetic resonance imaging (MRI) of the shoulder demonstrates synovial hypertrophy, diffuse oedema of the humeral head and of the glenoid, small marginal erosions and large subchondral cysts on both articular surfaces, larger on the superior part of the humeral head (22 mm), effusion in the subacromial bursa
Fig. 4.
Magnetic resonance imaging (MRI) of the pelvis, showing oedema on the right femoral head and neck, and on the acetabulum; small marginal erosions and large subchondral cysts on both articular surfaces, hypertrophy of the synovia with T2 hyper signal on the left, as well as small marginal erosions with subchondral cysts on the femoral head and acetabulum
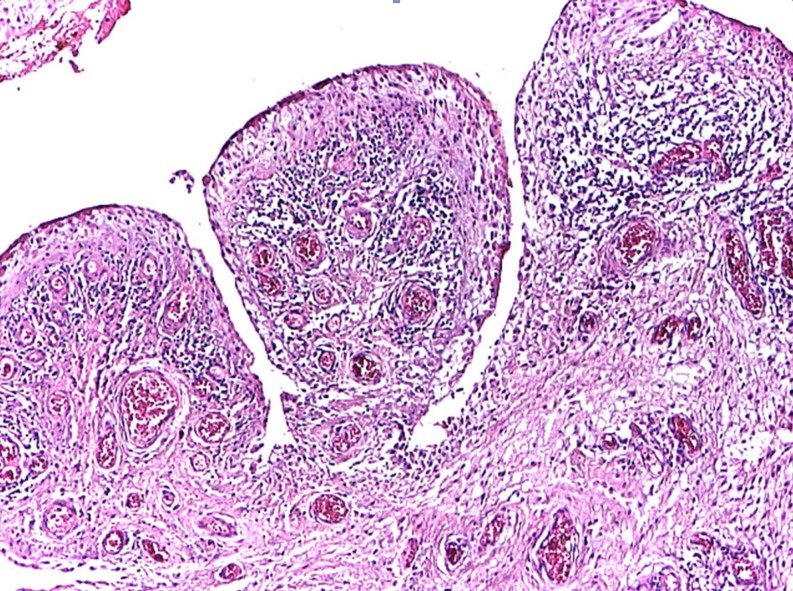
The exact diagnosis can be made by anatomo-pathological examination. Macro examination shows dark nodular lesions associated with hemorrhagic effusion. Microscopic examination reveals the matrix made of proliferated synovial cells, macrophages loaded with hemosiderin, histiocytes, multinuclear giant cells similar to osteoclasts, plasmatic cells, and xantomatous cells. [1]. The histopathological examination also shows bone fragments with chronic lymphocytic inflammation within the superficial osteomedular surfaces associating osteoclasts and sclerohyalinosis; synovial fragments with villi hypertrophy and synoviocytes hyperplasia with ulcerous site; granular tissue covered by fibrinous exudate; chronic follicular inflammation; frequent plasmacytes, Russel bodies, marked congestion and fibrosis; synovial hypertrophy with villositary structure; congestion (dilated vessels) in the villi axis; chronic diffuse or follicular inflammation and oedema (Fig. 5).
Fig. 5.
Microscopic presentation: synovial hypertrophy with villositary structure
Pigmented villonodular synovitis is a benign proliferation of the synovial membrane affecting the tendon’s joint, bursa or sheath. The proliferation of synovial cells raised many opinions during the years, mainly concerning the aetiology of the disease. Many theories were formulated, yet only two (inflammatory and tumoral) remain under discussion. The presence of increased cellularity and the tendency to recurrence may also suggest neoplastic origin. Equally, the presence of infiltrations with macrophages, histiocytes and plasmatic cells raises the possibility of an inflammatory origin [3]. Several studies bring into discussion the importance of genetic factors in the occurrence of PVNS. Wendt et al. proved the presence of synovitis in members of the same family affected by a rare condition with dominant autosomal transmission [28]. The same idea appears in cases describing multifocal PVNS associated with congenital abnormalities. Other studies revealed the existence of trisomy 7 associated with PVNS, suggesting the neoplastic aspect of the proliferation [29] (trisomy 7 is associated with a large variety of neoplastic conditions [30, 31]). Also, c-erb B oncogenes codify the reception of the epidermal growth factor EGFR and the growth factor PDGF, which are found on chromosome 7; the increase activity of these growth factors may contribute to the inflammatory aspect of synovitis.
The proliferation of synovial membrane can occur diffusely or under the shape of nodules, hence the term villonodular. The most frequent form is one that is mixed [33]. The destruction of the articular cartilage and the peri-articular bone erosions are mediated by metalloproteinases (MMPs). The expression of MMPs seems to be stimulated by the local production of cytokines, especially the specific ones TNFα, IL-1, IL-6 that play an important part in stimulating the osteoclastic bone resorption. Giant cells, synovial cells and histiocytes secrete TNFα, IL-1, and IL-6, stimulating the formation and activation of osteoclasts, and thus causing bone destruction. TNFα and IL-1 stimulate the degradation of cartilaginous matrix, while MMP-9, produced by tumoral cells, directly participates in its destruction. MMP-9 produced by tumoral cells and native osteoclasts also participate in bone resorption.
The differential diagnosis includes: synovitis from recurrent hemorrhages, as in the first stage of hemophilia, degenerative pathology, specific and non-specific infectious arthritis, neoplastic pathology, and rheumatoid polyarthritis.
Therapeutic strategy varies depending on the case, is modulated by the evolution of the lesions, and has two main objectives: excision of pseudotumoral lesions (arthroscopic synovectomy or open arthrotomy) and preservation of the joint in the absence of secondary arthritic lesions. The efficiency of the treatment can be correlated with the quality of the synovectomy. Some studies claim that the possibility of recurrence after synovectomy usually varies between 0 % and 12 % [32], while others show 20 %–40 % [33]. In the case of PVNS of the hip, the literature presents a lower rate of recurrence in association with total arthroplasty, despite a more severe synovial proliferation due to the evolution of the condition. The preservation of the joint in the absence of an athritic degradation seems hypothetical. Arthritic degeneration is constant, influenced by the evolution of the arthritic lesion prior to the synovectomy, the arthritic character of all extensive arthrotomies and the delay of total arthroplasty. The recommended surgical treatment consists of total arthroplasties of the affected joints with articular destruction. Following surgery, the outcome is uneventful with excellent and complete recovery of the operated joints.
The purpose of this presentation is to describe the rarity of multiple location PVNS that included multifocal arthritic complications, the late start of the disease, and to encourage the prosthetic arthroplasty of large joints.
Acknowledgments
Conflict of interest
No funds were received in support of this study. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Byers PD, Cotton RE, Deacon UW. The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Am. 1968;50:290–305. [PubMed] [Google Scholar]
- 2.Smith JH, Pugh DG. Roentgenographic aspects of articular pigmented villonodular synovitis. AJR Am J Roentgenol. 1962;87:1146–1156. [PubMed] [Google Scholar]
- 3.Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis, and tenosynovitis. Arch Pathol. 1941;31:731–765. [Google Scholar]
- 4.Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59:2030–2036. doi: 10.1097/00005792-198005000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Nilsonne U, Moberger G. Pigmented villonodular synovitis of joints. Acta Orthop Scand. 1969;40:448–460. doi: 10.3109/17453676909046531. [DOI] [PubMed] [Google Scholar]
- 6.Gehweiler JA, Wilson JW. Diffuse biarticular pigmented villonodular synovitis. Radiology. 1969;93:845–850. doi: 10.1148/93.4.845. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg RL, Hedgcock MW. Bilateral pigmented villonodular synovitis of the hip. Br J Radiol. 1978;51:916–917. doi: 10.1259/0007-1285-51-611-916. [DOI] [PubMed] [Google Scholar]
- 8.Jamieson TW, Curran JJ, Desmet AA, Cotelingam JD, Kimmich H. Bilateral pigmented villonodular synovitis of the wrists. Orthop Rev. 1990;19:432–436. [PubMed] [Google Scholar]
- 9.Zuber M, Braun C, Pfreundschuh M, Piischel W. Ulnar deviation is not always rheumatoid. Ann Rheum Dis. 1996;55:786–788. doi: 10.1136/ard.55.11.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotten A, Flipo RM, Chastanet P, Desvigne-Noulet MC, Duquesnoy B, Delcambre B. Pigmented villonodular synovitis of the hip: review of radiographic features in 58 patients. Skeletal Radiol. 1995;24(1):1–6. doi: 10.1007/BF02425936. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Mahroof S, Pringle J, Short SC, Briggs TW, Cannon SR. Diffuse pigmented villonodular synovitis of the foot and ankle treated with surgery and radiotherapy. Int Orthop. 2005;29(6):403–405. doi: 10.1007/s00264-005-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh KH, Lim KS, Yoo JC. Arthroscopic treatment of pigmented villonodular synovitis involving bilateral shoulders. Orthopedics. 2010;33(6):442. doi: 10.3928/01477447-20100429-31. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Kwon JW, Ahn JH, Chang MJ, Cho EY. Localized tenosynovial giant cell tumor in both knee joints. Skeletal Radiol. 2010;39(9):923–926. doi: 10.1007/s00256-010-0910-8. [DOI] [PubMed] [Google Scholar]
- 14.Akinci O, Akalin Y, Kayali C. Results of total knee arthroplasty in patients with gonarthrosis resulting from the delayed diagnosis of PVNS: a case series. Eur J Orthop Surg Traumatol. 2012;22:51–56. doi: 10.1007/s00590-011-0784-z. [DOI] [Google Scholar]
- 15.Graf J, Bernd L, Pauschert R, Niethard FU. Rare occurrence of villonodular synovitis of both shoulder joints. Z Rheumatol. 1991;50(1):46–48. [PubMed] [Google Scholar]
- 16.Patkar D, Prasad S, Shah J, Patankar T, Kothari S. Pigmented villonodular synovitis: magnetic resonance features of an unusual case of bilateral hip joint involvement. Australas Radiol. 2000;44(4):458–459. doi: 10.1046/j.1440-1673.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Krause FG, Wroblewski JA, Younger AS. Pigmented villonodular synovitis in both hindfeet. Can J Surg. 2009;52(2):E38–E39. [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay K, Smith M, Hughes PM. Multifocal PVNS in a child—followed over 25 years. Skeletal Radiol. 2006;35:539–542. doi: 10.1007/s00256-005-0013-0. [DOI] [PubMed] [Google Scholar]
- 19.Leszczynski J, Huckell JR, Percy JS, LeRiche JC, Lentle BC. Pigmented villonodular synovitis in multiple joints. Ann Rheum Dis. 1975;34:269–272. doi: 10.1136/ard.34.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walls JP, Nogi J. Multifocal pigmented villonodular synovitis in a child. Casereport. J Pediatr Orthop. 1985;5:229–231. [PubMed] [Google Scholar]
- 21.Martin RC, II, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413–419. [PubMed] [Google Scholar]
- 22.Pavlica L, Nikolic D, Tadic J, Tatic V, Bralovic S, Panajotovic L. Pigmented villonodular synovitis—analysis of 50 patients. Vojnosanit Pregl. 1997;543:209–216. [PubMed] [Google Scholar]
- 23.Kay RM, Eckardt JJ, Mirra JM. Multifocal pigmented villonodular synovitis in a child: a case report. Clin Orthop Relat Res. 1996;322:194–197. doi: 10.1097/00003086-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Vedantam R, Strecker WB, Schoenecker PL, Salinas-Madrigal L. (1998) Polyarticular pigmented villonodular synovitis in a child. Clin Orthop Relat Res 348:208–211 [PubMed]
- 25.Chandan K, Mangat G, Balakrishnan C. RK chinoy, a bhaduri pauciarticular villonodular synovitis in a child. J Indian Rheumatol Assoc. 2003;11:14–15. [Google Scholar]
- 26.Tavangar SM, Ghafouri M. Multifocal pigmented villonodular synovitis in a child. Singapore Med J. 2005;46(4):193–195. [PubMed] [Google Scholar]
- 27.Gaubert J, Mazabraut A, Verdié J, Cheneau J. Les synovites villo-nodulaires hemo-pigmentees des grosses articulations. Rev Chir Orthop. 1974;60:265–298. [PubMed] [Google Scholar]
- 28.Wendt RG, Wolfe F, McQueen D, Murphy P, Solomon H, Housholder M. Polyarticular pigmented villonodular synovitis in children: evidence for a genetic contribution. JRheumatol. 1986;13(92):1–926. [PubMed] [Google Scholar]
- 29.De Jong B, Castedo SMMJ, Oosterhuis JW, Dam A. Trisomy 7 in a case of angiomyolipoma. Cancer Genet Cytogenet. 1988;34:219–222. doi: 10.1016/0165-4608(88)90263-4. [DOI] [PubMed] [Google Scholar]
- 30.Bridge J, Sanger W, Shaffer B, Neff J. Cytogenetic findings in malignant fibrous histiocytoma. Cancer Genet Cytogenet. 1987;29:197–202. doi: 10.1016/0165-4608(87)90035-5. [DOI] [PubMed] [Google Scholar]
- 31.Rao A, Vigorita VJ. Pigmented villonodular synovitis (giant cell tumor of the tendons sheaths and synovial membrane). a review of eighty-one cases. J Bone Joint Surg (Am) 1984;66:76–94. [PubMed] [Google Scholar]
- 32.Della Valle GA, Piccaluga F, Potter H, Salvati E, Pusso R. Pigmented villonodular synovitis of the hip: 2 to 23 year followup study. Clin Orthop. 2001;388:187–199. doi: 10.1097/00003086-200107000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Hs S, Kk U, Dj P. Pigmented villonodular synovitis. A retrospective review of affected large joints. Clin Orthop. 1989;247:243–255. [PubMed] [Google Scholar]