Abstract
Purpose
Though anti-infectives have been used for a long time in surgical procedures, the effect on bone tissue has not been determined for most antibiotics and antiseptics.
Methods
In our in vitro study, 4x4x8 mm3 blocks of rabbit cancellous bone tissue were incubated with Ringer’s solution, gentamicin and Lavasorb® each for time intervals of 15 minutes, 30 minutes, one hour, four hours and eight hours. Samples were examined double blinded through optical and electron microscopy.
Results
Tissue degeneration was observed in all samples. It was low in Ringer’s solution. Samples with Lavasorb showed a moderate degeneration after 15 and 30 minutes, which was accelerated after one hour. Gentamicin led to a moderate degeneration of bone tissue after 15 and 30 minutes and to a more accelerated degeneration after one hour. The effect of gentamicin on bone tissue was more pronounced than the effect of Lavasorb.
Conclusions
This investigation showed that local application of Lavasorb or gentamicin on bone tissue should be restricted to 30 minutes, while Lavasorb showed a better tissue tolerability. This finding could have clinical implications for the management of wounds with open osseous tissue and should be further investigated by in vivo studies.
Introduction
Antibiotics have been used for a long time in radical surgical procedures to avoid local infections. Their efficacy was demonstrated in many studies [1–3]. However, the use of local antibiotics is hampered by some disadvantages: Local antibiotics often have a narrow specificity, enhance the risk for development of biological resistance as well as cross reactions, and show cytotoxic potential, if administered for extended periods [4, 5].
Modern antiseptics can replace antibiotics in these settings, since they have a broader spectrum of efficacy. Also, pathogens cannot develop resistance mechanisms against them. Further advantages are the low costs compared to antibiotics and the broader therapeutic window.
To be applicable as local drugs, antiseptics and antibiotics must therefore meet several terms [6]:
Good tissue compatibility
Low toxic potential if adsorbed
Low anaphylactic potential
Adequate activity against the expected microbiological spectrum
Lacking inactivation by biological material (e.g. pus)
For the treatment of cartilage in general, as well as knees and other joints in particular, studies have demonstrated that not all antiseptics and antibiotics fulfil these prerequisites [7]; especially the tissue compatibility often turns out to be a problem. Therefore, toxicological tests of antibiotics and antiseptics in an experimental model comparable to the site of application in humans are mandatory [6].
In this study, we examined the effect of gentamycin (Refobacin®, Merck) and the antiseptic Lavasorb® (Fresenius Kabi) on cancellous bone tissue.
The antibiotic gentamicin belongs to the group of the aminoglycosides and has a broad antibiotic spectrum [8]. Due to the high polarity of the drug, it is not well absorbed through the skin or via the intestine [4, 9]. Earlier studies have found a potential of gentamicin for an inhibition of bone metabolism, especially in osteoblasts [10, 11]. However, it is not clear if there is an incubation period with gentamicin that can be regarded as safe for bone tissue.
Lavasorb is a solution of polyhexanide and macrogol that has been in use as an antiseptic for almost thirty years, and is recommended as first choice for the treatment of chronic wounds and bone infections [4] due to its proven efficacy [12–15] and its high tissue compatibility [16]. For use in soft tissue wounds, Lavasorb showed even better tissue compatibility than Ringer’s solution [5]. Yet, a polyhexanide solution did inhibit epiphysical growth and enhanced the reduction of cartilage in an in vitro experiment [16], though the magnitude of the effect and the time period safe for polyhexanide treatment still have to be determined.
The aim of this study was to find out how long cancellous bone tissue can be incubated with gentamicin and Lavasorb without toxic effects.
Materials and methods
Extraction of cancellous bone blocks
Six female adult rabbits (strain Chbb:CH, Thomae, Biberach) were anaesthetized and 4x4x8 mm3 blocks of cancellous bone of the lateral femur condyle were removed as described previously [17, 18]. Each block was dissected equally into six smaller blocks, which were randomised for incubation with gentamicin (80 mg/l Refobacin® (Merck) in Ringer’s solution) or Ringer’s solution.
In a second approach, six female adult rabbits (strain Chbb:CH, Thomae, Biberach) underwent the same preparation procedures. The blocks were dissected equally into six smaller blocks and these were randomised to incubation with Lavasorb® (0.04 % polyhexanide, macrogol 4,000 0.002 %; Fresenius Kabi) or Ringer’s solution.
Incubation of samples
The small cancellous bone blocks were each incubated with 10 ml of the test solutions (gentamicin or Ringer’s solution; Lavasorb or Ringer’s solution) for 15 minutes, 30 minutes, one hour, four hours or eight hours at 8 °C.
All samples were treated in a blinded manner: Different staff members were responsible for treatment and assessment of the samples. At the time of assessment, the researchers examining the samples were unaware of group assignments.
Production of thin-sections
After incubation, the blocks were fixated for three days in a glutaraldehyde/formaldehyde solution (KARNOVSKY) and washed for 24 hours in cacodylic acid buffer solution (pH 7.0–7.2). Blocks were contrasted with osmium 1 % (Merck, Darmstadt), washed several times with cacodylic acid buffer solution and dehydrated with alcohol. After this, the blocks were transfered into pure propylenoxide, which was replaced with a 3:1 mixture of propylenoxide and Polybed® 812 (Embedding Media/DMP-30, Polysciences Inc., Warrington, USA) followed by a 1:1 and 1:3 mixture. The blocks were then incubated in a pure Polybed® 812 solution for 12–24 hours. Polymerisation was completed after two days at 40 °C and four days at 70 °C.
1 μm thin-sections were sliced from the hardened blocks with an Ultramikrotom (Ultra-Cut, S Reichert-Jung), dyed with toluidine blue and analysed with optical microscopy. In parallel, 0.1 μm thin-sections were sliced from the blocks, contrasted with uranyl acetate and lead citrate, put on copper nets and analysed with an electron microscope T-400 (Philips, Eindhoven, NL).
Analysis
In optical microscopy, quantity and width of different cell types were analyzed; in electron microscopy, changes of the cellular substructures were analyzed. Analysis was conducted in a descriptive, semiquantitative manner. Endpoints were quantity of osteoblasts, osteoclasts and osteocyts, rER (rough endoplasmatic reticulum) and mitochondria of osteoblasts and osteocytes, width and lamellar layering of osteoid and mineralisation. The cells were quantified as numerously present, moderately present, scarcely present or not present. In case of osteoid width, they were quantified analogical as thick, moderately thick, thin and not present.
Results
15 minutes incubations
The effect of gentamicin and Lavasorb on cancellous bone compared to Ringer’s solution after 15 minutes of incubation was only marginal (Table 1, Fig. 1). There were no distinct differences between gentamicin and Lavasorb exposure to tissue sections in the overall picture, although disparities were seen in different parameters. The most pronounced effect occurred on the rough endoplasmic reticulum (rER) of osteoblasts and lamellar layering in the presence of with gentamicin, and on lamellar layering of osteoid and mineralisation after exposure to Lavasorb.
Table 1.
Characteristics after 15 min
| Gentamicin | Ringer’s | Lavasorb® | Ringer’s | |
|---|---|---|---|---|
| osteoblasts, quantity | ++/+ | ++ | ++ | ++ |
| osteoblasts, rER | +/− | ++ | ++ | ++ |
| osteoblasts, mitochondria | +/− | + | + | + |
| osteoclast, quantity | −− | − | − | − |
| osteocytes, quantity | + | + | + | + |
| osteocytes, rER | − | − | − | − |
| osteocytes, mitochondria | − | − | − | − |
| osteoid, width | ++/+ | + | + | + |
| osteoid, lamellar layering | +/− | ++ | −− | ++ |
| mineralisation | + | ++ | − | ++ |
++ numerously present, + moderately present, − scarcely present, −− not present)
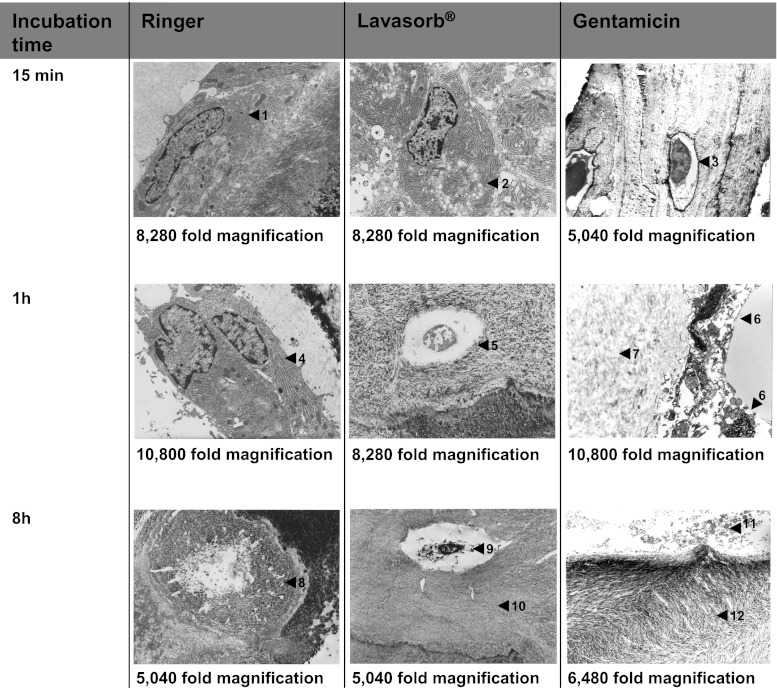
Fig. 1.
Electron microscopic views of bone tissue. 15 minutes: intact osteoblast with lamellar osteoid (Ringer) (1), osteoblast with inclusions (2) (Lavasorb) and intact osteocyte (gentamycin) (3). one hour: osteoblast with dilated rER (Ringer) (4), osteocyte within reduced mineralised bone matrix (Lavasorb) (5), damaged osteoblasts (6) and fragments of cells within demineralised bone matrix (gentamycin) (7). eight hours: remains of a dead osteocyte within mineralised matrix (Ringer) (8), degenerated osteocytes (9) and demineralisation of bone matrix (Lavasorb) (10), fragments of osteoblasts (11) and demineralised bone matrix (gentamycin) (12) (electron microscope)
30 minute incubations
After 30 minute, samples incubated in gentamicin and Lavasorb differed markedly from their controls (Table 2). Changes in the gentamicin samples were less pronounced than in the Lavasorb samples. Ringer’s solution also had a distinct effect on the samples, when compared to the result after 15 minutes of incubation.
Table 2.
Characteristics after 30 minutes
| Gentamicin | Ringer‘s | Lavasorb® | Ringer‘s | |
|---|---|---|---|---|
| osteoblasts, quantity | − | +/− | −/−− | +/− |
| osteoblasts, rER | +/− | − | −/−− | − |
| osteoblasts, mitochondria | +/− | − | −/−− | − |
| osteoclast, quantity | −− | − | −− | − |
| osteocytes, quantity | − | +/− | +/− | +/− |
| osteocytes, rER | − | − | − | − |
| osteocytes, mitochondria | − | − | − | − |
| osteoid, width | − | + | − | + |
| osteoid, lamellar layering | − | − | − | − |
| mineralisation | +/– | + | + | + |
++ numerously present, + moderately present, − scarcely present,−− not present
One hour incubations
Samples after one hour of incubation showed no further degradation (Table 3, Fig. 1). In fact, the effects of the incubation in Ringer’s solution were less pronounced than after 30 minutes.
Table 3.
Characteristics after one hour
| Gentamicin | Ringer‘s | Lavasorb® | Ringer‘s | |
|---|---|---|---|---|
| osteoblasts, quantity | +/− | ++/+ | − | ++/+ |
| osteoblasts, rER | − | ++ | − | ++ |
| osteoblasts, mitochondria | − | + | − | + |
| osteoclast, quantity | −− | − | −− | − |
| osteocytes, quantity | + | + | +/− | + |
| osteocytes, rER | −/−− | − | − | − |
| osteocytes, mitochondria | −− | − | − | − |
| osteoid, width | +/− | + | −/−− | + |
| osteoid, lamellar layering | + | + | + | + |
| mineralisation | +/− | + | + | + |
++ numerously present, + moderately present, − scarcely present, −− not present
Four hour incubations
A further degradation after four hours was observed only in the gentamicin samples (Table 4, Fig. 1). The blocks with Ringer’s solution stayed unchanged on average compared with baseline, while the samples incubated with Lavasorb showed less prominent changes. Compared with gentamicin, Lavasorb affected the bone tissue less after four hours.
Table 4.
Characteristics after Four hours
| Gentamicin | Ringer‘s | Lavasorb® | Ringer‘s | |
|---|---|---|---|---|
| osteoblasts, quantity | − | +/− | ++/+ | + |
| osteoblasts, rER | −− | ++ | − | ++/+ |
| osteoblasts, mitochondria | −− | + | − | +/− |
| osteoclast, quantity | −− | −/−− | −− | −− |
| osteocytes, quantity | − | +/− | + | +/− |
| osteocytes, rER | −− | +/− | − | − |
| osteocytes, mitochondria | −− | +/− | − | − |
| osteoid, width | +/− | ++/+ | + | ++/+ |
| osteoid, lamellar layering | + | ++ | − | ++ |
| mineralisation | + | ++/+ | − | +/+ |
++ numerously present, + moderately present, − scarcely present, −− not present
Eight hour incubations
After eight hours of incubation, gentamicin samples showed a distinct degradation of bone tissue, whereas Lavasorb and Ringer’s solution showed only a mild degradation compared to gentamicin samples and baseline assessment after 15 minutes (Table 5, Fig. 1).
Table 5.
Characteristics after Eight hours
| Gentamicin | Ringer‘s | Lavasorb® | Ringer‘s | |
|---|---|---|---|---|
| osteoblasts, quantity | −− | − | ++/+ | + |
| osteoblasts, rER | −− | + | − | ++/+ |
| osteoblasts, mitochondria | −− | +/− | − | +/− |
| osteoclast, quantity | −− | −− | −− | −− |
| osteocytes, quantity | − | +/− | + | +/− |
| osteocytes, rER | −− | −/−− | − | − |
| osteocytes, mitochondria | −/−− | −/−− | − | − |
| osteoid, width | − | −/−− | + | ++/+ |
| osteoid, lamellar layering | +/− | −/−− | − | ++ |
| mineralisation | +/− | +/− | − | ++/+ |
++ numerously present, + moderately present, − scarcely present, −− not present
Overall effect
For analysis of the overall effect, different parameters were summarized and plotted for comparison (Tables 1, 2, 3, 4, 5). From 15 minutes to eight hours, degradation of bone cells was seen in all samples. The overall degradation over eight hours was less distinct for Lavasorb and Ringer’s solution, but more severe in gentamicin samples. It should be noted that after one hour, there was more degradation present in the Lavasorb samples than in the other samples, yet for longer incubation periods with Lavasorb, this effect was reversed and gentamicin caused greater harm to the bone cells.
Discussion
Toxicity of Ringer’s solution on bone tissue
The results demonstrate that even prolonged incubation in Ringer’s solution resulted in a degradation of cancellous tissue and cell death after approximately four to eight hours. Incubation periods up to one hour led to moderate signs of degradation.
Other studies with cancellous tissue of rabbits revealed that the changes seen upon exposure to Ringer’s solution occur generally, as an epiphenomenon of bone necrosis [19].
Samples exposed to Ringer’s solution showed no signs of a dissolving of bone tissue due to storage outside of the normal environment. However, the degradation effects could be related to the absence of nutrients, oxygen, and hormones. Furthermore, specific interaction with ions in Ringer’s solution may have an affect on bone tissue as well, since in other settings even saline caused cell damage [16, 20].
Even more important than the amount of degradation is the question, which degree of degradation would be judged as tolerable and which degree corresponds to a lasting damage of cancellous tissue in a clinical setting? Since our in vitro experiments did not address this directly, it is necessary to rely on previous studies.
The changes seen in samples with Ringer’s solutions up to eight hours correspond to a low proliferating synovitis described in another study [21]. This state occurred postoperatively after repeated washing cycles during five days. An additional study showed a fast convalescence after only one wash cycle [22]. Therefore, tissue damages under Ringer’s solution were considered to be reversible.
Toxicity of Lavasorb on bone tissue
In our experiments, Lavasorb showed the second best tissue compatibility. In samples incubated with Lavasorb for 15 minutes or 30 minutes, the effect on bone tissue was comparable to the effect of Ringer’s solution for one to four hours and was considered reversible.
Incubation for one to eight hours with Lavasorb showed more severe tissue damage. A previous study on femur bone fragments from rabbits used comparable parameters in an ischemia model. In these experiments, irreversible damages to the tissue occurred in vitro after two hours of ischaemia [23]. Qualitatively changes in these tissue samples correspond to the changes seen with Lavasorb after one hour and more. These results are also supported by an in vivo experiment in rabbits. Test solutions of polyhexanide, PVP iodine and Ringer’s solution were administered by drainage (25 times) and by injections (one time) for gentamicin [21]. An irritation was found in all groups and was attributed to the pressure of the drainage. However, polyhexanide and gentamicin caused more damages than PVP iodine and Ringer’s solution. Similar results were reported by Kallenberger et al. for chlorhexidine, iodoform and taurolidine [16]. We conclude from this in vitro incubation of cancellous tissue with Lavasorb results in irreversible damages to the tissue, and exposure to Lavasorb should be restricted to less than an hour.
Toxicity of gentamicin on bone tissue
Gentamicin had the lowest tissue compatibility of the solutions tested in this experiments. As with Lavasorb, samples incubated with gentamicin for 15 minutes or 30 minutes showed an effect on bone tissue comparable to samples with Ringer’s solution for one to four hours.
However, the effects seen in samples with longer incubation times with gentamicin were more pronounced than in the corresponding Lavasorb samples, making these changes irreversible as well. In a previous study, the low pH value of gentamicin was held responsible for these effects [21].
Hence, one might assume that a short intermittent use of gentamicin and Lavasorb for 30 minutes affects the bone in a local and reversible fashion. For longer times of exposure, both substances led to irreversible damages of bone tissue. Therefore, exposure to gentamicin and Lavasorb should be restricted to less than an hour.
Further treatment alternatives
Though no other studies that are published examine the same endpoints as in our study, it is well known that other local antibiotics do affect bone tissue as well. Bone grafts have been treated with chloramphenicol, methicillin or polybactrin before transplantation in rats; in the following two weeks, the treated grafts produced little or no new bone material [24]. In spite of such results, these antibiotics are still considered a standard in bone infections, since the benefits of preventing an infection exceed the harm of slowing down recovery.
Another treatment option are antibiotic bone cements that gradually release their active compounds [25]. The mobilisation of small amounts of topical antibiotics circumvents the problems upon exposure to concentrated antibiotics and antibiotic bone cements have been proven effective in several clinical studies in arthroplasty surgery [25]. However, allergic reactions can be induced by longer exposure to these drugs and may require additional measures in serious cases. Furthermore, topical antibiotics possess a higher potential for inducing antibiotic resistance mechanisms, and their use might therefore be restricted in multimorbid patients.
Conclusion
Concluding from our data, physical cell damage starts to be recordable after 30 minutes to one hour of exposure to different antiinfective substances. In general, the antiseptic Lavasorb shows better tissue compatibility than the antibiotic gentamicin. However, both drugs inflict a considerable amount of tissue degradation if tissue is exposed for more than 30 minutes. To confirm the general meaning of these observations, the use of Lavasorb or gentamicin on cancellous tissue should be investigated under in vivo conditions.
References
- 1.Yarboro SR, Baum EJ, Dahners LE. Locally Administered Antibiotics for Prophylaxis Against Surgical Wound Infection. An in Vivo Study. The J Bone Joint Surg Am. 2007;89:929–933. doi: 10.2106/JBJS.F.00919. [DOI] [PubMed] [Google Scholar]
- 2.Zalavras CG, Patzakis MJ, Holtom P. Local Antibiotic Therapy in the Treatment of Open Fractures and Osteomyelitis. Clin Orthop. 2004;427:86–93. doi: 10.1097/01.blo.0000143571.18892.8d. [DOI] [PubMed] [Google Scholar]
- 3.Miller K, Lang B, Hell E. Local vs Systemic Antibiotics to Decrease Wound Complications following Vertical Banded Gastroplasty: Results of a Prospective Randomized Trial. Obes Surg. 1995;5:293–297. doi: 10.1381/096089295765557665. [DOI] [PubMed] [Google Scholar]
- 4.Kramer A, Daeschlein G, Kammerlander G, Andriessen A, Aspöck C, Bergemann R, Eberlein T, Gerngross H, Görtz G, Heeg P, Jünger M, Koch S, König B, Laun R, Peter RU, Roth B, Ruef C, Sellmer W, Wewalka G, Eisenbeiß W. Konsensusempfehlung zur Auswahl von Wirkstoffen für die Wundantiseptik. Hyg Med. 2004;5:147–57. [Google Scholar]
- 5.Schmit-Neuerburg KP, Bettag C, Schlickewei W, Fabry W, Hanke J, Renzing-Köhler K, Hirche H, Kock HJ. Wirksamkeit eines neuartigen Antisepticum in der Behandlung kontaminierter Weichteilwunden. Effectiveness of an improved antiseptic in treatment of contaminated soft tissue wounds. Chirurg. 2001;72:61–71. doi: 10.1007/s001040051269. [DOI] [PubMed] [Google Scholar]
- 6.Kramer A, Adrian V, Werner HP (1997) Prophylaxe und Therapie von Infektionen in der Orthopädie. in Ganzer D, Kramer A, Mayer, G (ed) Prophylaxe und Therapie von Infektionen in der Orthopädie, mhp, Wiesbaden, pp 11–17
- 7.Stutz G, Gächter A. Diagnostik und stadiengerechte Therapie von Gelenkinfekten. Unfallchirurg. 2001;104:682–686. doi: 10.1007/s001130170068. [DOI] [PubMed] [Google Scholar]
- 8.Alexander M, Estler CJ, Legler F (1995) Antibiotika und Chemotherapeutika, 2nd edn. Wissenschaftliche Verlags Gesellschaft, Stuttgart, pp 81–87 and 222–223
- 9.Knothe H. Aminoglycoside. 3. Frankfurt: Umwelt und Medizin Verlag; 1991. [Google Scholar]
- 10.Pedersen JG, Lund B. Effects of gentamicin and monomer on bone. An in vitro study. J Arthroplasty. 1988;3:63–68. doi: 10.1016/S0883-5403(88)80011-1. [DOI] [PubMed] [Google Scholar]
- 11.Isefuku S, Joyner CJ, Simpson AHRW. Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma. 2003;17:212–216. doi: 10.1097/00005131-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Rahn HD, Tolksdorff G. Abdominoperineale Rektumexstirpation. Chir Prax. 1989;41:637–643. [Google Scholar]
- 13.Roth B, Balzer K. Prophylaktische intraoperative Spülung bei Wundversorgung mit Lavasept. Z Unfallchir Vers Med. 1990;83:224–226. [PubMed] [Google Scholar]
- 14.Roth B, Müller J, Willenegger H. Intraoperative Wundspülung mit einem neuartigen lokalen Antiseptikum. Helv chir Acta. 1985;52:61–65. [PubMed] [Google Scholar]
- 15.Willenegger H. Klinische Erfahrungen mit einem neuen Antiinfektivum. Hyg Med. 1994;19:227–233. [Google Scholar]
- 16.Kallenberger A, Kallenberger C, Willenegger H. Experimentelle Untersuchung zur Gewebeverträglichkeit von Antiseptika. Hyg Med. 1991;16:383–395. [Google Scholar]
- 17.Kock HJ, Stürmer KM. Ein alternatives Modell zur standardisierten Untersuchung der Heilung von Knochendefekten. Osteologie. 2001;10:148–156. [Google Scholar]
- 18.Kock HJ, Werther S, Uhlenkott H, Taeger G. Beeinflussung der Knochenheilung durch unfraktionierte und niedermolekulare Heparine: eine tierexperimentelle Studie. Unfallchirurg. 2002;105:791–796. doi: 10.1007/s00113-002-0419-2. [DOI] [PubMed] [Google Scholar]
- 19.Drenhaus U, Imhoff M, Tassler H. Über lagerungsbedingte Veränderungen autologer Spongiosa. Unfallchirurg. 1988;91:165–173. [PubMed] [Google Scholar]
- 20.Kallenberger A, Willenegger H, Roth W. Ein jod- und metallsalzfreies Desinfektionsmittel für die Wundbehandlung. Langenbecks Arch Chir. 1982;358:481. doi: 10.1007/BF01271891. [DOI] [Google Scholar]
- 21.Ganzer D, Völker L, Follak N, Wolf E, Granzow H. Reaktion des hyalinen Gelenkknorpels und der Synovialis auf eine intraartikuläre Instillation von verschiedenen Antiinfektiva. Arthroskopie. 2001;14:31–44. doi: 10.1007/s001420050195. [DOI] [Google Scholar]
- 22.Marshall GJ, Kirchen ME, Sweeney JR, Snyder S. Synovisol as an irrigant for electrosurgery of joints. Arthroscopy. 1988;4:187–193. doi: 10.1016/S0749-8063(88)80025-2. [DOI] [PubMed] [Google Scholar]
- 23.James J, Steijn-Myagkaya GL. Death of Osteocytes. J Bone Joint Surg. 1986;68-B:620–624. doi: 10.1302/0301-620X.68B4.3733842. [DOI] [PubMed] [Google Scholar]
- 24.Gray JC, Elves MW. Osteogenesis in bone grafts after short term storage and topical antibiotic treatment. An experimental study in rats. J Bone Joint Surg. 1981;63:441–445. doi: 10.1302/0301-620X.63B3.7021562. [DOI] [PubMed] [Google Scholar]
- 25.Block JE, Stubbs HA. Reducing the risk of deep wound infection in primary joint arthroplasty with antibiotic bone cement. Orthopedics. 2005;28:1334–45. doi: 10.3928/0147-7447-20051101-13. [DOI] [PubMed] [Google Scholar]