Abstract
Purpose
Ideally, a classification should have some prognostic value, and should therefore include precise information upon extent and location of the Achilles tendon disorders. We propose a new imaging and anatomical system to classify Achilles tendon disorders at imaging using US and MRI.
Approach
We consider the non-insertional region as the tendon mid-portion, and distinguish the insertional component into a pre-insertion site, located about two centimetres above the calcaneum, and a calcaneal insertion, where the tendon is attached to the bone. On sagittal scans, we introduced a new classification which considers two main portions: “musculotendinous” and “insertional”. In the context of the muscolotendinous portion, it is possible to find muscle fibres proximally, and the free tendon distally. This latter is made up of proximal, middle and distal portions. We also propose a 5 grade Doppler classification system to quantify blood flow, in which Grades I and II are respectively characterised by the presence of one and two vessels within the tendon; in Grades III, IV and V, the neovascularisation respectively involves less than 50 %, from 50 to 90 %, and more than 90 % of the tendon tissue. These proposed systems will require validation and possible modification to be applied to different tendons.
Introduction
The Achilles tendon, though strong, is one of the most frequently injured tendons of the human body [25, 30]. The terminology of Achilles tendon ailments is often unclear, but a recent nomenclature proposal has clarified some concerns about these disorders [44]. Achilles tendinopathy, a major cause of chronic pain at the heel [26, 29], presents histological features of failed healing response, with no evidence of intratendinous inflammation [27]. Tendinopathy is a failed healing response in which the tendon attempts to heal, but the healing process is not targeted and is disorganised, with haphazard proliferation of tenocytes, intracellular abnormalities in tenocytes, disruption of collagen fibres, and increase in non-collagenous matrix [44]. These changes may predispose the tendon to develop mechanical instability, and make it more susceptible to damage [27]. Previously described classifications do not allow to specifically define the exact site of pathology. Ideally, a classification should have some prognostic value, and should therefore include precise information upon extent and location of the Achilles tendon disorders. We first describe the anatomy and functional anatomy of the Achilles tendon, we then propose a new anatomical classification, and finally we report a modification of the power Doppler classification of neovascularity currently used in the field of tendinopathy [35].
Normal anatomy
The Achilles tendon (AT) is the thickest and strongest tendon in the human body. Its origin lies close to the middle of the calf, and fuses with the gastrocnemius muscle proximally [14]. The gastrocnemius is a fusiform muscle formed by two heads, medial and lateral, each separately crossing the knee joint. The medial and lateral heads fuse in a single muscle belly occupying the posterior superficial compartment of the lower leg [41, 45]. Deep to the gastrocnemius there is the soleus, a large flat, pennate muscle [14]. Together with the gastrocnemius, it forms the three-headed triceps surae, which acts to plantarflex the ankle joint via its conjoint tendon, the Achilles tendon. Finally, the plantaris is a small and thin vestigial muscle, originating from the popliteal surface of the femur. Its tendon inserts onto the medial aspect of calcaneum, anterior to the AT. It is absent in up to 8 % of individuals [34]. The blood supply of the tendon, from the musculotendinous junction, surrounding connective tissues, and the osteotendinous junction [1], is age dependent, and decreases with age [20]. The AT presents three main vascular areas: the peroneal artery supplies the midsection, while the posterior tibial artery supplies the proximal and distal sections. The relatively poor vascularisation of the mid-substance of the tendon might explain the frequent incidence of pathology at this site [5, 10].
Achilles tendon and insertion
The average length of the AT is 15 cm, ranging from 11 to 26 cm. The mean width of 6.8 cm (4.5–8.6 cm) at its origin gradually decreases at the midsection (1.8 cm, range 1.2–2.6 cm). The AT becomes more rounded at an average of 4 cm above the calcaneus, and has a width of 3.4 cm (2.0–4.8 cm) at its insertion site over the posterior surface of the calcaneus [2]. The relative contribution of the soleus and gastrocnemius fibres to the AT is variable, and the exact degree of contribution can be difficult to appreciate because of the changing orientation of the tendon fibres. From cadaver studies, in 52 % of the subjects 52 % of the Achilles tendon fibres come from the soleus and 48 % from the gastrocnemius, an equal contribution is provided in 35 %, and more than 60 % of contribution arises from the gastrocnemius in 13 % of cadavers [14].
Functional anatomy
The gastrocnemius, soleus, and plantaris muscles act as ankle flexors, while the gastrocnemius is also a knee flexor [19]. The gastrocnemius muscle is active in walking, jumping, and running, and it is composed predominantly of type II fibres [17]. The soleus muscle works as a stabiliser of the foot while standing, and consists primarily of type I fibres [18]. Consequently, muscle fibre atrophy of the soleus occurs more rapidly than that of the gastrocnemius, making the soleus muscle a more sensitive indicator of atrophy as a result of injury or denervation [6].
Anatomical classification
The lack of consistency about univocal and clear definition of the Achilles tendon disorders drove us to introduce a terminology which considers anatomical location, symptoms, clinical findings, and histopathology. Accordingly, we distinguished four main pathologies of the AT: mid-portion tendinopathy, rupture, paratendinopathy, and insertional tendinopathy [44]. In this article, we do not consider rupture. We propose a new imaging and anatomical model to classify the Achilles tendon disorders at imaging, US and MRI. The Achilles tendon is anatomically made up of non-insertional (proximal) and insertional (distal) portions [11] (Fig. 1). This anatomical description is somewhat generic and confusing, and we therefore suggest several modifications. We consider the non-insertional region as the tendon mid-portion, and distinguish the insertional component into a pre-insertion site, located about two centimetres above the calcaneum, and a calcaneal insertion, where the tendon is attached to the bone. On sagittal scans, we introduced a new classification which considers two main portions, which we describe as “musculotendinous” and “insertional” (Fig. 2) (Tables 1 and 2). In the context of the muscolotendinous portion, it is possible to find muscle fibres proximally, and the free tendon distally. This latter is formed by proximal, middle and distal portions (Fig. 3).
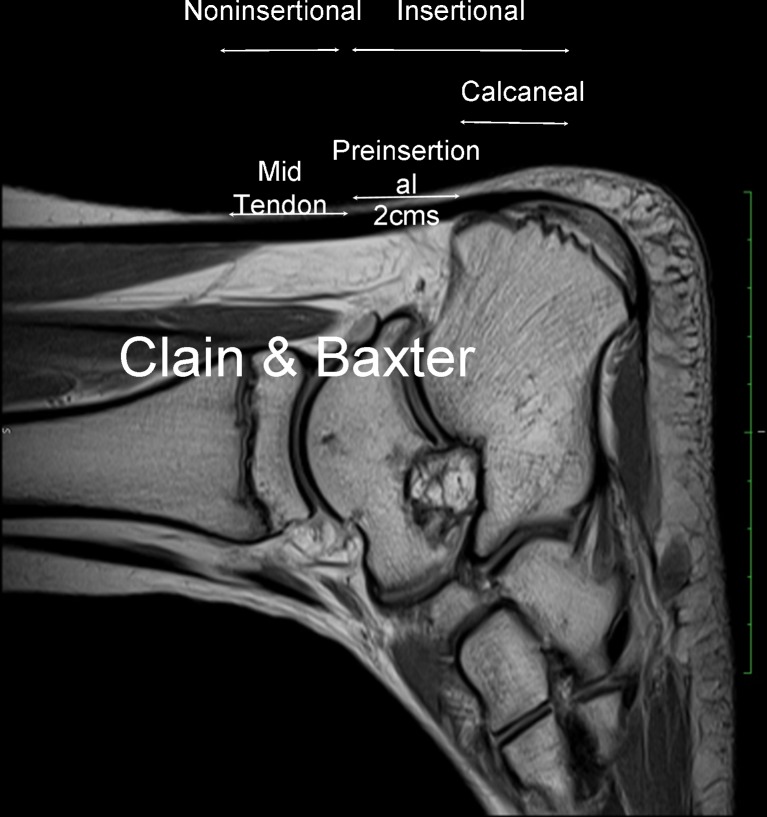
Fig. 1.
Sagittal T2 MRI scan in a 29-year-old track runner with 2-year history of Achilles tendinopathy. Calin & Baxter classification according which the Achilles tendon contains two main portions: non-insertional (proximal) and insertional (distal)
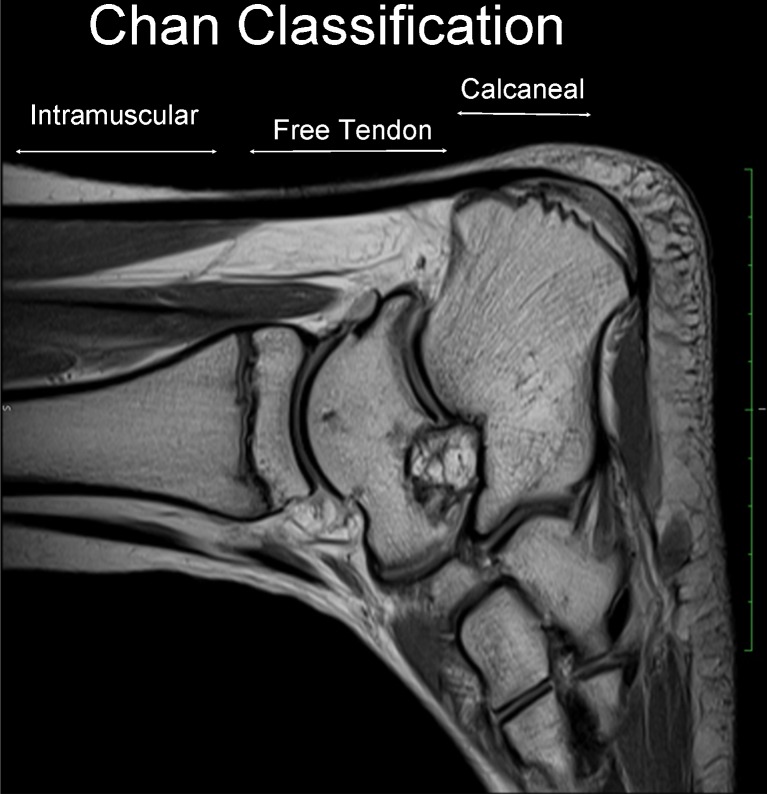
Fig. 2.
Sagittal T2 MRI scan. We present the new classification by Chan et al. that considers three main portions: “intramuscular”, “insertional” and “calcaneal” insertion
Table 1.
Anatomical classification
| Site of location |
|---|
| 1. Intramuscular |
| 2. Free tendon |
| A. Proximal |
| B. Middle |
| C. Distal |
| 3. Calcaneal |
Table 2.
Vascularisation grading system
| Vascularisation grading system | Imaging pattern |
|---|---|
| Grade I | One vessel into the tendon |
| Grade II | Two vessels into the tendon |
| Grade III | Neovascularisation involving less than 50 % of tendon thickness |
| Grade IV | Neovascularisation involving from 50 % to 90 % of tendon thickness |
| Grade V | Neovascularisation involving more than 90 % of tendon thickness |
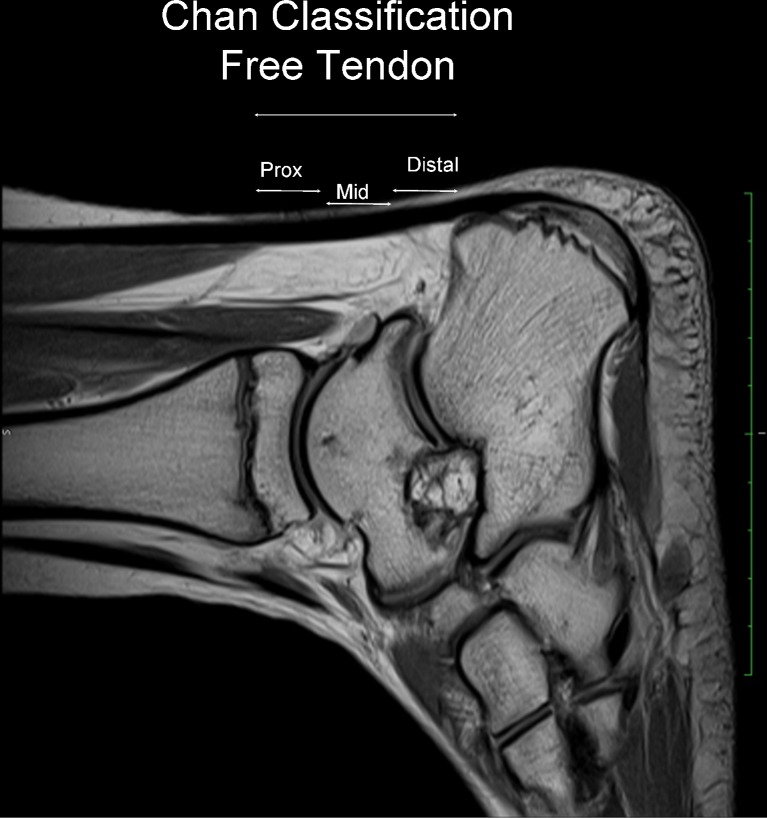
Fig. 3.
Sagittal T2 MRI scan. In the context of the free tendon, Chan et al. distinguished proximal, middle, and distal components, according to the involvement of which management changes
Imaging and Achilles tendon disorders
New technological advances have introduced sophisticated imaging tools to improve both diagnosis and prognosis of Achilles tendon disorders. Achilles tendon problems should be diagnosed clinically, and MRI and US should be used to better define and understand the location of the lesion.
MRI
On sagittal images, the anterior and posterior margins of the tendon should be parallel below the soleus insertion; on axial images, the anterior margin of the Achilles tendon is concave for most of its course. In the sagittal plane, the margin is straight or convex proximally, typically convex and at times bulbous at the soleus insertion, whilst both sides are fairly straight on coronal views. The normal retrocalcaneal bursa is visible on MR imaging, and should measure less than 6 mm of longitudinal length (superior to inferior), 3 mm medial to lateral, and 2 mm anterior to posterior [7]. In normal conditions, the Achilles tendon presents uniform low signal intensity in all commonly used MR sequences, but increased intratendinous signal intensity spots may be appreciated on axial T1-W and proton density-weighted images [20]. The anteroposterior diameter of the Achilles tendon, oval or ellipse shaped on cross-sectional images, is still undefined [23, 42]. A normal Achilles tendon has no internal signal intensity on any sequence, except at its insertion, but signal intensity changes may occur in the distal portion on axial FLASH images [7, 20], probably because of the presence of vessels in the context of tissue septa. On the other hand, the small ground-glass areas sometimes observed in asymptomatic tendons on axial FLASH sequences could be the earliest sign of tendinopathy around more severe intratendinous lesions [15]. In addition, the small percentage of tendon fibres not parallel to the main axis could provide an oblique orientation, the magic angle phenomenon [32]. Paratenon and paratendinous adhesions have to be differentiated from soft tissue swelling, inflammation of the paratenon, and true tendinous disease. The paratenon is a distinct layer over the dorsal aspect of the tendon, normally indistinct toward the calcaneal insertion, fused with the posterior subcutaneous structures. Its higher signal intensity on STIR images at the proximal and distal portions of the Achilles tendon may indicate decreased vascularisation at the level of the “critical zone” [8, 38], but caution should be used in interpreting a high signal intensity in this region. The Achilles tendon fat pad (the Kager’s triangle), low and high intense on STIR and T1-weighted images respectively, contain thin septa and vessels, and a signal abnormality should prompt the observer to exclude pathology. Increased signal intensity of the distal soleus muscle on STIR images could be a normal variant not related to the Achilles tendon or peritendinous disease, most likely attributed to the rich vascularity of the musculotendinous junction. A tendinopathic tendon is nodular and yellow, loses its usual glistening luster, and is often oedematous or fibrillated [4]. In insertional Achilles tendinopathy, the tendon is thickened distally, with vaguely defined longitudinal high signal, fairly intense, especially on fat suppressed image. In insertional tendinopathy, the typical internal signal observed on sagittal MRI scans may mimic a tear, which usually is, conversely, more proximal. Most Achilles tendon tears occur two to six centimetres from the insertion, but they can also occur distally and proximally [40]. Proximal Achilles tendon tears usually involve the musculotendinous junction and the medial head of the gastrocnemius of the dominant leg [31]. On axial images, when the myotendinous junction is involved, the musculotendinous junction of the Achilles tendon contains fluid which can extend along the distal margins of the muscle belly. On the other hand, the traditional U appearance of these tears on coronal images is expression of the fluid that dissects down, along the fascial plane of a distal muscle belly of the gastrocnemius [15]. The oedema seen within the muscle fibres may be diagnostic of a strain injury or of acute atrophy. In the latter case, although the medial head of the gastrocnemius is more commonly involved than the lateral head, the atrophy usually affects both heads because they act in concert [30]. Finally, haematoma could be another indirect sign of muscle injury.
Ultrasound
At US, the normal tendon is composed of fascicles of collagen fibres running in parallel, which appear as fibrillar hyperechoic (brighter) bands, and a closely adherent paratenon, often imperceptible, which appears as a synovial sheath filled with fluid when a paratendinopathy is present. Most tears and tendinopathy lesions occur approximately two to six centimetres from the insertion of the Achilles tendon on the calcaneus [39]. In this area, complete tears are easily differentiated from partial tears, but the nearby plantaris tendon, lying medially, can be mistaken for an intact Achilles tendon [37]. When diagnosis is doubtful, US examination may provide dynamic imaging, and ankle dorsiflexion may be helpful to detect tendon discontinuity [24]. At times, small tendon tears may be undetected, but gentle cyclical pressure with the probe or dorsi- and plantar-flexion of the ankle allows further tendon separation, and allows recognition of AT complete tears poorly demarcated by the presence of haematoma and associated debris [24]. In Achilles tendon disorders, US and MRI have similar accuracy, mostly when patients are referred for surgery, when the great sensitivity of US (81 %) and MRI (96 %) may reflect the great severity of symptoms [22]. The volume of intratendinous abnormalities seen on US may be used as a parameter of imaging severity, which however has to be corroborated by clinical symptoms. Some pitfalls may arise from the fact that some asymptomatic patients, especially when very active, may show positive US imaging findings, and US may be unable to provide prognostic indications, as the association between imaging changes over time and development of clinical symptoms is at best weak [12, 13, 21, 22]. Therefore, baseline US appearance is not able to predict clinical outcome. In Achilles tendinopathy, grey-scale ultrasonography is a cost-effective method to examine the Achilles tendon [3, 28, 33, 36]. As grey scale sonography is operator dependent, and relies on the detection of hypoechoic lesion and assessment of tendon thickening and widening, it was hoped that the addition of colour or power Doppler assessment would add objective evidence of pathology. Colour Doppler sonography is an established technique useful in the investigation of tendinopathy, with 92 % sensitivity and 100 % specificity [46]. Some authors believe that the value of US in Achilles tendinopathy is not enhanced by the addition of colour and power Doppler examination [22]. However, positive correlations have been shown between colour flow and power Doppler detections and positive findings on grey scale, as well as between neovascularisation and pain. Ohberg et al. found vessels through the entire thickness of the widened part of the tendon of patients with advanced tendinopathy, demonstrating with colour Doppler the correlation between occurrence of neovascularisation and ventral side widening of the tendon [35]. Until the histological cause of positive colour and power Doppler sonography is identified, the mechanism of this increased flow remains speculative [24]. Eccentric calf strengthening exercises [16] decreases these infiltrating vessels possibly by repeated constriction but, when patients are unresponsive, the injection under US guidance of sclerosing chemical substances or high volume formulation may remove these aberrant vessels, possibly normalising the Achilles tendon structure, and significantly relieving clinical symptoms [9].
Doppler classification
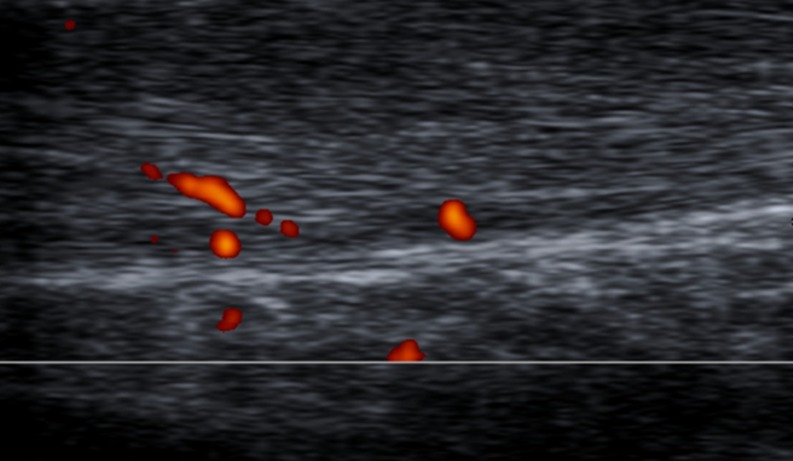
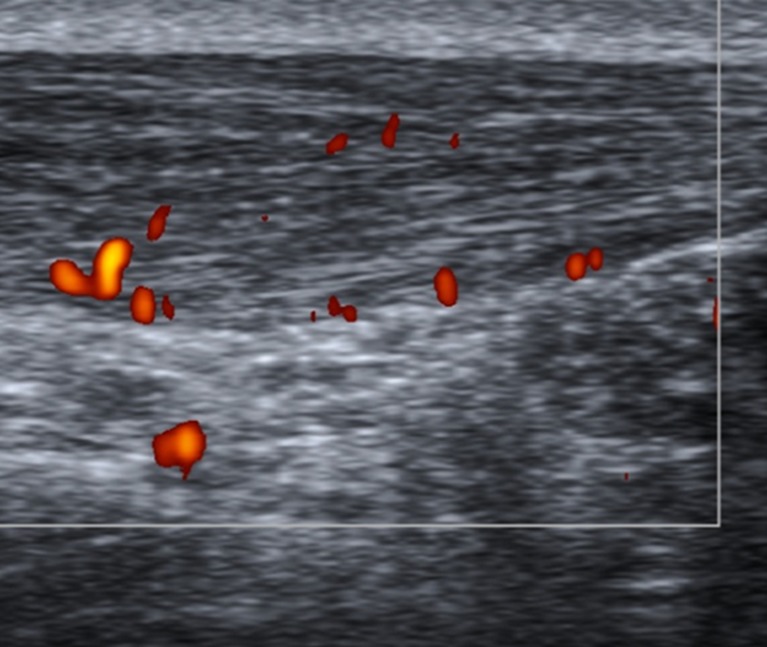
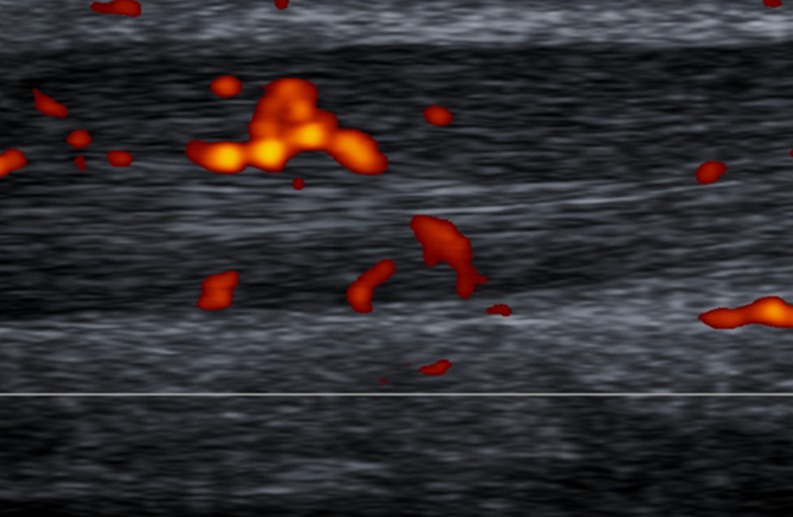
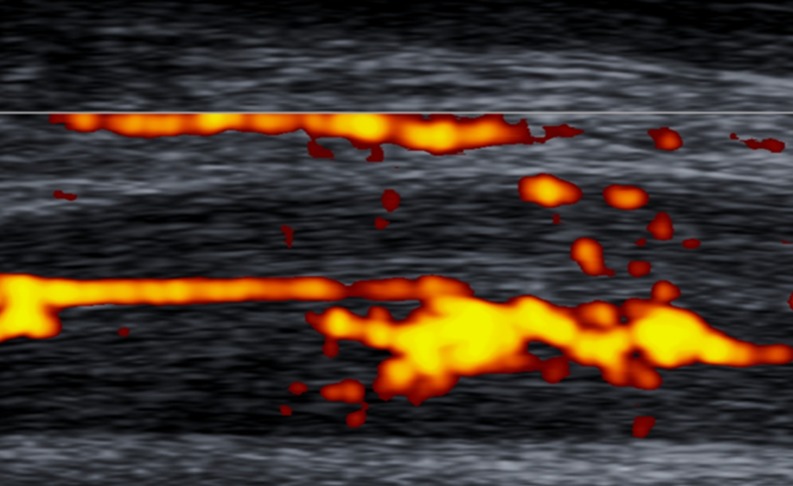
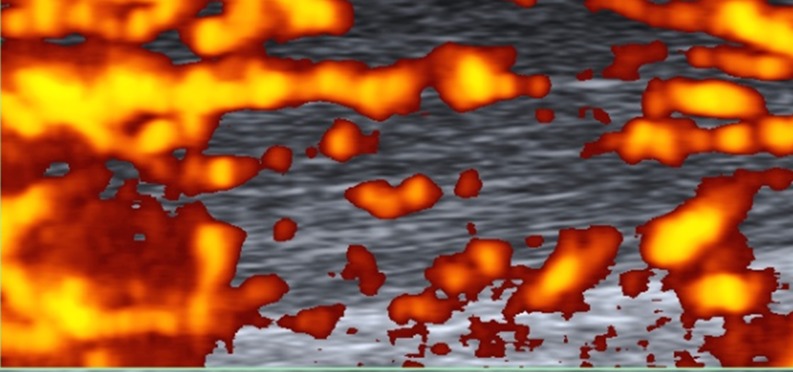
To standardise the pattern of vascularisation in Achilles tendinopathy, we propose a system to classify Doppler findings. The above-mentioned system consists of five grades: Grades I (Fig. 4) and II (Fig. 5) are respectively characterised by the presence of one and two vessels within the tendon; in Grades III, IV and V the neovascularisation involves less than 50 % (Fig. 6), from 50 to 90 % (Fig. 7), and more than 90 % of the tendon tissue (Fig. 8), respectively.
Fig. 4.
Sagittal US scan of the Achilles tendon in a 33-year-old male who was an asymptomatic soccer player. New Doppler classification By Chan et al.; Grade I vascularisation: only a single vessel may be appreciated within the tendon
Fig. 5.
Sagittal US scan of the tendon in a 27-year-old male rugby player with six months history of recurrent tendinopathy, located at the proximal and middle portions of the free tendon. On Doppler assessment, evidence of two vessels into the tendon (Grade II vascularisation)
Fig. 6.
Sagittal US Doppler assessment in a 31-year-old male who was an elite jumper with 15-month history of pain and stiffness to the free portion of the left Achilles tendon. The presence of vessels in more than 50 % of the thickness of the Achilles tendon is indicative of Grade III vascularisation
Fig. 7.
Sagittal US Doppler imaging in a 47-year-old female who was an amatuer runner complaining of symptoms to the insertion and free portion of the Achilles tendon. Grade IV vascularisation: evidence of vascularisation within 50–90 % of the tendon thickness
Fig. 8.
Sagittal US Doppler assessment in a 34-year-old male basketball player who was symptomatic for more than 18 months. Imaging shows tendon thickening and vascularisation involving more than 90 % of the tendon itself (Grade V)
Conclusions and perspectives
An inconsistent nomenclature of disorders of the Achilles tendon has resulted in a large number of confusing definitions and terms. A uniform and clear classification is necessary for research, diagnostic purposes and management planning. We propose a new classification, based on the anatomical location, pattern, and severity of the most common Achilles tendon disorders. These classifications consider the anatomical site, pattern, and severity of the lesion. We are aware that proposed systems will require validation and possible modification to be applied to different tendons. The anatomical classification should allow planning of more specific strategies of management. The power Doppler classification system may be positively correlated with clinical symptoms and US grey-scale findings [34]. However, in the light of recent evidence, the role of neovascularisation is questionable and, specifically, neo-vessels may not, in themselves, provide additional value for diagnosis and prognosis, i.e. appropriate clinical examination remains a mainstay for diagnosis and management [43]. All the above-mentioned limitations should be the object of research in studies which aim to test the reliability and validity of our proposed system.
References
- 1.Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J Orthop Res. 1998;16:591–596. doi: 10.1002/jor.1100160511. [DOI] [PubMed] [Google Scholar]
- 2.Apaydin N, Bozkurt M, Loukas M, Vefali H, Tubbs RS, Esmer AF. Relationships of the sural nerve with the calcaneal tendon: an anatomical study with surgical and clinical implications. Surg Radiol Anat. 2009;31(10):775–780. doi: 10.1007/s00276-009-0520-0. [DOI] [PubMed] [Google Scholar]
- 3.Astrom M, Gentz CF, Nilsson P, Rausing A, Sjoberg S, Westlin N. Imaging in chronic achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol. 1996;25:615–620. doi: 10.1007/s002560050146. [DOI] [PubMed] [Google Scholar]
- 4.Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. [PubMed] [Google Scholar]
- 5.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth FW. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol. 1977;43:656–661. doi: 10.1152/jappl.1977.43.4.656. [DOI] [PubMed] [Google Scholar]
- 7.Bottger BA, Schweitzer ME, El-Noueam KI, Desai M. MR imaging of the normal and abnormal retrocalcaneal bursae. AJR Am J Roentgenol. 1998;170:1239–1241. doi: 10.2214/ajr.170.5.9574592. [DOI] [PubMed] [Google Scholar]
- 8.Carden DG, Noble J, Chalmers J, Lunn P, Ellis J. Rupture of the calcaneal tendon. The early and late management. J Bone Joint Surg Br. 1987;69:416–420. doi: 10.1302/0301-620X.69B3.3294839. [DOI] [PubMed] [Google Scholar]
- 9.Chan O, O’Dowd D, Padhiar N, et al. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil. 2008;30:1697–1708. doi: 10.1080/09638280701788225. [DOI] [PubMed] [Google Scholar]
- 10.Chen TM, Rozen WM, Pan WR, Ashton MW, Richardson MD, Taylor GI. The arterial anatomy of the Achilles tendon: anatomical study and clinical implications. Clin Anat. 2009;22:377–385. doi: 10.1002/ca.20758. [DOI] [PubMed] [Google Scholar]
- 11.Clain MR, Baxter DE. Achilles tendinitis. Foot Ankle. 1992;13:482–487. doi: 10.1177/107110079201300810. [DOI] [PubMed] [Google Scholar]
- 12.Cook JL, Khan KM, Kiss ZS, Coleman BD, Griffiths L. Asymptomatic hypoechoic regions on patellar tendon ultrasound: a 4-year clinical and ultrasound followup of 46 tendons. Scand J Med Sci Sports. 2001;11:321–327. doi: 10.1034/j.1600-0838.2001.110602.x. [DOI] [PubMed] [Google Scholar]
- 13.Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000;19:473–479. doi: 10.7863/jum.2000.19.7.473. [DOI] [PubMed] [Google Scholar]
- 14.Doral MN, Alam M, Bozkurt M, et al. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc. 2010;18:638–643. doi: 10.1007/s00167-010-1083-7. [DOI] [PubMed] [Google Scholar]
- 15.Erickson SJ, Prost RW, Timins ME. The “magic angle” effect: background physics and clinical relevance. Radiology. 1993;188:23–25. doi: 10.1148/radiology.188.1.7685531. [DOI] [PubMed] [Google Scholar]
- 16.Fahlstrom M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327–333. doi: 10.1007/s00167-003-0418-z. [DOI] [PubMed] [Google Scholar]
- 17.Fugl-Meyer AR, Nordin G, Sjostrom M, Wahlby L. Achilles tendon injury. 5. A model of isokinetic strength training using biofeedback. Scand J Rehabil Med. 1979;11:37–44. [PubMed] [Google Scholar]
- 18.Garrett WE, Califf JC, Bassett FH. Histochemical correlates of hamstring injuries. Am J Sports Med. 1984;12:98–103. doi: 10.1177/036354658401200202. [DOI] [PubMed] [Google Scholar]
- 19.Grumbine NA, Santoro JP. The tendo Achillis as it relates to rearfoot position. A new classification for evaluation of calcaneal stance position. Clin Podiatr Med Surg. 1990;7:203–216. [PubMed] [Google Scholar]
- 20.Hastad K, Larsson LG, Lindholm A. Clearance of radiosodium after local deposit in the Achilles tendon. Acta Chir Scand. 1959;116:251–255. [PubMed] [Google Scholar]
- 21.Khan KM, Cook JL, Kiss ZS, et al. Patellar tendon ultrasonography and jumper’s knee in female basketball players: a longitudinal study. Clin J Sport Med. 1997;7:199–206. doi: 10.1097/00042752-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Khan KM, Forster BB, Robinson J, et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med. 2003;37:149–153. doi: 10.1136/bjsm.37.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagergren C, Lindholm A. Vascular distribution in the Achilles tendon; an angiographic and microangiographic study. Acta Chir Scand. 1959;116:491–495. [PubMed] [Google Scholar]
- 24.Lento PH, Primack S. Advances and utility of diagnostic ultrasound in musculoskeletal medicine. Curr Rev Musculoskelet Med. 2008;1:24–31. doi: 10.1007/s12178-007-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maffulli N. Rupture of the Achilles tendon. J Bone Joint Surg Am. 1999;81:1019–1036. doi: 10.2106/00004623-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Maffulli N. Augmented repair of acute Achilles tendon ruptures using gastrocnemius-soleus fascia. Int Orthop. 2005;29:134. doi: 10.1007/s00264-004-0632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/S0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 28.Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21:158–162. doi: 10.1136/bjsm.21.4.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maffulli N, Spiezia F, Longo UG, Denaro V. Z-shortening of healed, elongated Achilles tendon rupture. Int Orthop. 2012;36:2087–2093. doi: 10.1007/s00264-012-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med. 1999;9:157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Millar AP. Strains of the posterior calf musculature (“tennis leg”) Am J Sports Med. 1979;7:172–174. doi: 10.1177/036354657900700306. [DOI] [PubMed] [Google Scholar]
- 32.Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:479–481. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- 33.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68:170–175. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005;10:225–238. doi: 10.1016/j.fcl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 36.Paavola M, Paakkala T, Kannus P, Jarvinen M. Ultrasonography in the differential diagnosis of Achilles tendon injuries and related disorders. A comparison between pre-operative ultrasonography and surgical findings. Acta Radiol. 1998;39:612–619. doi: 10.3109/02841859809175485. [DOI] [PubMed] [Google Scholar]
- 37.Patel S, Fessell DP, Jacobson JA, Hayes CW, van Holsbeeck MT. Artifacts, anatomic variants, and pitfalls in sonography of the foot and ankle. AJR Am J Roentgenol. 2002;178:1247–1254. doi: 10.2214/ajr.178.5.1781247. [DOI] [PubMed] [Google Scholar]
- 38.Sandelin J, Kiviluoto O, Santavirta S, Honkanen R. Outcome of sports injuries treated in a casualty department. Br J Sports Med. 1985;19:103–106. doi: 10.1136/bjsm.19.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller AD, Kasser JR, Quigley TB. Tendon injuries about the ankle. Orthop Clin North Am. 1980;11:801–811. [PubMed] [Google Scholar]
- 40.Schweitzer ME, Karasick D. MR imaging of disorders of the Achilles tendon. AJR Am J Roentgenol. 2000;175:613–625. doi: 10.2214/ajr.175.3.1750613. [DOI] [PubMed] [Google Scholar]
- 41.Seebacher JR, Inglis AE, Marshall JL, Warren RF. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64:536–541. [PubMed] [Google Scholar]
- 42.Soma CA, Mandelbaum BR. Achilles tendon disorders. Clin Sports Med. 1994;13:811–823. [PubMed] [Google Scholar]
- 43.Tol JL, Spiezia F, Maffulli N. Neovascularisation in Achilles tendinopathy: have we been chasing a red herring? Knee Surg Sports Traumatol Arthrosc. 2012;20:1891–1894. doi: 10.1007/s00167-012-2172-6. [DOI] [PubMed] [Google Scholar]
- 44.van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011;19:835–841. doi: 10.1007/s00167-010-1374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe Y, Moriya H, Takahashi K, et al. Functional anatomy of the posterolateral structures of the knee. Arthroscopy. 1993;9:57–62. doi: 10.1016/S0749-8063(05)80344-5. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg EP, Adams MJ, Hollenberg GM. Color Doppler sonography of patellar tendinosis. AJR Am J Roentgenol. 1998;171:743–744. doi: 10.2214/ajr.171.3.9725308. [DOI] [PubMed] [Google Scholar]