Abstract
Purpose
Blood-derived proliferative factors such as platelet rich plasma or activated plasma are promising adjuvants for bone grafts. Our earlier studies showed that serum albumin itself can markedly enhance the proliferation of stem cells on bone allograft and postulated that albumin coating alone may improve bone graft integration in vivo.
Methods
Two femoral defect models were performed in adult male Wistar rats. In the critical size model a six millimetre gap was created in the midshaft of the femur and fixed with plate and screws, while a nonunion model was established by the interposition of a spacer in the osteotomy for four weeks which resulted in compromised healing and nonunion. Albumin coated and uncoated grafts were placed into the defects. Bone healing and morphometry were evaluated by μCT and histology four weeks after implantation of the grafts.
Results
In the critical size model none of the bone grafts were able to bridge the defect, and graft resorption was the typical outcome. In the nonunion model regular uncoated grafts had a low union rate (two out of six), which increased markedly when albumin coating was applied (six out of eight). Trabecular thickness and pattern factor improved significantly in the albumin coated group versus uncoated or empty controls.
Conclusions
Our results showed that serum albumin coating of bone grafts can enhance the remodelling and efficacy of treatment in a nonunion model.
Introduction
Despite modern bone grafting techniques, management of large segmental bone defects remains a significant surgical challenge. An ideal bone graft should feature good mechanical strength and significant osteoinductive, osteoconductive and osteogenic capabilities [1]. Autologous human bone graft possesses all these characteristics, but it is not available in large quantities, and complications of the donor site also limit its use [2].
Many different osteoinductive materials have been tested in an attempt at improving bone healing, bone morphogenic proteins (BMPs), demineralised bone matrix (DBM) and platelet rich plasma (PRP) being the most widely studied varieties [3]. However, despite the significant efforts to find an appropriate osteoinductive agent none of them has gained general acceptance in everyday use. Bone morphogenic proteins are known to have a notable osteoinductive effect [4–6], but they are rather expensive and a suitable carrier material for their optimal application is still missing [7]. Though demineralised bone matrix provided encouraging results in experimental setups [8, 9], the concentration of BMPs in DBM products is highly variable leading to unreliable clinical outcomes [10]. Being a natural source of growth factors, the use of PRP is a reasonable option [11, 12]. However, recent studies have questioned its efficacy and report limited ability to enhance bone healing [13, 14]. In addition, the exact mode of action of PRP is unknown and some studies suggested that a significant proportion of its effect is attributable to other components than platelets or growth factors [15].
We have previously shown that serum albumin, which is a well-known proliferative factor in cell culture, has the ability to induce mesenchymal stem cell growth on the surface of bone allografts [16]. Since it is obvious that the proliferation of bone-forming cells is the rate-limiting factor in graft remodelling, one may postulate that serum addition to bone substitutes may improve the colonisation of the graft by host cells. In this study we investigated whether simple serum albumin coating of bone grafts may be enough to improve graft integration in a compromised bone-healing model [17].
Materials and methods
Study design
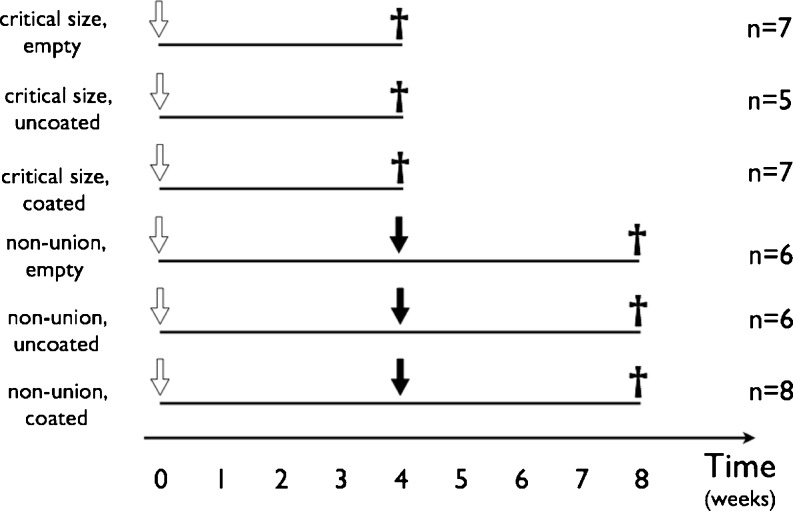
All animal procedures were approved by the Scientific Research Committee of Semmelweis University. Adult male Wistar rats (n = 39) weighing 496–692 g were housed and maintained at 12/12 day/night cycles and were provided with water and lab chow ad libitum. The animals were separated into six groups summarised in Fig. 1. In the first half of the animals the classical critical size model was established by creating a six millimetre wide midshaft defect [18], which was either left empty or filled with an uncoated or albumin coated graft. In the second half a nonunion model was created by blocking bone healing with a spacer [17]. The interposed spacer was removed after four weeks and the osteotomy gap was either left empty or filled with uncoated or albumin coated bone graft. The detailed technique of the albumin coating of the grafts was described previously [16]. Each animal was sacrificed four weeks after the implantation procedure.
Fig. 1.
Experimental protocol. White arrow indicates the time of the first procedure in each group. Black arrow shows the time of the second procedure, i.e. removal of the spacer in the control nonunion group, or removal of the spacer and implantation of the uncoated graft or coated graft. The black cross represents the time when the animals were sacrificed and the bone harvested for CT micromorphometry
Surgical technique
The detailed surgical technique was described previously [17]. Briefly, after halothane, N2O and O2 general anaesthesia, a lateral approach to the femur was used. A five hole steel plate (Mini plate; Sanatmetal, Eger, Hungary) was fixed to the diaphysis of the femur by four 1.5-mm wide and 8-mm long cortical screws (Sanatmetal, Eger, Hungary) using two proximal and two distal holes, but leaving the middle hole empty. After that, an osteoperiosteal segment was removed at the level of the middle hole. The size of the defect was six millimetres in the critical size group and two millimetres in the nonunion group. In the critical size groups the defect was either left empty, or filled with an uncoated or albumin coated graft. In the nonunion group a preformed bone cement spacer was interposed into the gap for four weeks, when a second procedure was performed to allow bone grafting. Four weeks after completion of the protocol the animals were sacrificed by exsanguination under anaesthesia and the femora were harvested; the plate and screws were removed to allow radiographic analysis.
Ex vivo μCT analysis
The detailed method of the analysis was described earlier [17]. The femora were scanned using a μCT scanner (Skyscan 1172 X-Ray microtomograph, Kontich, Belgium) and evaluated according to Verna and Schmidhammer [19, 20]. Bone area per total area was calculated in each slice of the CT scan, referred throughout this study as “relative bone volume”. Bone regeneration in the defect was related to the original bone stock, hence it was characterised by the ratio of the B.Ar/T.Ar values of the defect and that of the bone proximal and distal to the osteotomy site. Union/nonunion was assessed using 3D μCT reconstruction according to Schmidhammer [19]. Union was defined as a continuous bony bridge across the defect. Morphometric measurements calculated by the Skyscan software, such as trabecular separation (Tb.Sp. in μm), trabecular thickness (Tb.Th. in μm), and trabecular pattern factor (Tb.Pf.) were performed.
Statistical methods
Data are presented as mean ± standard error throughout the study. Statistical analysis was done by InStat 3.0 software (GraphPad Software, Inc. La Jolla, CA, USA). The differences among groups were analysed using ANOVA and Tukey post hoc tests. P values <0.05 were considered significant.
Results
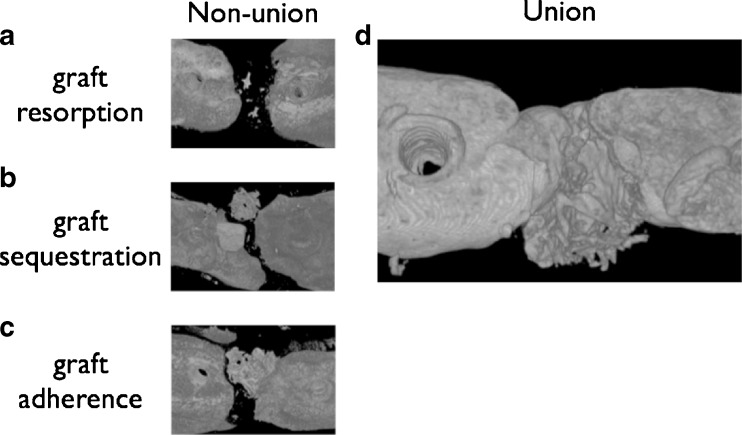
No complications were observed during or after surgery. Healing occured uneventfully in all animals, graft dislocation or infection was not observed. The analysis of the 3D μCT reconstructions of the graft–host interface allowed the distinction of three categories of nonunion (Fig. 2). The graft either resorbed completely (resorption), or remained visible without attachment to any of the bone ends indicating sequestration (sequestration) or attached to one side of the defect (adherence). Trabecular separation values clearly identified each case where the graft was resorbed (Fig. 3).
Fig. 2.
Different typical courses of the implanted grafts are presented. a A representative image, when the graft underwent resorption. b A sequestrated graft, i.e. when there is no bony union with either bone ends but the graft is clearly visible in the osteotomy gap. c The graft adheres to the bone but does not bridge the defect. d A representative image of a union. In this case the graft is clearly integrated into the host bone and new bone formation is evident
Fig. 3.
Using CT micromorphometry and measuring the trabecular separation values in the lesion site clearly confirmed graft resorption, however, there was no difference between sequestration or adherence
In the critical size model, where a relatively large gap was filled by a bone graft, healing was not observed in any of the groups either when the defect was left empty or when it was filled by any graft type. The relative bone volume in the defect site was comparable among the three groups (Table 1). In this model graft resorption was the most prominent outcome without consolidation of the defect.
Table 1.
Results of bone grafting in the critical size model
| Parameter | Empty | Uncoated | Albumin coated |
|---|---|---|---|
| Union rate | 0/7 | 1/5 | 0/7 |
| Relative bone volume | 57.78 ± 8.4 % | 39.13 ± 3.1 % | 25.36 ± 3.7 % |
When the nonunion model was used and the osteotomy gap was left empty, all the animals developed a nonunion (union rate 0/7) and relative bone volume in the defect site was 70.98 ± 10.2 %. If the osteotomy gap was filled with an uncoated bone graft, the union rate slightly increased to 2/6 and relative bone volume was 55.26 ± 6.1 %. However, when an albumin coated bone graft was applied the union rate increased markedly to 6/8 with a relative bone volume of 61.19 ± 8.7 % (Fig. 4). Micromorphometry data showed that trabecular thickness and trabecular pattern factor increased significantly when albumin coating was applied, further supporting the finding that albumin coating beneficially affected the bone remodeling in the defect (Fig. 4).
Fig. 4.
Results of bone grafting in the nonunion model. The implantation of the uncoated graft resulted in a 33 % union rate, albumin coating increased this ratio to 75 % (a). This improvement was reflected in micromorphometry parameters, such as trabecular pattern factor and trabecular thickness, which were significantly improved in albumin coated grafts (b and c). Asterisk represents significant difference (p < 0.05) between coated and uncoated grafts
Discussion
Our study shows that bone grafting of any type was insufficient to bridge a large defect. However, albumin coating of freeze-dried cancellous bone grafts improves the remodelling characteristics of the graft in a nonunion model. This improvement is so prominent at the macro level that it achieves consolidation of a nonunion in most cases where the uncoated grafts fail.
In our experimental setup two different animal models were used: the gold-standard critical size model [18] and an interposition femoral defect model [17]. As far as the former, there is an emerging concern regarding the accuracy of the critical size model, claiming that the size of the “critical gap” and also the lifetime of the investigated animal should be determined precisely, which is not feasible in most experimental protocols [21–25]. However appropriate this concern may be, we believe that the critical size model is still an adequate method for testing large cortical bone deficiencies. Knowing that the reconstruction of smaller bone defects with compromised regenerative capacity is a different, but not too rare event in the everyday surgical practice of human musculoskeletal diseases (i.e. revision arthroplasty), we also used an interposition femoral defect model, which is more suitable to mimic this problem. The difference between the two models was observed in our study, since bone union developed almost exclusively in the interposition model, while the critical size model resulted in nonunion in all but one case, regardless of the type of the implanted graft.
Comparing the relative bone volume in the different groups by the type of the graft, we found that if the uncoated graft was used, relative bone volume did not differ significantly between the critical size model and the interposition model, but in cases of albumin coated grafts the relative bone volume was significantly higher in the interposition model than that of the critical size model. The interpretation of this result is somewhat controversial. Lower bone volume measured in the critical size group could be a consequence of the fact that the osseous regenerative process is not satisfactory enough to fill a large, i.e. “critical” defect, while the smaller defect of the interposition model could be bridged easier, which would result in a higher relative bone volume. However, our previous results showed that bone regeneration in the interposition defect model is compromised [17], which should provide low bone volume values similarly to the critical size model. Therefore it can be assumed that the significantly higher relative bone volume measured in the interposition model can be the consequence of an increased healing capacity provided by the albumin coating of the graft.
In both animal models the highest relative bone volume was measured in those groups where the defect was left empty. If a bone graft of any kind was implanted into the defect, relative bone volume decreased. Since resorption of the bone substitute is part of the regenerative process, it can be postulated that lower bone volume refers to the onset of the osseous healing procedure. Further investigations are needed to prove this hypothesis.
It is somewhat surprising that a simple blood protein can increase the efficacy of bone grafting from almost complete failure to almost complete reconstitution, at least in one model form the two we tested. This effect is less of a surprise if we take into account that serum albumin is a key element in in vitro stem cell culture, without which stem cells cannot be grown effectively. Our earlier studies elaborated this concept further by showing that serum albumin coating boosts bone marrow derived stem cell proliferation in vitro without affecting the physical characteristics of the graft [16]. Therefore, it is plausible that the high concentration of albumin coating creates a milieu for stem cells migrating from the bone marrow which is similar to cell culture conditions. Since we know that this condition promotes cell proliferation, there are more cells which possibly undergo osteoblast transformation and remodel the dead bone scaffold.
In conclusion, we showed that regular freeze-dried bone grafts fail to bridge the defect in two animal models of compromised bone healing. However, adding a simple albumin coating to the graft resulted in consolidation of nonunions. The clinical significance of this result is that an easily applicable factor, serum albumin, can enhance the efficacy of bone grafting from failure to load-bearing callous if the defect is less than the diameter of the bone.
Acknowledgments
We are thankful for Lacerta Technologies Inc. and the West-Hungarian Tissue Bank for providing the bone grafts. The present work was funded by grants from TÉT-SIN-CELLTHER, TÁMOP-4.2.1/B09/1/KMR-2010-0001, OTKA 83803.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Bae JH, Kim YK, Myung SK. Effects of platelet-rich plasma on sinus bone graft: meta-analysis. J Periodontol. 2011;82:660–667. doi: 10.1902/jop.2010.100529. [DOI] [PubMed] [Google Scholar]
- 2.Bae HW, Zhao L, Kanim LEA, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine. 2006;31:1299–1306. doi: 10.1097/01.brs.0000218581.92992.b7. [DOI] [PubMed] [Google Scholar]
- 3.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper GM, Mooney MP, Gosain AK, Campbell PG, Losee JE, Huard J. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg. 2010;125:1685–1692. doi: 10.1097/PRS.0b013e3181cb63a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–S15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Gamradt SC, Lieberman JR. Bone graft for revision hip arthroplasty: biology and future applications. Clin Orthop Relat Res. 2003;417:183–194. doi: 10.1097/01.blo.0000096814.78689.77. [DOI] [PubMed] [Google Scholar]
- 7.Gosain AK, Song L, Yu P. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg. 2000;106:360–372. doi: 10.1097/00006534-200008000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Hao W, Dong J, Jiang M, Wu J, Cui F, Zhou D. Enhanced bone formation in large segmental radial defects by combining adipose-derived stem cells expressing bone morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop. 2010;34:1341–1349. doi: 10.1007/s00264-009-0946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZQ, Liu HY, Zhang LP, Wu ZQ, Shang DZ. Repair of calvarial defects in rabbits with platelet-rich plasma as the scaffold for carrying bone marrow stromal cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;113:327–333. doi: 10.1016/j.tripleo.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Johannes C, Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, Van Griensven M, Redl H, Hutmacher DW. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30:2149–2163. doi: 10.1016/j.biomaterials.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Kanthan SR, Kavitha G, Addi S, Choon DS, Kamarul T. Platelet-rich plasma (PRP) enhances bone healing in non-united critical-sized defects: a preliminary study involving rabbit models. Injury. 2011;42:782–789. doi: 10.1016/j.injury.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey RW, Gugala Z, Milne E, Sun M, Gannon FH, Latta LL. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J Orthop Res. 2006;24:1438–1453. doi: 10.1002/jor.20154. [DOI] [PubMed] [Google Scholar]
- 13.Muschler GF, Lane JM, Werntz J, Gebhardt M, Sandu H, Piergentili C, Nottebaert M, Baker C, Burstein A. Segmental femoral defect model in the rat. In: Aebi M, Regazzoni P, editors. Bone transplantation. New York: Springer; 1989. pp. 167–169. [Google Scholar]
- 14.Oakes DA, Lee C, Lieberman JR. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop. 2003;413:281–290. doi: 10.1097/01.blo.0000073347.50837.16. [DOI] [PubMed] [Google Scholar]
- 15.Pape HC, Evans A, Kobbe PJ. Autologous bone graft: properties and techniques. Orthop Trauma. 2010;24(Suppl 1):S36–S40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 16.Pecina M, Haspl M, Jelic M, Vukicevic S. Repair of a resistant tibia non-union with a recombinant bone morphogenetic protein-7 (rh-BMP-7) Int Orthop. 2003;27:320–321. doi: 10.1007/s00264-003-0475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peerbooms JC, Colaris JW, Hakkert AA, Van Appeldorn M, Bruijn DJ, Den Oudsten BL, Gosens T. No positive bone healing after using platelet rich plasma in a skeletal defect. An observational prospective cohort study. Int Orthop. 2012;36:2113–2119. doi: 10.1007/s00264-012-1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimondini L, Nicoli-Aldini N, Fini M, Guzzardella G, Tschon M, Giardino R. In vivo experimental study on bone regeneration in critical bone defects using an injectable biodegradable PLA/PGA copolymer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:148–154. doi: 10.1016/j.tripleo.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Schmidhammer R, Zandieh S, Mittermayr R, Pelinka LE, Leixnering M, Hopf R, Kroepfl A, Redl H. Assessment of bone union/nonunion in an experimental model using microcomputed technology. J Trauma. 2006;61:199–205. doi: 10.1097/01.ta.0000195987.57939.7e. [DOI] [PubMed] [Google Scholar]
- 20.Skaliczki G, Weszl M, Schandl K, Major T, Kovács M, Skaliczki J, Redl H, Szendrői M, Szigeti K, Máté D, Dobó-Nagy C, Lacza Z. Compromised bone healing following spacer removal in a rat femoral defect model. Acta Physiol Hung. 2012;99:223–232. doi: 10.1556/APhysiol.99.2012.2.16. [DOI] [PubMed] [Google Scholar]
- 21.Southerland D, Bostrom M (2005) Grafts and bone graft substitutes. In: Lieberman JR, Friedlaender GE (eds) Bone regeneration and repair. Humana Press, Totowa, NJ, pp 133–56
- 22.Verna C, Dalstra M, Wikesjo UM, Trombelli L. Healing patterns in calvarial bone defects following guided bone regeneration in rats. A micro-CT scan analysis. J Clin Periodontol. 2002;29:865–870. doi: 10.1034/j.1600-051X.2002.290912.x. [DOI] [PubMed] [Google Scholar]
- 23.Weszl M, Skaliczki G, Cselenyák A, Kiss L, Major T, Schandl K, Bognár E, Stadler G, Peterbauer A, Csönge L, Lacza Z. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J Orthop Res. 2012;30:489–496. doi: 10.1002/jor.21527. [DOI] [PubMed] [Google Scholar]
- 24.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz S, Kabadayi C, Ipci SD, Cakar G, Kuru B. Treatment of intrabony periodontal defects with platelet-rich plasma versus platelet-poor plasma combined with a bovine-derived xenograft: a controlled clinical trial. J Periodontol. 2011;82:837–844. doi: 10.1902/jop.2010.100503. [DOI] [PubMed] [Google Scholar]