Abstract
AIM: To facilitate engineering of suitable biomaterials to meet the challenges associated with myocardial infarction.
METHODS: Poly (glycerol sebacate)/collagen (PGS/collagen) core/shell fibers were fabricated by core/shell electrospinning technique, with core as PGS and shell as collagen polymer; and the scaffolds were characterized by scanning electron microscope (SEM), fourier transform infrared spectroscopy (FTIR), contact angle and tensile testing for cardiac tissue engineering. Collagen nanofibers were also fabricated by electrospinning for comparison with core/shell fibers. Studies on cell-scaffold interaction were carried out using cardiac cells and mesenchymal stem cells (MSCs) co-culture system with cardiac cells and MSCs separately serving as positive and negative controls respectively. The co-culture system was characterized for cell proliferation and differentiation of MSCs into cardiomyogenic lineage in the co-culture environment using dual immunocytochemistry. The co-culture cells were stained with cardiac specific marker proteins like actinin and troponin and MSC specific marker protein CD 105 for proving the cardiogenic differentiation of MSCs. Further the morphology of cells was analyzed using SEM.
RESULTS: PGS/collagen core/shell fibers, core is PGS polymer having an elastic modulus related to that of cardiac fibers and shell as collagen, providing natural environment for cellular activities like cell adhesion, proliferation and differentiation. SEM micrographs of electrospun fibrous scaffolds revealed porous, beadless, uniform fibers with a fiber diameter in the range of 380 ± 77 nm and 1192 ± 277 nm for collagen fibers and PGS/collagen core/shell fibers respectively. The obtained PGS/collagen core/shell fibrous scaffolds were hydrophilic having a water contact angle of 17.9 ± 4.6° compared to collagen nanofibers which had a contact angle value of 30 ± 3.2°. The PGS/collagen core/shell fibers had mechanical properties comparable to that of native heart muscle with a young’s modulus of 4.24 ± 0.7 MPa, while that of collagen nanofibers was comparatively higher around 30.11 ± 1.68 MPa. FTIR spectrum was performed to confirm the functional groups present in the electrospun scaffolds. Amide I and amide II of collagen were detected at 1638.95 cm-1 and 1551.64 cm-1 in the electrospun collagen fibers and at 1646.22 cm-1 and 1540.73 cm-1 for PGS/collagen core/shell fibers respectively. Cell culture studies performed using MSCs and cardiac cells co-culture environment, indicated that the cell proliferation significantly increased on PGS/collagen core/shell scaffolds compared to collagen fibers and the cardiac marker proteins actinin and troponin were expressed more on PGS/collagen core/shell scaffolds compared to collagen fibers alone. Dual immunofluorescent staining was performed to further confirm the cardiogenic differentiation of MSCs by employing MSC specific marker protein, CD 105 and cardiac specific marker protein, actinin. SEM observations of cardiac cells showed normal morphology on PGS/collagen fibers and providing adequate tensile strength for the regeneration of myocardial infarction.
CONCLUSION: Combination of PGS/collagen fibers and cardiac cells/MSCs co-culture system providing natural microenvironments to improve cell survival and differentiation, could bring cardiac tissue engineering to clinical application.
Keywords: Mesenchymal stem cells, Cardiac cells, Co-culture, Cardiac patch, Poly (glycerol sebacate), Core/shell fibers.
INTRODUCTION
Myocardial infarction (MI), known as “heart attack” leads to loss of cardiomyocytes and the injured heart tissue is replaced by scar tissue[1]. When the scar is large enough to interfere with the hearts normal rhythm, heart failure occurs. Nearly 8 million Americans each year experience myocardial infarction[2] and must cope with the consequences of compromised heart muscle function. The myocardial tissue lacks significant intrinsic regenerative capacity to replace the lost cells[3]. Moreover, the relative shortage of organ donors to recipients, and the ineligibility of many heart patients for transplantation revamp the search for new strategies to repair the injured myocardium. The current methods employed include cardiac restraint devices like acorn corcap heart mesh (knitted polyester)[4], marlex mesh (polypropylene)[5], and Merselene mesh (knitted polyester)[6]. Cell transplantation therapies have shown promise for improving heart function after myocardial infarction[7]. However, the cell engraftment efficiency is low due to significant loss of cells from the site of injury following transplantation.
One promising approach is to prevent the increase of heart failure after myocardial infarction is the implantation of engineered cardiac patch at the site of infarction. In addition of having enough elasticity for mechanical support, an ideal cardiac patch material must provide an excellent milieu for cell survival. Furthermore, the ideal biomaterial should be capable of being safely replaced by newly formed tissue and also degrade appropriate time period without producing any toxic products[8]. Naturally derived materials used in experimental or clinical treatment of infarcts include tumor-derived basement membrane matrix gel (matrigel)[9], alginate[10], collagen[11], laminin[12], fibrin[13] and decellularized extracellular matrix (ECM)[14], all of which can enhance cell and tissue function in the myocardial region. They provide a natural substrate for cellular attachment, proliferation, and differentiation in its native state. For the above-mentioned reasons, natural polymers like collagen could be a favourable substrate for tissue engineering applications[15,16]. Furthermore, collagen is the most abundant protein in the human body, and imparts structural integrity and tensile strength to tissues.
Tissue disruption following injury requires collagen for the repair and restoration of structure and function. In addition, collagens have a low antigenicity, being only weakly immunogenic largely due to their homology across species and are biodegradable due to their proteinaceous nature[17]. Using matrigel, a collagen-based multicomponent mixture of ECM proteins and growth factors, Zimmermann et al[18] have established one of the most convincing models of three-dimensional cardiac cell cultures, where differentiation status and functional parameters are similar to that of native myocardium. However, there are concerns about Matrigel’s safety because matrigel and basement membrane matrix are known to enhance tumorigenesis and tumor growth in vivo[19-22]. In 1997, Eschenhagen et al[23] reported for the first time, an artificial heart tissue, which was termed engineered heart tissue (EHT). The embryonic chick cardiomyocytes were mixed with collagen solution and allowed to form gel for EHT. By culturing the cardiomyocytes in the collagen matrix, they produced a spontaneously and coherently contracting 3D heart tissue construct in vitro. However, poor mechanical supportive ability of collagen gels was a major drawback associated with this approach. Hence a combination of poly (glycerol sebacate) (PGS) with collagen was suggested with an objective to overcome this drawback for cardiac tissue engineering (CTE). The elastomer PGS, recently developed for soft tissue engineering[24,25], represents a feasible substrate from the mechanical perspective; Collagen favours enhanced cell adhesion and prevents cells loss at the site of implantation. The conventional electrospinning technique is to dissolve the polymer in a solvent, which evaporates during the spinning process. However, this approach is not pragmatic with PGS. Although, there are solvents available for dissolving PGS[26], its low molecular weight results in such a low solution viscosity that even with a high concentration solution, the electrospinning of PGS fibers cannot occur. Hence, it was necessary to develop a core/shell electrospinning process[27] to produce PGS fibers with a protective shell polymer. The combination of PGS/collagen core/shell fibers with a unique ECM like topography has been suggested to be a potential cardiac patch material for MI. Fabricated core/shell material; the core material is solely responsible for mechanical properties, whereas the shell material is responsible for extrinsic factors like cell adhesion and proliferation. The optimal cell source to create an engineered myocardial patch should be easy to harvest, proliferative, non-immunogenic and has the ability to differentiate into mature, functional cardiomyocytes. Studies have shown that after expansion, stem cells can be directed to differentiate into cardiomyogenic lineages[28,29]. Cardiomyocytes have natural contractile and electrophysiological properties, are difficult to obtain, to expand, and are allogenic. In contrast, bone marrow derived stem cells have the ability to differentiate into any desired cell type in the presence of cues and are non-immunogenic, making them an ideal cell source.
In this study, we hypothesize that a combinatorial approach of PGS/collagen core/shell fibrous patch material and stem cell therapy is of potential interest for the treatment of heart failure rather than either strategy alone. Our approach takes advantage of the ability of an elastomeric biomaterial sheet comprising of PGS/collagen fibers to act as a flexible patch; with this approach: (1) Cells would remain adhered to the nanofibrous patch preventing cell loss and providing a more site-directed repair mechanism. It is increasingly accepted that physical cues play a key role in cell growth and tissue assembly[30,31]. These signals are important in stem cells during self-renewal, proliferation, and differentiation; (2) A softer substrate and the ability to tune the mechanical properties within a given range could be advantageous as cell differentiation was shown to be affected by substrate stiffness[32]; (3) Additionally, it has been estimated that a cell number on the order of one billion would need to be replaced in patients with heart failure[33]. The present study proposed PGS/collagen core/shell fibrous scaffold similar to cardiac ECM like topography, which promotes in situ regeneration and homing of cells; thereby reducing the number of requisite cells, is desirable for cardiac tissue engineering.
MATERIALS AND METHODS
Fabrication of core/shell fibers
PGS was synthesized by the procedure described by Wang et al[34] a mixture of glycerol and sebacic acid in the ratio of 1:1 was reacted at 120 °C under nitrogen for 24 h. The pressure was then reduced to 40 m Torr and the reaction held at 120 °C for 48 h to synthesise PGS. Collagen type I (8%) was dissolved in 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol (HFP) (Aldrich Chemical Company, Inc., St. Louis, United States) to form shell solution and PGS (15%) was dissolved in same solvent to form the core solution. The coaxial spinneret had an inner diameter of 1 mm and an outer diameter of 2.0 mm was designed such that the fluids were immiscible before exiting the nozzle. Fluid was loaded to the nozzle by two syringe pumps (KD Scientific Inc., MA, United States) that provide a constant-volume flow rate of 0.3 mL/h for core solution and 1.2 mL/h for shell solution. A high voltage electric field (DC high voltage power supply from Gamma High Voltage Research, FL, United States) of 15 kV was applied at the tip of the spinneret. A collector plate was placed at a distance of 15 cm from the tip of the spinneret to collect core/shell fibers. Collagen nanofibers were also fabricated using 8% w/v solution in HFP separately. The electrospinning conditions used were 1.2 mL/h flow rate, 12 cm distance between the needle tip and collector plate and 12 kV voltage supply. The fibers produced were subsequently vacuum dried to remove the residual solvents. The fibers were then cross-linked using 50% glutaraldehyde (Sigma) vapour for 24 h in order to improve its mechanical stability.
Material characterization
The surface morphology of electrospun nanofibrous scaffolds was studied under scanning electron microscope (JEOL JSM-5600LV) at an accelerating voltage of 10 kV, after gold coating (JEOL JFC-1200 fine coater, Japan). For calculating the fiber diameter of the nanofibers from the SEM images, n = 10 fibers were chosen randomly on each of the scaffolds. For each scaffold material n = 5 samples were chosen for measuring the fiber diameter. The average fiber diameter along with SD was then analyzed from the SEM images using image analysis software (Image J, National Institutes of Health, United States). Functional groups present in the scaffolds were analyzed using Fourier Transform Infrared (FTIR) spectroscopic analysis on avatar 380, (Thermo Nicolet Waltham, MA, United States) over a range of 400-4000 cm-1 at a resolution of 4 cm-1. The hydrophobic or hydrophilic nature of electrospun fibers was measured by sessile drop water contact angle measurement using VCA optima surface analysis system (AST products, Billerica, MA, United States). Tensile properties of electrospun fibrous scaffolds were determined using a tabletop tensile tester (Instron 3345, United States) at 10 mol/L load capacity under dry testing conditions. Rectangular specimens of dimensions 10 mm × 20 mm were used for testing at a rate of 10 mm/min. The data’s were recorded at room conditions 25 °C and 34% humidity. In order to avoid uncertainty of data, only the results of those samples which have failed in the centre; giving a dog bone like appearance have been used for the stress-strain curves calibration. The data’s of those samples which have failed at the grips, where the load was being applied, were not employed for the calculation. The tensile stress-strain curve was drawn using excel sheet.
Cell culture
The electrospun fibers collected on round glass cover slips of 15 mm in diameter, were placed in a 24-well plate with stainless steel rings to prevent lifting on the cover slips. The fibers were sterilized under ultraviolet (UV) light for 2 h, washed thrice with phosphate buffered saline (PBS) for 15 min each, in order to remove any residual solvent and prevent cytotoxicity from glutaraldehyde. The fibers were subsequently immersed in Dulbecco’s modified Eagle’s medium (DMEM) overnight before cell seeding. The rabbit cardiac cells were isolated using collagenase treatment. The rabbit heart was fragmented into tiny pieces and washed thoroughly with 2× and 3× antibiotic solutions made in PBS, thrice for 30 min. Subsequently, it was followed by treatment with 1% collagenase in PBS at 37 °C for 30 min. The fragmented tissues were cultured in DMEM media supplemented with 10% FBS (GIBCO Invitrogen, United States) and 1% antibiotic and antimycotic solutions (Invitrogen Corp, United States) in a 75 cm2 cell culture flask to isolate cardiomyocytes. The culture medium was changed once in every 2 d. MSCs (PT-2501, Lonza, United States) were cultured in low glucose DMEM media supplemented with 10% FBS (GIBCO Invitrogen, United States) and 1% antibiotic and antimycotic solutions (Invitrogen Corp, United States) in a 75 cm2 cell culture flask. Cells were incubated in CO2 incubator at 37 °C at 5% CO2. Before seeding the cells were detached by adding 1 mL of 0.25% trypsin containing 0.1% EDTA. Detached cells were centrifuged, counted by trypan blue assay using a hemocytometer and seeded on the scaffolds. The scaffolds were separated into three groups-the control tissue culture plate (TCP), collagen nanofibrous scaffolds and the PGS/collagen core/shell fibers. These were further segregated into (1) co-culture scaffolds, onto which both MSCs and cardiac cells were seeded in the ratio of 1:1 at a seeding density of 10 000 cells per well (5000 MSCs:5000 cardiac cells); (2) positive control scaffolds, onto which cardiac cells were seeded at the same seeding density of 10 000 cells per well and (3) The final batch comprised of the negative control scaffolds, onto which MSCs were seeded at the same seeding density of 10 000 cells per well.
Cell proliferation
The cell proliferation on different scaffolds was analyzed using MTS assay (CellTiter 96 Aqueous One solution reagent, purchased from Promega, Madison, WI, United States). The rationale behind the MTS assay involves the reduction of yellow tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium] in MTS to form purple formazan crystals by the dehydrogenase enzymes secreted by mitochondria of metabolically active cells. The formazan dye shows absorbance at 490 nm and the amount of formazan crystals formed is directly proportional to the number of cells. After 5 d of cell seeding, the media was removed from the well plates and the scaffolds were washed in PBS. The scaffolds were then incubated in a 1:5 ratio mixture of MTS assay and serum free DMEM medium for 3 h at 37 °C in a 5% CO2 incubator. After the incubation period, the samples were pipette out into 96 well plates. The absorbance reading was then taken at 490 nm using a microplate reader (Fluostar Optima, BMG Lab Technologies, Germany). The same procedure was repeated for day 10 and day 15 samples.
Cell morphology
The cell morphology was analyzed using SEM. After 15 d of seeding cells on the scaffolds, the media was removed from the wells and the samples were fixed with 3% glutaraldehyde in PBS for 3 h. The scaffolds were then rinsed with distilled water for 15 min and then dehydrated with a series of ethanol gradients starting from 30% to 50%, 75%, 90% and 100% (v/v). Subsequently the samples were treated with Hexamethyldisilazane (HMDS) solution (Sigma) and allowed to air-dry at room temperature in the fume hood. The samples were then gold coated and the cells morphology was analyzed using SEM.
Expression of cardiac marker protein
To observe whether the MSCs co-cultured with cardiac cells have undergone cardiogenic differentiation, immunofluorescent staining of the selected proteins of cardiomyocytes was performed. For confocal analysis, the cells were fixed with 100% ice cold methanol for 15 min. The samples were then washed with PBS once for 15 min and incubated in 0.5% Triton-X solution for 5 min to permeabilize the cell membrane. Non-specific sites were blocked by incubating the cells in 3% BSA (Sigma) for 1 h. Following which primary antibodies α-actinin and troponin-T (Sigma) were added into separate wells, at the dilution of 1:100 and incubated for 90 min at room temperature. This was followed by washing the samples thrice with PBS for 15 min, to remove the excess unbound primary antibodies; followed by incubation for 60 min with Alexa Fluor 488 secondary antibodies (Invitrogen) in the dilution of 1:250 at room temperature. The samples were again washed thrice with PBS for 15 min. Negative controls were also employed in each analysis to delete the disturbance of the primary or secondary antibody. The cell nuclei were stained using 1:5000 dilution of 4,6-diamidino-2-phenylindole hydrochloride (DAPI; Sigma) for 30 min at room temperature. The cells were again washed with PBS thrice to remove any excess staining. The samples were then removed and mounted over glass slides using H-1000 vectashield mounting medium (Vector Laboratories, United States). The edges of the coverslips were sealed using fluoromount. The samples were then viewed using fluorescence microscopy for the cardiac marker protein expression (Olympus FV 1000).
Double immunofluorescent staining was further performed on the co-culture scaffolds to confirm the differentiation of MSCs into cardiomyocytes. The MSC-cardiac cells co-culture cells cultured on TCP, collagen nanofibers and PGS/collagen core/shell fibers were stained with MSC specific marker CD 105 (abcam, United States) in the dilution 1:100 for 90 min. at room temperature; prior to which the non-specific sites were blocked with 3% BSA. This was followed by the addition of secondary antibody Alexa Fluor 488 (green) in the dilution 1:250 for 60 min. at room temperature. The samples were washed with PBS thrice to remove the excess staining. The samples were then treated with cardiac specific marker protein actinin in the dilution 1:100 for 90 min at room temperature. This was followed by the addition of the secondary antibody Alexa Fluor 594 (red) (Invitrogen) in the dilution 1:250 for 60 min. at room temperature. The samples were washed with PBS thrice to remove the excess staining. The samples were then incubated with DAPI in the dilution 1:5000 for 30 min at room temperature. The samples were then removed and mounted over a glass slide using vectashield mounting agent and examined under the fluorescent microscope (Olympus FV 1000).
Statistical analysis
For each experiment n = 5 samples were tested and all the data presented are expressed as mean ± SD and were analyzed using Student’s t-test for the calculation of statistical significance. Differences were considered statistically significant at P ≤ 0.05 and P ≤ 0.01.
RESULTS
Material characterization
Electrospinning is a versatile technique for bio-mimicking the natural environment similar to that of ECM for better cell adhesion and tissue growth[35]. It has been estimated that a cell number on the order of one billion would need to be replaced in patients with heart failure[33]. The proposed scaffold with ECM like topography is desirable as it has been suggested that the ECM scaffold itself contains components that are chemo-attractants to endogeneous stem cells[36]. Collagen lacks mechanical integrity upon hydration in an electrospun form for tissue engineering. Without cross-linking electrospun structure it does not have sufficient mechanical strength. Previously established methods of cross-linking with glutaraldehyde, was successful in increasing the strength of electrospun structures[37]. Moreover, the existence of collagen on the surface provides uninterrupted cell recognition signals with polymer degradation, which is essential for cell function and development[38]. SEM micrographs (Figure 1) of electrospun fibrous scaffolds revealed porous, beadless, uniform fibers with a diameter in the range of 380 ± 77 nm and 1192 ± 277 nm for collagen fibers and PGS/collagen core/shell fibers respectively. The PGS/collagen core/shell fibers have a contact angle of 17.9 ± 4.6° compared to collagen 30 ± 3.2°. FTIR spectrum was carried out to confirm the functional groups present in electrospun scaffolds. Amide I and amide II of collagen were detected at 1638.95 cm-1 and 1551.64 cm-1 in the electrospun collagen fibers and at 1646.22 cm-1 and 1540.73 cm-1 for PGS/collagen core/shell fibers respectively as shown in Figure 2. The characteristic peak of PGS at 1740 cm-1 is absent in the PGS/collagen core/shell FTIR spectrum because PGS has been incorporated as the core material in the core/shell system.
Figure 1.
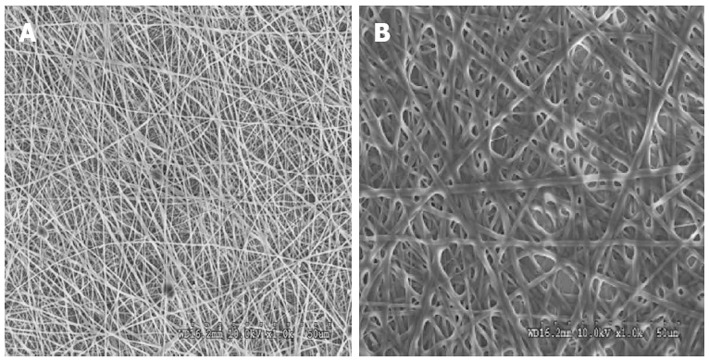
Scanning electron microscope image showing the fiber morphology of (A) collagen fibers with a fiber diameter of 380 ± 77 nm (B) poly (glycerol sebacate)/collagen core/shell fibers with a fiber diameter of 1192 ± 277 nm at 1000 × magnification.
Figure 2.
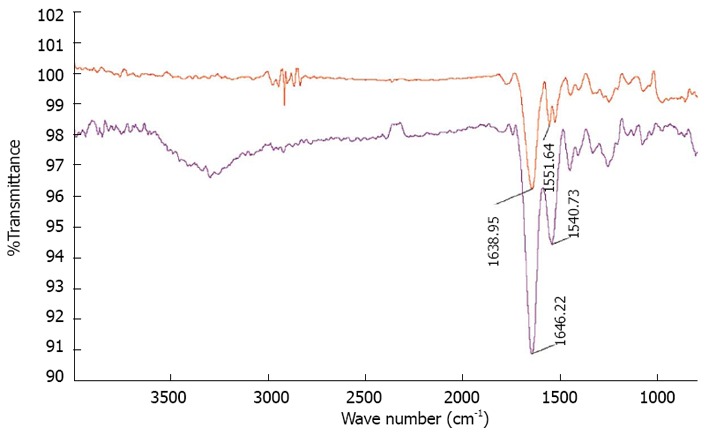
Fourier transform infrared spectroscopy images of red curve-collagen fibers showing characteristic amide peaks at 1638.95 cm-1and 1551.64 cm-1 blue curve-poly (glycerol sebacate)/collagen core/shell fibers showing characteristic amide peaks at 1646.22 cm-1 and 1540.73 cm-1.
For any tissue-engineered construct to be functional, it is imperative to determine its material properties and compare against the native heart muscle. A comparison of tensile properties of nanofibers with respect to tensile stress and strain is illustrated in Table 1. We found that the Young’s modulus of collagen decreases further in the presence of PGS from 30.11 ± 1.68 MPa to 4.24 ± 0.7 MPa. The principal structural protein of adult myocardium is collagen and of the total collagen in the myocardium 85% is type I and 11% is type III[39]. The roles of collagens I and III are different: collagen I is thicker and provides stiffness and structural support while collagen III is thinner and provides flexibility and elastic recovery[40]. The two types of collagens jointly support and maintain the myocyte alignment, whereas their tensile strength and resilience resist the deformation, thereby contributing to the passive and active stiffness of the myocardium[41]. Radisic et al[42] applied electrical signals onto ultrafoam collagen sponges using matrigel seeded with rat cardiomyocytes to mimic the native heart for cardiac tissue engineering. However, poor mechanical properties, the lack of stability and large degree of swelling (approximately 30%) immediately following hydration in culture medium[43], hampered its clinical applications. Figure 3, we observed that the elastic modulus of PGS/collagen core/shell fibers is 4.24 ± 0.7 MPa, which is nearing to native myocardium, while that of collagen nanofibers was comparatively higher around 30.11 ± 1.68 MPa. Hence, PGS/collagen core/shell fibers are a novel concept for improving the mechanical stability as well as not compromising on the biocompatibility of the patch material. Additionally, PGS has other desirable properties including control of its mechanical properties, which can match those of native myocardium[44], and support of adhesion and phenotypic protein expression for a variety of primary cell types in vitro[45].
Table 1.
Tensile properties of collagen and poly (glycerol sebacate)/collagen fibrous membranes
| Membrane | Tensile stress (MPa) | Tensile strain at maximum load | Tensile strain at break | Young’s modulus (MPa) | Water contact angle mean ± SD |
| Collagen | 2.79 | 13.59% | 47.92% | 30.11 | 30 ± 3.2 |
| PGS/collagen | 2.06 | 57.87% | 83.65% | 4.24 | 17.9 ± 4.6 |
PGS: Poly (glycerol sebacate).
Figure 3.
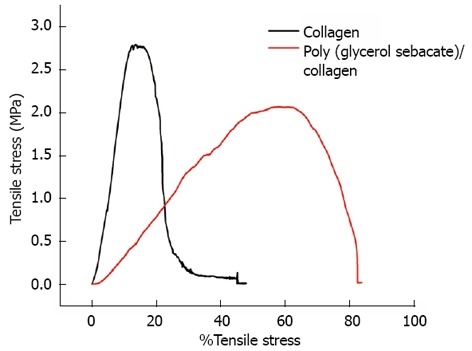
Tensile stress-strain curves of collagen fibers and poly (glycerol sebacate)/collagen core/shell fibers.
DISCUSSION
The scaffold properties play an important role in influ-encing the cell responses for cardiac tissue engineering. Cell behaviour such as adhesion and proliferation represent the initial phase of cell-scaffold communication that subsequently influences the cell differentiation[46]. The cell proliferation results as shown in Figure 4, we observed that the MSC and co-culture cells adhesion and proliferation were significantly (P ≤ 0.05) higher on day 10 and day 15 on PGS/collagen scaffold compared to the TCP. This was because of the nanofibrous scaffold which resembles the ECM and thereby provides necessary cues, promoting cell proliferation and differentiation as discussed in our previous studies[46-49]. Moreover, studies have reported that cardiomyocyte adhesion and organization into a contractile tissue have been far superior on natural scaffolds compared to synthetic scaffolds[50]. The results observed that the proliferation of co-culture cells was significantly (P ≤ 0.01) higher on PGS/collagen core/shell fibers compared to collagen nanofibers on day 10 and day 15 (Figure 4). Additionally, the MSCs proliferation was also significantly higher on day 10 and day 15 on PGS/collagen scaffolds compared to collagen fibers. The percentage increase in the rate of proliferation of co-culture cells from day 5 to day 15 has been calculated to be 87.45% and 118.91% on collagen nanofibers and PGS/collagen core/shell fibers respectively. Enhanced cell population on the co-culture group maybe due to the synergistic effect of cardiac cells and MSCs. The MSCs provide the necessary paracrine signals to prevent apoptosis of the cardiac cells. Recent studies suggest that the transplanted MSCs interact with local tissues in the heart, releasing paracrine factors that support the regenerative process. Thus the beneficial effects of MSCs transplantation are probably mediated primarily through the preservation of cardiac myocytes within the infarction[51]. Additionally, large amount of protective cytokines secreted by MSCs, functioned as the limitation of inflammation, inhibition of apoptosis, and stimulating myoangiogenic differentiation[52]. Stem cell/cardiomyocyte interactions regulate not only cardiac development[53] but also cardiomyocyte function in the adult heart[54]. In MSC-cardiomyocyte co-culture environment, stem cells may promote cardiomyocyte survival[55]. Given the rapid loss of cardiomyocytes after ischemic injury, promoting cardiomyocyte survival is an efficient strategy for preserving viable myocardium[56].
Figure 4.
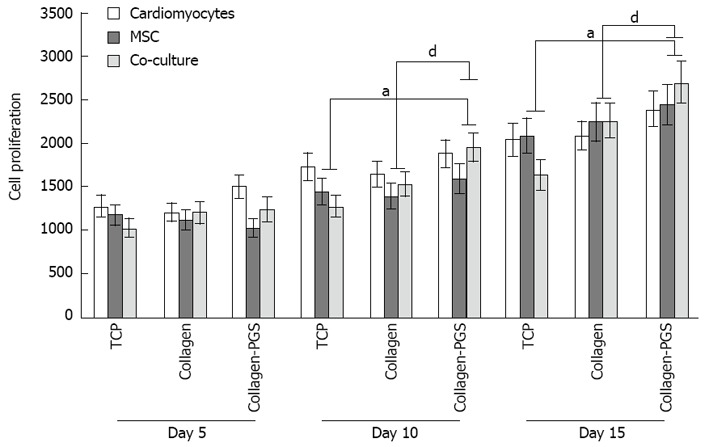
Cell proliferation study for days 5, 10 and 15 on tissue culture plate, poly (glycerol sebacate)/collagen core/shell fibers and collagen nanofibers using cardiomyocytes, mesenchymal stem cells and cardiomyocytes-mesenchymal stem cells co-culture. Denotes statistical significant difference MSC aP ≤ 0.05 vs co-culture cells cultured on TCP and PGS/collagen core/shell fibers; Denotes statistical significant difference MSC dP ≤ 0.01 vs co-culture cells cultured on collagen and PGS/collagen core/shell fibers. TCP: Tissue culture plate; PGS: Poly (glycerol sebacate); MSCs: Mesenchymal stem cells.
To observe the cellular responses of the patches to the myocardiogenic differentiations of MSCs, the immunofluorescence stains of specific proteins of cardiomyocytes like troponin T and α-actinin were selected[57-59]. Troponin-T is important for effective cardiomyocytes which contain contractile proteins, as it regulates the force and velocity of myocardial contraction[57], and actinin is an important constituent of the contractile apparatus. Many investigators agree that hMSCs also exhibit some cardiogenic potential but the frequency at which cardiac differentiation occurs without any added induction factors is small[60]. Figure 5D-F and Figure 6D-F, demonstrated that the cardiogenic choice can be enhanced in vitro by co-culturing MSCs with cardiac cells. The biological cues secreted by cardiac cells would drive the differentiation of MSCs into cardiac lineage. It has been reported that, in the presence of certain physical and chemical cues, MSCs differentiate into cells that resemble cardiac myocytes and may be applicable for cardiac regeneration[46]. Figure 5 observed that the expression of cardiac proteins actinin (Figure 5D-F) and troponin (Figure 6D-F) are higher in co-culture environment compared to individual cell culture systems of MSCs (Figure 5G-I and Figure 6G-I and cardiac cells (Figure 5A-C and Figure 6A-C). As shown in Figure 5G-I and Figure 6G-I, the MSCs did not express the marker proteins actinin and troponin-T in their undifferentiated state. Hence they express DAPI alone which stains the nucleus of MSCs. It is increasingly accepted that physical cues play a role in cell growth and tissue assembly[30,31]. These signals are important in stem cells (SCs) during self-renewal, proliferation and differentiation. Hence the presence of cardiac cells in the stem cell-cardiac cells co-culture provides the necessary cues to trigger the cardiogenic differentiation of MSCs, in the absence of any other soluble differentiating factors. Furthermore, the cardiac marker expression was higher on the PGS/collagen scaffold compared to the collagen nanofibers. This increase in protein expression is because of the difference in the mechanical property between PGS/collagen and collagen substrates. It has been shown that a softer substrate and the ability to tune the mechanical properties within a given range could be advantageous as cell differentiation was shown to be affected by substrate stiffness[32]. Owing to the favourable mechanical properties of PGS, the cell differentiation was more on PGS/collagen scaffolds compared to collagen scaffolds. There was no protein expression in MSC culture group (Figure 5G-I and Figure 6G-I) proving that in the absence of cues, the cardiogenic differentiation of MSCs may not occur. However in the co-culture environment we found that more cells express the cardiac specific marker proteins, indicating that the MSCs have differentiated into cardiac cells and therefore express troponin T and actinin markers. This cardiogenic differentiation of MSCs was further confirmed by dual immunostaining. Figure 7A, D, G shows the expression of MSC specific marker protein CD 105 by the MSCs cultured in the co-culture environment on TCP, collagen and PGS/collagen core/shell fibers. Figure 7B, E, H shows the expression of cardiac marker protein actinin. The MSCs which have undergone cardiogenic differentiation, express both CD 105 and the cardiac specific marker protein actinin. This result in dual expression of both CD 105 and actinin by the MSCs which have undergone cardiogenic differentiation, as shown in Figure 7C, F, I. We observed that PGS/collagen core/shell fibers (Figure 7I) express higher level of actinin expression in differentiated MSCs compared to collagen scaffolds (Figure 7F). However, the differentiated MSCs did not exhibit any contraction. A similar study was reported[61], where stem cells were delivered to the canine RV on an ECM patch, and tracked with quantum dots, some of these cells were demonstrated to differentiate to mature myocytes. Similar to our study, these cells were considered “cardiogenic”, since they express cardiac markers like troponin T, but do not contract in vitro and have not fully differentiated into functional cardiac myocytes. Moreover, the study also suggested that since the cardiogenic cells did not exhibit the complete cardiac phenotype in vitro, it remained possible that the ‘committed’ cells retained the capacity to proliferate in vitro. Ideally, it has been reported that a full cardiogenic differentiation of stem cells should not occur until the cells are transplanted into the heart, since different regions of the heart have unique ion currents and the expression of these currents may be affected by the local environment[62].
Figure 5.
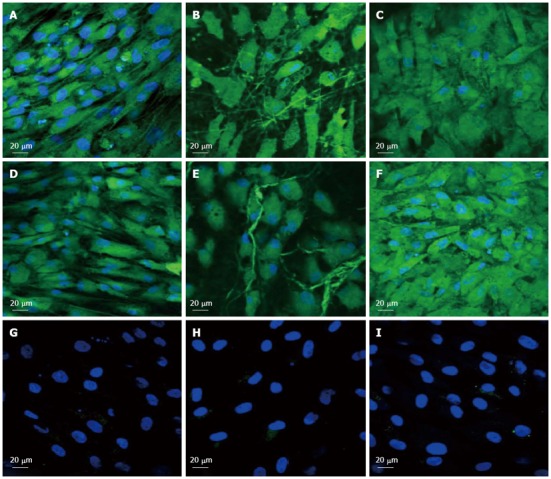
Immunocytochemical analysis for the expression of cardiac marker protein actinin at 60 × magnification on the tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) comprising of cardiomyocytes (A-C), mesenchymal stem cells-cardiomyocytes co-culture group (D-F) and mesenchymal stem cells (G-I). Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
Figure 6.
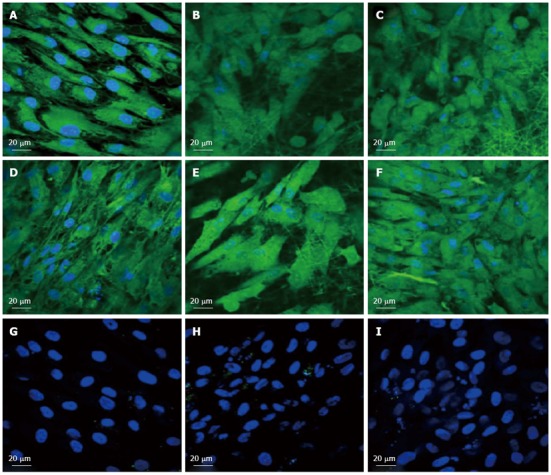
Immunocytochemical analysis for the expression of the cardiac marker protein Troponin at 60 × magnification on the tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) comprising of cardiomyocytes (A-C), mesenchymal stem cells-cardiomyocytes co-culture group (D-F) and mesenchymal stem cells (G-I). Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
Figure 7.
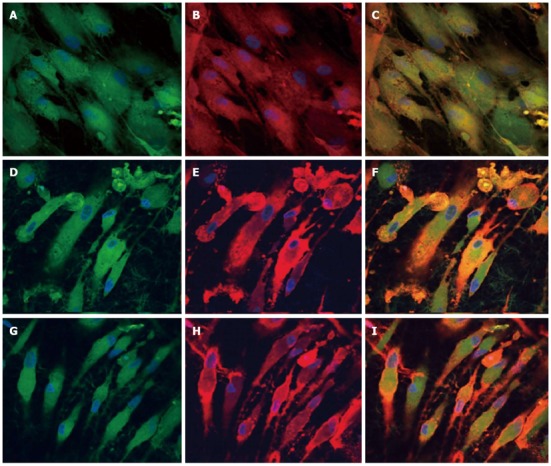
Dual immunocytochemical analysis for the expression of mesenchymal stem cells marker protein CD 105 (A, D, G) and cardiac marker protein Actinin (B, E, H) in the co-culture samples and the merged image showing the dual expression of both CD 105 and Actinin (C, F, I); on the tissue culture plate (A, B, C), collagen nanofibers (D, E, F) and poly (glycerol sebacate)/collagen core/shell fibers (G, H, I) at 60 × magnification. Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
The ability of seeded cells to adhere, survive and migrate within a scaffold is crucial when trying to regenerate a tissue in vivo. Previous reports of cardiac tissue engineering noted that even if the surface layers of the construct were filled with cells, its interior was frequently devoid of tissue regeneration[63]. Since the fibrous scaffolds are highly porous, it favours the cells to penetrate deep within the scaffold. This was further confirmed by 3D confocal images, as shown in Figure 8, processed using Imaris software. It was observed that the cells penetrated more profoundly within the PGS/collagen fibers (542 nm) when compared to the collagen scaffolds (475 nm). This is because of the favourable mechanical property and flexibility of PGS, which favours easier cell migration, causing the cells to crawl inside, towards the interior of the scaffold.
Figure 8.
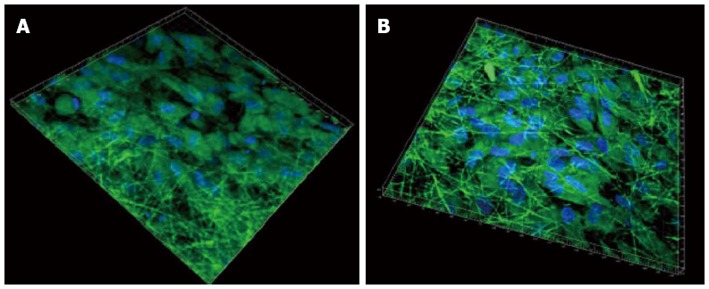
3D image using Imaris software of cardiomyocytes-mesenchymal stem cells co-culture group stained with cardiac specific marker protein troponin at 60 × magnification on (A) collagen fibers (B) poly (glycerol sebacate)/collagen core/shell fibers. Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
Quantification of the number of cells present on nanofibrous scaffolds and cellular behaviour is also another pivotal indicator to determine the potential application of a material construct for any tissue engineering application. The cell morphology was studied using SEM images as shown in Figure 9. It showed that the nanofiber contour allowed the cardiac cells to make extensive use of available cues for isotropic or anisotropic growth, and to some degree even to crawl inside and pull the fibers, as evidenced in Figure 9A-C. The results showed that the cell-to-cell interaction between the MSC and cardiac cells group, leading to cell fusion, was observed in co-culture group (Figure 9D-F). This cell-to-cell interaction favoured the MSCs to undergo cardiogenic differentiation. The differentiated MSCs in the co-culture system acquired the cardiomyocyte phenotype as revealed in Figure 9D-F. MSCs also showed favourable growth on the nanofibers with the greater cell-to-cell contact and extension of filopodia as evidenced in Figure 9G-I.
Figure 9.
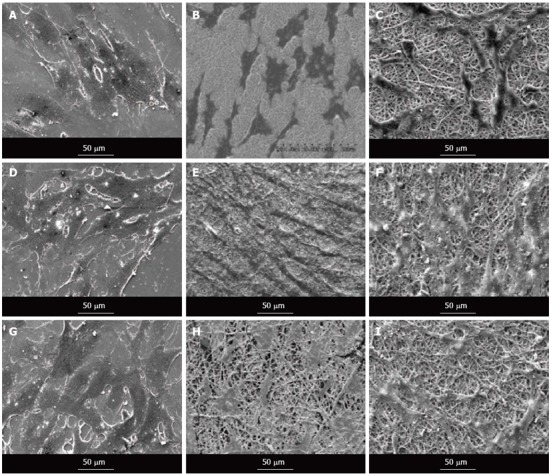
Scanning electron microscope images showing the cell morphology of cardiomyocytes (A-C), cardiomyocytes-mesenchymal stem cells co-culture cells (D-F) and mesenchymal stem cells (G-I) grown on tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) on day 15 at 500 × magnification.
We have employed the use of co-culture system of stem cells with scaffold microenvironments engineered to improve tissue survival and enhance differentiation. Transplanted MSCs may differentiate in situ into cells of cardiomyogenic lineage, promoted by the local microenvironment. These in turn could integrate into the myocardium and help to regenerate damaged tissue. We have reported that direct cell-to-cell contact between MSC and adult cardiac cells is necessary for the differentiation of MSC into cardiac cells. This integrative approach of PGS/collagen core/shell nanofibrous cardiac patch material, to prevent cell loss, and stem cell therapy, would maximize the capacity of myocardial tissue regeneration.
In conclusion, even though the implanted stem cells survived and regenerated the infarcted myocardium, the site of MI is a poor environment for cell growth. To increase cell viability, some factors to improve such an infertile environment are desirable. We aimed at improving the quality of the local microenvironment by trapping the cells within the nanofiber mesh and then transplanting to the infarcted site, which may in turn improve survival of the cells and facilitate the biological behaviour of implanted cells. Electrospun fibrous scaffolds provide both flexibility and guidance for cardiac cells growth and MSC differentiation into cardiac lineage and thus can be successfully applied to obtain structurally and functionally competent cardiac tissue constructs. There have been several studies of different scaffolds for cardiac tissue engineering, but there has been few research focused on elastomeric scaffolds like PGS for CTE. In fact, no research has been done so far on the PGS/collagen scaffold material for MI. New solutions including the recruitment, in vitro proliferation and homing of the patients own stem cells, combined with biocompatible and bio-mimicking materials will open new doors in the field of cardiac tissue engineering for MI. The biomaterial employed should be able to interact on the molecular level, with the cells in a precise and controlled manner, similar to the natural interactions existing between cells and the native ECM. We have shown that the direct cell-to-cell contact between MSCs and adult cardiac cells, governed the differentiation of MSCs into cardiac cells. This novel combinatorial paradigm of a PGS/collagen mechanical construct with MSC-cardiac cells co-culture environment might ultimately bring cardiac tissue engineering into clinical application.
COMMENTS
Background
Myocardial infarction leads to weakening of collagen extracellular matrix leading to loss of ventricular function and the injured heart tissue ultimately becomes scar tissue. When the scar is large enough to interfere with the hearts normal rhythm, heart failure occurs. One promising approach is to prevent the increase of heart failure after myocardial infarction is the implantation of engineered cardiac patch at the site of infarction.
Research frontiers
Poly (glycerol sebacate) (PGS) is an elastomeric material recently applied in the area of soft tissue engineering. In the area of cardiac tissue engineering, the research hotspot is how to fabricate PGS nanofibers by electrospinning technique to engineer a cardiac patch with mechanical properties similar to that of the heart tissue.
Innovations and breakthroughs
The conventional electrospinning technique is to dissolve the polymer in a solvent, which evaporates during the spinning process. However, this approach is not pragmatic with PGS, due to its low molecular weight which results in such a low solution viscosity that even with a high concentration solution, the electrospinning of PGS fibers cannot occur. Hence, the authors developed a core/shell electrospinning process to produce PGS fibers with a protective shell polymer.
Applications
The combination of PGS/collagen core/shell fibers with a unique extracellular matrix like topography has been suggested to be a potential cardiac patch material for myocardial infarction.
Terminology
Myocardial Infarction, known as “heart attack” leads to weakening of collagen extracellular matrix leading to the loss of left ventricular function and the injured heart tissue ultimately becomes scar tissue. When the scar is large enough to interfere with the hearts normal rhythm, heart failure occurs.
Peer review
The article by Ravichandran et al is an interesting article that addresses the important issue of optimizing biomaterials as scaffolding for stem cells. The methodology and data is well presented.
Footnotes
Supported by NRF-Technion, No. R-398-001-065-592; Ministry of Education, No. R-265-000-318-112; and NUSNNI, National University of Singapore
P- Reviewers Takano M, Feher G, Joseph J, Miyasaka Y S- Editor Song XX L- Editor A E- Editor Zhang DN
References
- 1.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. J Tissue Eng Regen Med. 2007;1:327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 2.LaNasa SM, Bryant SJ. Influence of ECM proteins and their analogs on cells cultured on 2-D hydrogels for cardiac muscle tissue engineering. Acta Biomater. 2009;5:2929–2938. doi: 10.1016/j.actbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Baig MK, Mahon N, McKenna WJ, Caforio AL, Bonow RO, Francis GS, Gheorghiade M. The pathophysiology of advanced heart failure. Heart Lung. 1999;28:87–101. doi: 10.1053/hl.1999.v28.a97762. [DOI] [PubMed] [Google Scholar]
- 4.Walsh RG. Design and features of the Acorn CorCap Cardiac Support Device: the concept of passive mechanical diastolic support. Heart Fail Rev. 2005;10:101–107. doi: 10.1007/s10741-005-4637-x. [DOI] [PubMed] [Google Scholar]
- 5.Kelley ST, Malekan R, Gorman JH, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, Edmunds LH. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto Y, Gorman JH, Moainie SL, Jackson BM, Parish LM, Plappert T, Zeeshan A, St John-Sutton MG, Gorman RC. Early ventricular restraint after myocardial infarction: extent of the wrap determines the outcome of remodeling. Ann Thorac Surg. 2005;79:881–87; discussion 881-87;. doi: 10.1016/j.athoracsur.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: 10 years of research survey. Mater Sci Eng R-Rep. 2008;59:1–37. [Google Scholar]
- 8.Jeong SI, Kim SH, Kim YH, Jung Y, Kwon JH, Kim BS, Lee YM. Manufacture of elastic biodegradable PLCL scaffolds for mechano-active vascular tissue engineering. J Biomater Sci Polym Ed. 2004;15:645–660. doi: 10.1163/156856204323046906. [DOI] [PubMed] [Google Scholar]
- 9.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–I177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 10.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 11.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 12.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–277. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Zünd G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, Hubbell JA, Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17:587–591. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 14.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15 Suppl 1:S29–S40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 15.Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng R-Rep. 2001;34:147–230. [Google Scholar]
- 16.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput. 2000;38:211–218. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann WH, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 19.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 20.Grant DS, Kibbey MC, Kinsella JL, Cid MC, Kleinman HK. The role of basement membrane in angiogenesis and tumor growth. Pathol Res Pract. 1994;190:854–863. doi: 10.1016/S0344-0338(11)80989-1. [DOI] [PubMed] [Google Scholar]
- 21.Bonfil RD, Vinyals A, Bustuoabad OD, Llorens A, Benavides FJ, Gonzalez-Garrigues M, Fabra A. Stimulation of angiogenesis as an explanation of Matrigel-enhanced tumorigenicity. Int J Cancer. 1994;58:233–239. doi: 10.1002/ijc.2910580215. [DOI] [PubMed] [Google Scholar]
- 22.Fridman R, Kibbey MC, Royce LS, Zain M, Sweeney M, Jicha DL, Yannelli JR, Martin GR, Kleinman HK. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 1991;83:769–774. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- 23.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schäfer H, Bishopric N, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 25.Li RK, Mickle DA, Weisel RD, Zhang J, Mohabeer MK. In vivo survival and function of transplanted rat cardiomyocytes. Circ Res. 1996;78:283–288. doi: 10.1161/01.res.78.2.283. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Ensley AE, Nerem RM, Wang Y. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J Biomed Mater Res A. 2007;83:1070–1075. doi: 10.1002/jbm.a.31434. [DOI] [PubMed] [Google Scholar]
- 27.Sun ZC, Zussman E, Yarin AL, Wendorff JH, Greiner A. Compound core-shell polymer nanofibers by co-electrospinning. Adv Mater. 2003;15:1929–1932. [Google Scholar]
- 28.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 29.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 31.Heidemann SR, Wirtz D. Towards a regional approach to cell mechanics. Trends Cell Biol. 2004;14:160–166. doi: 10.1016/j.tcb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 35.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 37.Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv Drug Deliv Rev. 2009;61:1007–1019. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 38.He W, Yong T, Teo WE, Ma Z, Ramakrishna S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005;11:1574–1588. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- 39.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2003;66:192–197. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 41.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 42.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 44.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Expression of cardiac proteins in neonatal cardiomyocytes on PGS/fibrinogen core/shell substrate for Cardiac tissue engineering. Int J Cardiol. 2012:Epub ahead of print. doi: 10.1016/j.ijcard.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 46.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Poly(Glycerol sebacate)/gelatin core/shell fibrous structure for regeneration of myocardial infarction. Tissue Eng Part A. 2011;17:1363–1373. doi: 10.1089/ten.TEA.2010.0441. [DOI] [PubMed] [Google Scholar]
- 47.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials. 2012;33:846–855. doi: 10.1016/j.biomaterials.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Ravichandran R, Ng CCh, Liao S, Pliszka D, Raghunath M, Ramakrishna S, Chan CK. Biomimetic surface modification of titanium surfaces for early cell capture by advanced electrospinning. Biomed Mater. 2012;7:015001. doi: 10.1088/1748-6041/7/1/015001. [DOI] [PubMed] [Google Scholar]
- 49.Ravichandran R, Liao S, Ng CCh, Chan CK, Raghunath M, Ramakrishna S. Effects of nanotopography on stem cell phenotypes. World J Stem Cells. 2009;1:55–66. doi: 10.4252/wjsc.v1.i1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 52.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 53.Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 55.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing Y, Huang PJ, Zhang KM. [Cardiac troponin T and I: application in myocardial injury and forensic medicine] Fa Yi Xue Zazhi. 2003;19:242–244. [PubMed] [Google Scholar]
- 58.Lemonnier M, Buckingham ME. Characterization of a cardiac-specific enhancer, which directs {alpha}-cardiac actin gene transcription in the mouse adult heart. J Biol Chem. 2004;279:55651–55658. doi: 10.1074/jbc.M411082200. [DOI] [PubMed] [Google Scholar]
- 59.Brand NJ, Roy A, Hoare G, Chester A, Yacoub MH. Cultured interstitial cells from human heart valves express both specific skeletal muscle and non-muscle markers. Int J Biochem Cell Biol. 2006;38:30–42. doi: 10.1016/j.biocel.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJ, Lu Z, Kochupura PV, Robinson RB, Rosen MR, Brink PR, et al. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol. 2008;295:H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 63.Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, Matheny RG, Badylak SF. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I135–I143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]


