Abstract
Purpose
Remelted highly crosslinked polyethylenes (HXLPEs) were introduced in total knee replacement (TKR) starting in 2001 to reduce wear and particle-induced lysis. The purpose of this study was to investigate the damage mechanisms and oxidative stability of remelted HXLPEs used in TKR.
Methods
A total of 186 posteriorly stabilised tibial components were retrieved at consecutive revision operations. Sixty nine components were identified as remelted HXLPE. The conventional inserts were implanted for 3.4 ± 2.7 years, while the remelted components were implanted 1.4 ± 1.2 years. Oxidation was assessed using Fourier transform infrared spectroscopy.
Results
Remelted HXLPE inserts exhibited lower oxidation indices compared to conventional inserts. We were able to detect slight regional differences within the HXLPE cohort, specifically at the bearing surface.
Conclusion
Remelted HXLPE was effective at reducing oxidation in comparison to gamma inert sterilised controls. Additional long-term HXLPE retrievals are necessary to ascertain the long term in vivo stability of these materials in TKR.
Introduction
Remelted highly crosslinked polyethylenes (HXLPEs) were introduced in total knee arthroplasty (TKA) starting in 2001 to reduce wear, oxidation, and particle-induced osteolysis [1]. In the hip, where HXLPE has been in use longer, the literature shows that HXLPEs reduce wear and osteolysis, at least within the first decade of service [2]. However, because elevated radiation crosslinking and remelting reduces the fracture toughness of polyethylene, the use of HXLPE in the knee has been considered controversial [3–6]. Over time, HXLPE has gained acceptance as a candidate biomaterial for TKR [1, 7]. While there are accepted radiographic techniques to measure wear in the hip, there are no readily available techniques to measure the wear of tibial inserts in vivo, due to their comparatively complex geometry. Thus, few studies have reported on the clinical performance of HXLPE knees [2, 8]. Now, more than one decade following its introduction, knowledge of in vivo damage mechanisms and oxidative stability of remelted HXLPEs in the knee remains incomplete.
Until recently, remelted highly crosslinked polyethylene has been considered oxidatively stable due to the lack of measurable free radicals [1]. Researchers have recently observed elevated oxidation in retrieved HXLPE hip liners and knee inserts, particularly at the articulating surface, despite the absence of free radicals [9, 10]. The mechanisms of in vivo oxidation of remelted HXLPEs remains poorly understood, but in vivo loading and cyclic stress levels have been suggested as potential factors. Thus, due to different loading paradigms between hip and knee components, it is unclear if remelted highly crosslinked polyethylene will oxidize in total knee replacements to a greater extent than in hips.
The purpose of this study was to investigate the damage mechanisms and oxidative stability of remelted polyethylenes in a consecutive series of retrieved tibial components. We postulated that due to the relative lack of free radicals, remelted highly crosslinked polyethylenes would have lower oxidation levels than gamma inert sterilised controls. We also postulated that the remelted components would remain oxidatively stable over time.
Materials and methods
A total of 186 posteriorly stabilised tibial components were retrieved at consecutive revision operations at seven surgical centres. Sixty-nine components were identified as remelted highly crosslinked polyethylene (Prolong; Zimmer, Warsaw, IN), while the remainder (n = 117) were conventional gamma inert sterilised polyethylene. The sterilisation method was confirmed by tracing the lot numbers with the manufacturer. The conventional inserts were implanted for 3.4 ± 2.7 years (Range: 0.0–10.1 years), while the remelted components were implanted 1.4 ± 1.2 years (Range: 0.0–4.2 years). Patient records were reviewed to determine the reasons for revision, patient demographics, and activity scores (Table 1). The predominant reasons for revision were loosening, instability, infection, and stiffness for both cohorts (Fig. 1). None of the highly crosslinked tibial inserts were revised for osteolysis or component fracture.
Table 1.
Summary of patient demographics for the conventional (Gamma Inert) and highly crosslinked (HXLPE) polyethylene cohorts
| Cohort | n | Age (years) | Gender | BMI (kg/m2) | Implantation time (Years) | Maximum UCLA score average (Range) |
|---|---|---|---|---|---|---|
| Gamma Inert | 117 | 62 ± 10 | 67 % F | 33.8 ± 8.7 | 3.4 ± 2.7 | 5 (1–10) |
| HXLPE | 69 | 65 ± 10 | 53 % F | 31.6 ± 5.4 | 1.4 ± 1.2 | 6 (1–10) |
Fig. 1.
Reasons for revision for the HXLPE and Gamma Inert cohorts. Loosening, infection, and instability were the predominant reasons for revision
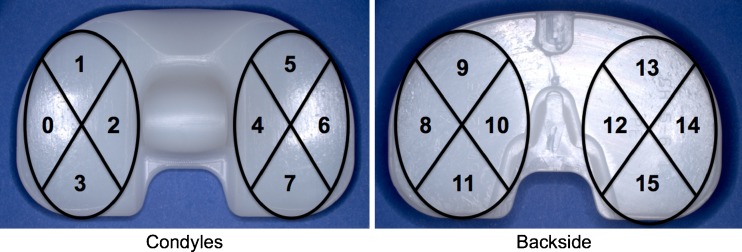
Surface damage was assessed using a previously described semi-quantitative scoring method [11]. The condyles, post and backside, were inspected for seven damage mechanisms, including scratching, pitting, burnishing, abrasion, delamination, embedded debris, and surface deformation [11]. Each condyle was split into four zones, the backside was split into eight zones, and each post face was scored as a zone, resulting in 20 total zones per tibial insert. Within each zone, a score of 0 was given when the damage mode was not present; a 1 was given when the damage mode covered less than 10 %; a 2 was given when the damage mode was present between 10 % and 50 % of the surface; and a score of 3 was given when the damage mode covered more than 50 % of the zone. Thus, the maximum score per tibial insert for each damage mode was 60. (Fig. 2)
Fig. 2.
Photographic representation of the condyle and backside zones used for surface damage assessment
For oxidation analysis, all of the remelted HXLPE inserts and a subset of the gamma inert sterilised implants were evaluated in accordance with ASTM 2102. Forty-one conventional and 69 remelted inserts were available for oxidation analysis. Thin slices (∼200 μm) were taken from the medial condyle and the central spine. The slices were boiled for six hours in heptane to extract any absorbed lipids and subsequently air-dried. Spectra were taken at 100 μm increments (32 repeat scans per location) perpendicular to each region of interest using transmission Fourier transform infrared spectroscopy (FTIR). Regions of interest were the bearing surface, the backside surface, the anterior and posterior faces, and the stabilising post. For each spectrum, an oxidation index was calculated as the ratio of areas under the curves at 1,650–1,850 cm−1 and 1,330–1,396 cm−1.
Following oxidation analysis, the slices were exposed to NO for 16 hours. Nitric oxide gas converts hydroperoxides (the precursors to oxidation) to nitrates, which are more readily identified and measured using FTIR spectroscopy. A hydroperoxide index was defined as the ratio of the integral of the curve between 1,600 and 1,670 cm−1 and the integral of the curve between 1,330 and 1,396 cm−1.
Distributions of continuous variables were tested for normality using the Shapiro-Wilk Test and found to be non-normal. Thus, differences between the retrieved inserts and the control inserts were calculated using the Mann–Whitney U test. To determine if metrics correlated with implantation time, we relied upon the Spearman’s Rank correlation test. All statistics were performed using commercial statistical software (JMP 9.0, SAS Institute, Cary, NC).
Results
Pitting, scratching, and burnishing were the predominant damage mechanisms within both material groups. Delamination was only present on one gamma inert insert, and was not present in any of the inserts in the highly crosslinked group. The prevalence of condylar pitting was similar between the material groups (p = 0.269); however, pitting scores were greater at the backside surface in the HXLPE retrievals (p < 0.001).
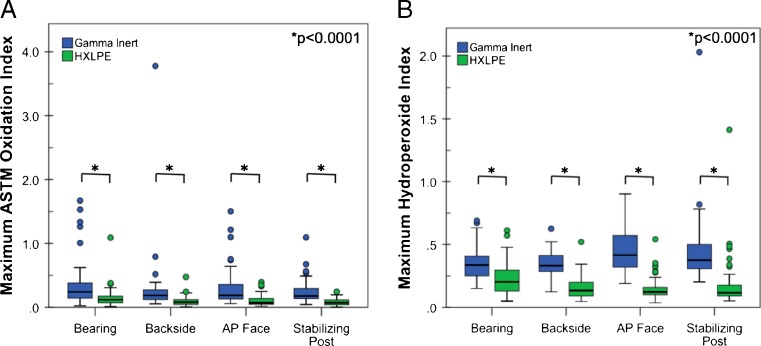
In the Gamma Inert cohort, oxidation was generally low (average OI: 0.3–0.5, depending on the location) and slight variation was observed between regions of the insert. Namely, the backside surface had lower oxidation indices than both the bearing surface (p = 0.02) and the anteroposterior (AP) face (p = 0.03). Likewise, in the HXLPE cohort, oxidation was low (average OI: 0.1–0.2, depending on the location). Regional variation was also observed in the HXLPE group, where the bearing surface had higher oxidation indices than the backside surface (p < 0.0001), the post (p < 0.0001), and the AP face (p < 0.0001). Oxidation was lower in the HXLPE group when compared to the gamma inert group at the bearing surface (mean difference = 0.2; p < 0.0001), the backside surface (mean difference = 0.2; p < 0.0001), the post (mean difference = 0.2; p < 0.0001), and the AP face (mean difference = 0.4; p < 0.0001; Fig. 3). In the gamma inert inserts, oxidation indices were positively correlated with implantation time at the bearing surface, AP face, and the post (Spearman’s Rho = 0.27–0.47; p ≤ 0.02). In the HXLPE group, oxidation correlated positively only at the bearing surface (Spearman’s Rho = 0.36; p = 0.003).
Fig 3.
Oxidation (a) and hydroperoxide (b) indices for both the Gamma Inert and highly crosslinked polyethylenes (HXLPE) cohorts
Hydroperoxide indices varied regionally in the Gamma Inert group with the AP face having higher hydroperoxide indices than the bearing surface (p < 0.0001) and backside surfaces (p = 0.0004). The post of the Gamma Inert inserts also had higher hydroperoxide indices than the bearing (p = 0.0008) and backside surfaces (p = 0.01). Similar to the oxidation results, the hydroperoxide index for the HXLPE group was higher at the bearing surface than all other locations (p < 0.0001). Hydroperoxide indices were greater in the gamma inert cohort at all regions (p < 0.0001; Mann–Whitney U Test) when compared to the HXLPE inserts (Fig. 3). The hydroperoxide index in the Gamma Inert group correlated positively with implantation time at both the AP face and the Post (Spearman’s Rho = 0.5 and 0.4, respectively; p = 0.002 and 0.04, respectively). Only the hydroperoxide index at the bearing surface of the HXLPE cohort was correlated with implantation time (Spearman’s Rho = 0.3; p = 0.03).
Discussion
This multi-centre study evaluated the surface damage mechanisms, oxidative stability, and reasons for revision for 1st generation remelted highly crosslinked polyethylene in a large collection of retrieved tibial inserts. Due to the remelting thermal treatment, this highly crosslinked polyethylene contains undetectable amounts of free radicals. Due to the lack of free radicals, remelted highly crosslinked polyethylenes were considered oxidatively stable. However, the remelting treatment also reduces the fatigue strength of the polymer that heightens concerns about component fractures. These factors contribute to the controversy of using highly crosslinked polyethylenes in total knee arthroplasty, although there is little in the literature describing the in vivo performance of these materials. We postulated that due to the relative lack of free radicals, remelted highly crosslinked polyethylenes would have lower oxidation levels than gamma inert-sterilised controls and would remain oxidatively stable over time.
The strengths of this study include the relatively large sample size, the multi-centre consecutive series of patients, and the use of standardised methods. However, this study has several limitations, including the short-term implantation of the highly crosslinked samples. Nevertheless, even at these short implantation times, we were able to detect low to moderate amounts of oxidation. Another limitation was that we used a semi-quantitative method to evaluate surface damage. While this method does not allow us to appreciate any improvement in wear performance, if any, it does permit us to distinguish between abrasive/adhesive and fatigue wear mechanisms.
The data in this study support the hypothesis that oxidation would be lower in the highly crosslinked group as compared to the gamma inert group. However, we were able to measure detectable levels of oxidation at the bearing surface (mean oxidation index = 0.2) of the highly crosslinked group. In the Gamma Inert group, locations with access to bodily fluids (i.e. the AP Faces and the post) led to higher oxidation indices. This is in contrast to the HXLPE inserts, where the bearing surface of the HXLPE group exhibited higher oxidation levels than the backside surface, AP face, and the stabilising post of the insert. The oxidation indices at the bearing of surface of the highly crosslinked group correlated with implantation time. This is similar to a recent study that found subtle increases in the oxidation index at the surface of acetabular liners, but not at other locations of the liners [12]. Given the location of this increase in oxidation, it appears that oxidation in remelted highly crosslinked polyethylene is probably facilitated by in vivo loading and cyclic stress levels in the insert. However, it is not clear what the implications of these findings are. An oxidation index (calculated using the ASTM method) of 1 is generally considered moderate oxidation and not expected to affect the mechanical properties of the polymer, while an oxidation index greater than 3 is considered dangerously high. At those levels, the mechanical integrity of the polymer is severely impaired and may result in catastrophic failure of the material [1, 13, 14].
Due to the non-conforming surface geometry, the condylar surfaces experience high stresses, and consequently fatigue damage is more likely. However, the damage mechanisms observed in this cohort of retrievals exhibited mainly adhesive/abrasive wear mechanisms, as opposed to fatigue wear mechanisms. This is similar to a short-term (average implantation time = 1.1 years) study that found the major damage mechanisms of remelted highly crosslinked polyethylene tibial inserts were abrasion, machine mark loss, and scratching [8]. This study also did not report delamination of the 13 HXLPE inserts that were analysed. Interestingly, Willie et al. did not observe any pitting in their retrieved specimens, although this may be attributed to the short implantation times [8]. Within both material groups in our collection, pitting, scratching, and burnishing were the predominant damage mechanisms. Pitting is thought to be caused by two distinct methods. The first method has been traditionally thought to be a fatigue mechanism from cyclic loading [1, 15, 16], while the second is an abrasive mechanism where third body particles embed and dislodge in the articulating surfaces of the insert [16–19]. We microscopically observed embedded debris in approximately 15 % of the inserts. Consequently, it appears that at least some of the pitting is due to an abrasive wear mechanism, as opposed to a fatigue wear mechanism. Further research is necessary to fully describe the aetiology of the pitting seen in these inserts.
In conclusion, this study investigated the in vivo performance of remelted highly crosslinked polyethylene used in total knee replacement. In our collection, we did not observe any component fractures in first generation highly crosslinked polyethylenes. Remelted highly crosslinked polyethylenes proved to have reduced oxidation indices as compared to conventional inserts. We were able to detect oxidation levels in the highly crosslinked group at the bearing surface, as well as a correlation with implantation time. Due to the relatively short implantation time of these retrievals, the clinical significance of the levels of oxidation seen here remains unknown. Additional long term highly crosslinked retrievals are necessary to ascertain the long term in vivo stability of these materials in total knee replacement.
Acknowledgments
This study was supported by the National Institutes of Health (NIAMS) R01 AR47904. Institutional support has been received from Zimmer, Stryker, Stelkast, Sulzer, and the Wilbert J. Austin Professor of Engineering Chair (CMR).
References
- 1.Kurtz SM. UHMWPE biomaterials handbook: ultra-high molecular weight polyethylene in total joint replacement and medical devices. 2. Burlington: Academic; 2009. [Google Scholar]
- 2.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262–2277. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66:146–154. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]
- 4.Huot JC, Van Citters DW, Currier JH, Currier BH, Mayor MB, Collier JP. Evaluating the suitability of highly cross-linked and remelted materials for use in posterior stabilized knees. J Biomed Mater Res B Appl Biomater. 2010;95:298–307. doi: 10.1002/jbm.b.31714. [DOI] [PubMed] [Google Scholar]
- 5.Jasty M, Rubash HE, Muratoglu O. Highly cross-linked polyethylene: the debate is over–in the affirmative. J Arthroplasty. 2005;20:55–58. doi: 10.1016/j.arth.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Ries MD. Highly cross-linked polyethylene: the debate is over–in opposition. J Arthroplasty. 2005;20:59–62. doi: 10.1016/j.arth.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Hodrick JT, Severson EP, McAlister DS, Dahl B, Hofmann AA. Highly crosslinked polyethylene is safe for use in total knee arthroplasty. Clin Orthop Relat Res. 2008;466:2806–2812. doi: 10.1007/s11999-008-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willie BM, Foot LJ, Prall MW, Bloebaum RD. Surface damage analysis of retrieved highly crosslinked polyethylene tibial components after short-term implantation. J Biomed Mater Res B Appl Biomater. 2008;85:114–124. doi: 10.1002/jbm.b.30923. [DOI] [PubMed] [Google Scholar]
- 9.Currier BH, Van Citters DW, Currier JH, Collier JP. In vivo oxidation in remelted highly cross-linked retrievals. J Bone Joint Surg Am. 2010;92:2409–2418. doi: 10.2106/JBJS.I.01006. [DOI] [PubMed] [Google Scholar]
- 10.Muratoglu OK, Wannomae KK, Rowell SL, Micheli BR, Malchau H. Ex vivo stability loss of irradiated and melted ultra-high molecular weight polyethylene. J Bone Joint Surg Am. 2010;92:2809–2816. doi: 10.2106/JBJS.I.01017. [DOI] [PubMed] [Google Scholar]
- 11.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J Biomed Mater Res. 1983;17:829–842. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald D, Sakona A, Ianuzzi A, Rimnac CM, Kurtz SM (2010) Do first-generation highly crosslinked polyethylenes oxidize in vivo? Clin Orthop Relat Res. doi:10.1007/s11999-010-1728-3 [DOI] [PMC free article] [PubMed]
- 13.Currier BH, Currier JH, Mayor MB, Lyford KA, Van Citters DW, Collier JP. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J Arthroplasty. 2007;22:721–731. doi: 10.1016/j.arth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Hozack W, Marcolongo M, Turner J, Rimnac C, Edidin A. Degradation of mechanical properties of UHMWPE acetabular liners following long-term implantation. J Arthroplasty. 2003;18:68–78. doi: 10.1016/S0883-5403(03)00292-4. [DOI] [PubMed] [Google Scholar]
- 15.Lewis G. Polyethylene wear in total hip and knee arthroplasties. J Biomed Mater Res. 1997;38:55–75. doi: 10.1002/(SICI)1097-4636(199721)38:1<55::AID-JBM8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Medel FJ, Kurtz SM, Parvizi J, Klein GR, Kraay MJ, Rimnac CM. In vivo oxidation contributes to delamination but not pitting in polyethylene components for total knee arthroplasty. J Arthroplasty. 2011;26:802–810. doi: 10.1016/j.arth.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornwall GB, Hansson CM, Bowe AJ, Bryant JT. Surface degradation features and microstructural properties of ultra-high molecular weight polyethylene (UHMWPe) J Mater Sci Mater Med. 1997;8:303–309. doi: 10.1023/A:1018564412753. [DOI] [PubMed] [Google Scholar]
- 18.Crowninshield RD, Wimmer MA, Jacobs JJ, Rosenberg AG. Clinical performance of contemporary tibial polyethylene components. J Arthroplasty. 2006;21:754–761. doi: 10.1016/j.arth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 19.McKellop HA. The lexicon of polyethylene wear in artificial joints. Biomaterials. 2007;28:5049–5057. doi: 10.1016/j.biomaterials.2007.07.040. [DOI] [PubMed] [Google Scholar]