Abstract
AIM: To investigate age- and gender-related differences in non-culprit versus culprit coronary vessels assessed with virtual histology intravascular ultrasound (VH-IVUS).
METHODS: In 390 patients referred for coronary angiography to a single center (Luzerner Kantonsspital, Switzerland) between May 2007 and January 2011, 691 proximal vessel segments in left anterior descending, circumflex and/or right coronary arteries were imaged by VH-IVUS. Plaque burden and plaque composition (fibrous, fibro-fatty, necrotic core and dense calcium volumes) were analyzed in 3 age tertiles, according to gender and separated for vessels containing non-culprit or culprit lesions. To classify as vessel containing a culprit lesion, the patient had to present with an acute coronary syndrome, and the VH-IVUS had to be performed in a vessel segment containing the culprit lesion according to conventional coronary angiography.
RESULTS: In non-culprit vessels the plaque burden increased significantly with aging (in men from 37% ± 12% in the lowest to 46% ± 10% in the highest age tertile, P < 0.001; in women from 30% ± 9% to 40% ± 11%, P < 0.001); men had higher plaque burden than women at any age (P < 0.001 for each of the 3 age tertiles). In culprit vessels of the lowest age tertile, plaque burden was significantly higher than that in non-culprit vessels (in men 48% ± 6%, P < 0.001 as compared to non-culprit vessels; in women 44% ± 18%, P = 0.004 as compared to non-culprit vessels). Plaque burden of culprit vessels did not significantly change during aging (plaque burden in men of the highest age tertile 51% ± 9%, P = 0.523 as compared to lowest age tertile; in women of the highest age tertile 49% ± 8%, P = 0.449 as compared to lowest age tertile). In men, plaque morphology of culprit vessels became increasingly rupture-prone during aging (increasing percentages of necrotic core and dense calcium), whereas plaque morphology in non-culprit vessels was less rupture-prone and remained constant during aging. In women, necrotic core in non-culprit vessels was very low at young age, but increased during aging resulting in a plaque morphology that was very similar to men. Plaque morphology in culprit vessels of young women and men was similar.
CONCLUSION: This study provides evidence that age- and gender-related differences in plaque burden and plaque composition significantly depend on whether the vessel contained a non-culprit or culprit lesion.
Keywords: Coronary vessels, Anatomy and histology, Coronary artery, Ultrasonography, Coronary artery disease, Atherosclerosis, Etiology, Age factors
INTRODUCTION
Age and gender have well-established relations to the prevalence of coronary artery disease (CAD) and the incidence of coronary events[1-5]. A few pathologic studies evaluated age- and gender-related differences in coronary plaques and found significant differences[6-9]. However, these studies were restricted to patients who died from a coronary event. Only two studies so far characterized age- and gender-related differences in coronary plaques in vivo with the use of virtual histology (VH) intravascular ultrasound (IVUS)[10,11]. Both studies reported that plaque burden as well as necrotic core and dense calcium increased with increasing patient age, that at any age men had a more rupture-prone plaque morphology than women and that gender differences diminished with increasing age. However, in these studies the compositional characteristics of non-culprit and culprit lesions were not distinguished. The present study therefore examines age- and gender-related differences in non-culprit versus culprit coronary vessels assessed with VH-IVUS.
MATERIALS AND METHODS
Study population
Consecutive patients referred for coronary angiography during daytime to a single center (Luzerner Kantonsspital, Switzerland) between May 2007 and January 2011 were evaluated for this study. Patients presented with stable angina or acute coronary syndromes (ACS) (i.e., unstable angina, non-ST-elevation myocardial infarction or ST-elevation myocardial infarction). The decision to perform coronary angiography was taken according to the local guidelines. Patients in cardiogenic shock and hemodynamically unstable patients depending on inotropes were excluded. Patients with total or subtotal stenosis of the proximal left anterior descending (LAD) or circumflex (CX) artery as well as high-grade left main coronary artery stenosis with potential for hemodynamic instability during the IVUS procedure were also excluded. Finally, 390 patients qualified for inclusion in the study and were willing to participate. All patients provided written informed consent. The institutional ethical committee approved the study, which was conducted in accordance with the declaration of Helsinki.
Measurements
In all 390 patients, clinical characteristics (age, sex, cardiovascular risk factors, clinical presentation) were assessed at baseline. Hypertension was defined as a repeatedly elevated blood pressure > 140/90 mmHg according to current guidelines[12-14]. Dyslipidemia was defined as total cholesterol > 234 mg/dL (6.0 mmol/L) or low-density lipoprotein cholesterol > 117 mg/dL (3.0 mmol/L)[15]. Diagnosis of diabetes mellitus was made if fasting plasma glucose was ≥ 7 mmol/L on at least two different days or if postprandial plasma glucose was ≥ 11.1 mmol/L[16]. Patients were considered as smokers, if they currently smoked ≥ 1 cigarette per week. Patients who previously stopped smoking were considered non-smokers[17]. A positive family history of CAD was defined as evidence of CAD in a parent or sibling before 60 years of age[18]. ACS was defined according to guidelines of the European Society of Cardiology and the American College of Cardiology/American Heart Association[19,20].
IVUS procedure and outcome
In all 390 participating patients, IVUS was performed in the proximal 4 cm of LAD, CX and right coronary artery (RCA). If the coronary anatomy was unsuitable for the IVUS procedure (e.g., small or tortuous vessels), the IVUS procedure was not performed in the corresponding vessel. IVUS was acquired using an Eagle Eye® Gold Catheter (20 MHz) and an automatic continuous pullback device (Volcano Corp., Rancho Cordova, CA, United States)[21-24]. Pullback velocity was 1 mm/s. Frames were acquired ECG-gated/R-wave triggered. The vessels in which the IVUS was performed were classified into either vessels containing a culprit lesion or vessels containing non-culprit lesions. To classify a vessel as vessel containing the culprit lesion, the following criteria had to be fulfilled: (1) the patient had an ACS; and (2) the IVUS was performed in the vessel segment containing the culprit lesion according to conventional coronary angiography. All other vessels were classified as vessels containing non-culprit lesions.
Analysis of the IVUS procedure was performed offline by a specially trained single investigator who was blinded to the clinical and angiographic data. The analysis embraced the whole vessel segment in which the IVUS was performed. Contours of lumen and media-adventitia interface were detected semi-automatically. For every cross-sectional area, vessel and lumen diameters as well as the area for the different plaque components were calculated using a special software package (pcVH2.2, Volcano Corp., Rancho Cordova, CA, United States). Spectral analysis of IVUS radiofrequency signals provided a histology of the plaque identifying 4 major plaque components, namely fibrous, fibro-fatty, necrotic core and dense calcium[25]. After manual detection, the software calculated the absolute volumes of each of the 4 plaque components as well as their relative amounts expressed as percentages of the total volume of the 4 components within the 4 cm segment. Total plaque volume was calculated as the sum of the volumes of all 4 plaque components and of the media-adventitia. Plaque burden was defined as the ratio between plaque volume and the sum of plaque and lumen volumes.
Statistical analysis
Data were analyzed using Stata software (Stata 11.2, StataCorp LP, College Station, TX, United States). For two-group comparisons, Student’s t test was used after checking for normal distribution; Mann-Whitney rank-sum test was used for non-normally distributed continuous variables. Distributional differences between categorical variables were assessed by a χ2 test and Fisher’s exact test. The P value for a trend across age groups was calculated using linear regression. In the regression model, age was analyzed as tertile. For all statistical comparisons, a P value < 0.05 was considered significant.
RESULTS
Baseline characteristic are shown in Table 1. Mean age was 59.4 ± 10.7 years with a range from 20.5 to 83.7 years. Hypertension, dyslipidemia and diabetes were increasingly prevalent with increasing patient age. Fewer patients in the highest age tertile were current smokers as compared to younger patients. Prevalence of CAD increased with increasing patient age, whereas patients with ACS were similarly prevalent in all age tertiles (Table 1).
Table 1.
Baseline characteristics (n = 130) n (%)
| Characteristic | Patients in the lowest age tertile | Patients in the middle age tertile | Patients in the highest age tertile | P value1 |
| Age, mean ± SD (range), yr | 47.6 ± 6.8 (20.5-55.5) | 59.6 ± 2.4 (55.5-64.2) | 70.9 ± 4.8 (64.3-83.7) | < 0.001 |
| Female sex | 29 (22.3) | 36 (27.7) | 43 (33.1) | 0.053 |
| Body mass index, mean ± SD, kg/m2 | 26.9 ± 5.1 | 28.0 ± 4.9 | 27.4 ± 3.6 | 0.408 |
| Obesity2 | 25 (19.2) | 37 (28.5) | 30 (23.1) | 0.466 |
| Systolic BP, mean ± SD, mmHg | 128 ± 19 | 134 ± 19 | 133 ± 20 | 0.017 |
| Diastolic BP, mean ± SD, mmHg | 77 ± 12 | 77 ± 11 | 73 ± 11 | 0.007 |
| Cardiovascular risk factors | ||||
| Hypertension | 58 (44.6) | 85 (65.4) | 95 (73.1) | < 0.001 |
| Dyslipidemia | 77 (59.2) | 101 (77.7) | 90 (69.2) | 0.082 |
| Current smoker | 47 (36.2) | 30 (23.1) | 16 (12.3) | < 0.001 |
| Diabetes | 11 (8.5) | 23 (17.7) | 30 (23.1) | 0.001 |
| Positive family history | 47 (36.2) | 48 (36.9) | 51 (39.2) | 0.609 |
| Manifest atherosclerosis | ||||
| CAD | 78 (60.0) | 101 (77.7) | 112 (86.2) | < 0.001 |
| ACS | 35 (26.9) | 32 (24.6) | 33 (25.4) | 0.777 |
| Previous stroke | 1 (0.8) | 5 (3.9) | 7 (5.4) | 0.038 |
| Peripheral artery disease | 2 (1.5) | 11 (8.5) | 6 (4.6) | 0.250 |
| Medication3 | ||||
| ACE inhibitor | 29 (22.3) | 46 (35.4) | 52 (40.0) | 0.002 |
| Betablocker | 47 (36.2) | 60 (46.2) | 67 (51.5) | 0.013 |
| Statin | 36 (27.7) | 57 (43.9) | 62 (47.7) | 0.001 |
| Other measurements | ||||
| LDL, mean ± SD, mmol/L | 2.8 ± 1.0 | 2.7 ± 1.2 | 2.6 ± 1.0 | 0.263 |
| LVEF, mean ± SD, % | 70 ± 11 | 68 ± 13 | 70 ± 12 | 0.875 |
P value for trend across age groups was calculated using linear regression;
Defined as body mass index ≥ 30 kg/m2;
Long-term medication in the two weeks before intravascular ultrasound. ACE: Angiotensin converting enzyme; ACS: Acute coronary syndrome; BP: Blood pressure; CAD: Coronary artery disease; LDL: Low-density lipoprotein cholesterol.
Overall, 691 vessel segments in LAD, CX and/or RCA were imaged by IVUS in the 390 participating patients. The LAD was imaged in 319 patients, the CX in 214 patients, and the RCA in 158 patients. All three vessels were depicted in 84 patients (21.5%), two vessels in 133 patients (34.1%), and only one vessel in 173 patients (44.4%). 618 vessels (89.4%) contained non-culprit lesions and 73 vessels (10.6%) contained culprit lesions of patients with ACS. Age- and gender-related differences of plaque components in vessels containing non-culprit or culprit lesions are summarized in Table 2 and in Figure 1.
Table 2.
Age- and gender-related differences of plaque burden and plaque components in vessels containing non-culprit or culprit lesions (mean ± SD)
| Plaque component |
Patients in the lowest age tertile |
Patients in the middle age tertile |
Patients in the highest age tertile |
P value1 | P value2 | ||||||
| Male | Female | P value | Male | Female | P value | Male | Female | P value | |||
| Non-culprit vessels | |||||||||||
| Segments available for analysis | n = 154 | n = 44 | n = 159 | n = 54 | n = 140 | n = 67 | |||||
| Plaque burden, % | 37 ± 12 | 30 ± 9 | < 0.001 | 42 ± 11 | 35 ± 12 | < 0.001 | 46 ± 10 | 40 ± 11 | < 0.001 | < 0.001 | < 0.001 |
| Fibrous volume, % | 53 ± 12 | 44 ± 20 | < 0.001 | 51 ± 12 | 54 ± 13 | 0.161 | 52 ± 11 | 53 ± 12 | 0.947 | 0.949 | 0.005 |
| Fibro-fatty volume, % | 13 ± 11 | 17 ± 19 | 0.966 | 11 ± 9 | 17 ± 15 | 0.053 | 13 ± 9 | 12 ± 10 | 0.365 | 0.992 | 0.060 |
| NC volume, % | 18 ± 8 | 15 ± 10 | 0.033 | 21 ± 9 | 16 ± 10 | < 0.001 | 20 ± 8 | 19 ± 9 | 0.646 | 0.13 | 0.017 |
| DC volume, % | 17 ± 14 | 24 ± 22 | 0.324 | 17 ± 12 | 13 ± 14 | 0.003 | 15 ± 10 | 16 ± 12 | 0.814 | 0.323 | 0.028 |
| Lumen volume, mm3 | 398 ± 182 | 342 ± 115 | 0.053 | 348 ± 133 | 308 ± 130 | 0.057 | 310 ± 133 | 332 ± 127 | 0.267 | < 0.001 | 0.815 |
| Culprit vessels | |||||||||||
| Segments available for analysis | n = 22 | n = 5 | n = 19 | n = 3 | n = 16 | n = 8 | |||||
| Plaque burden, % | 48 ± 6 | 44 ± 18 | 0.376 | 43 ± 11 | 47 ± 14 | 0.578 | 51 ± 9 | 49 ± 8 | 0.724 | 0.523 | 0.449 |
| Fibrous volume, % | 57 ± 9 | 58 ± 8 | 0.795 | 51 ± 10 | 67 ± 12 | 0.026 | 44 ± 12 | 57 ± 11 | 0.024 | < 0.001 | 0.727 |
| Fibro-fatty volume, % | 13 ± 9 | 13 ± 6 | 0.683 | 9 ± 5 | 8 ± 2 | 0.562 | 7 ± 5 | 11 ± 7 | 0.069 | 0.006 | 0.561 |
| NC volume, % | 19 ± 8 | 19 ± 7 | 0.930 | 23 ± 9 | 17 ± 8 | 0.285 | 28 ± 7 | 20 ± 7 | 0.018 | 0.003 | 0.697 |
| DC volume, % | 11 ± 8 | 10 ± 8 | 0.851 | 17 ± 8 | 8 ± 7 | 0.113 | 22 ± 12 | 13 ± 12 | 0.040 | 0.001 | 0.655 |
| Lumen volume, mm3 | 340 ± 144 | 398 ± 340 | 0.541 | 385 ± 171 | 236 ± 133 | 0.168 | 271 ± 82 | 307 ± 116 | 0.394 | 0.201 | 0.505 |
P value for trend across age groups in men. 1P value was calculated using linear regression;
P value for trend across age groups in women. P value was calculated using linear regression. DC: Dense calcium; NC: Necrotic core.
Figure 1.
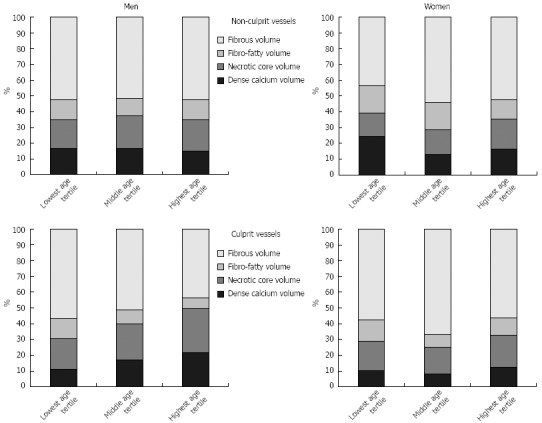
Plaque composition in men and women during aging in non-culprit and culprit vessels.
Age- and gender-related differences in non-culprit vessels
In non-culprit vessels plaque burden as well as fibrous and necrotic core volumes were higher in young men than in young women (Table 2). The plaque burden in men increased significantly with age; however, the plaque component characteristics of young age were preserved until old age. Women also had a significant increase in plaque burden during aging, but plaque burden remained generally lower than in men. In contrast to men, plaque composition of non-culprit vessels in women altered during aging with increasing volumes of fibrous and necrotic tissue, so that differences in plaque composition between women and men disappeared in the elderly. Old women had a plaque composition that was similar to men, even compared to young men. Interestingly, the most important change of plaque composition in women occurred between the lowest and middle age tertile (i.e., after menopause).
Age- and gender-related differences in culprit vessels
Table 2 summarizes plaque burden and composition of culprit vessels. The plaque composition of culprit vessels in young men and women presenting with ACS was very similar. In contrast to women, plaque composition in culprit vessels of men changed significantly during aging with increasing volumes of necrotic core and dense calcium (i.e., old men need a rupture-prone plaque morphology to present as ACS). Plaque composition in old women was similar to that in young women, therefore leading to significant differences in plaque composition between men and women in the highest age tertile. Old women also presented as ACS with less necrotic core and dense calcium than men. Plaque burden remained similar between men and women and also between the three age groups.
Differences between non-culprit and culprit vessels
In the lowest and middle age tertiles, differences between non-culprit and culprit vessels were similar for men and women. In the lowest age tertile, plaque burden in culprit vessels was significantly higher than that in non-culprit vessels (P < 0.001 for men and P = 0.004 for women), but no significant differences in plaque composition were found between non-culprit and culprit vessels. In the middle age tertile of men and women, there were no significant differences of plaque burden and composition between non-culprit and culprit vessels. In men of the highest age tertile, plaque composition in non-culprit and culprit vessels differed significantly with more necrotic core (P < 0.001), more dense calcium (P = 0.012), less fibrous (P = 0.003) and less fibro-fatty (P = 0.004) volume in the culprit vessel, whereas plaque burden was not significantly (P = 0.110) different. In women in the highest age tertile, plaque burden in culprit vessels was significantly higher than in non-culprit vessels (P = 0.017), whereas no significant differences in plaque composition were found.
Analysis of combined non-culprit and culprit vessels
If non-culprit and culprit vessels are combined for analysis, plaque burden increased significantly in men (P < 0.001) and women (P < 0.001). Men had more plaque burden than women in every age tertile (P < 0.001 for lowest, middle or highest age tertile). Percentages of necrotic core increased with increasing patient age in both men (P = 0.019) and women (P = 0.016). Plaque composition significantly differed between men and women in the lowest and middle age tertile, whereas in the highest age tertile no significant differences were found.
DISCUSSION
This study provides evidence that age- and gender-related differences in plaque burden and plaque composition significantly depend upon whether the vessel contained a non-culprit or culprit lesion. Effects of pathophysiologic processes during aging are presumably better observable in non-culprit lesions where acute processes play a minor role. In non-culprit vessels of men, plaque burden increases with increasing age, but plaque composition remains constant during aging. Compared with men, young women have a significantly lower plaque burden and lower amount of necrotic core which may reflect protective effects of female hormones. After menopause the plaque composition in non-culprit vessels of women approximates that of men. The plaque burden of women increases during aging like men, but it remains lower than in men until old age. In culprit vessels the situation is different. Culprit lesions reflect an acute stage of disease and influencing factors other than the long-term pathophysiologic processes which occur during aging presumably come to the fore. Thus, culprit vessels of young women and men exhibit similar rupture-prone plaque morphology with a high percentage of necrotic core and a relatively high plaque burden. In men, a significant increase of necrotic core and dense calcium is required to result in a rupture-prone morphology at old age. In old women, morphology similar to young women and men is sufficient to result in a rupture-prone culprit lesion.
Qian et al[10] reported that both women and men had an increase in plaque with increasing age, that at any age, men had more plaque than women, that percentages of dense calcium and necrotic core increased with age in both men and women, and that gender differences were lowest in the oldest tertile. Our study confirms these findings, if non-culprit and culprit lesions are analyzed together. However, if non-culprit and culprit lesions are analyzed separately, then age- and gender-related differences are more complex.
Our study is in accordance with previous pathologic studies although comparability of IVUS and pathologic studies is limited due to several reasons[6-9]. Dollar et al[6] reported that young women had more fibrous and fibro-fatty tissue and less necrotic core than old men. Our study confirms this finding. Burke et al[8] reported that plaque erosion, the major substrate for thrombosis in premenopausal women, does not appear to be inhibited by estrogen. Presumably this could explain why we did not find any differences in the plaque composition of culprit vessels between young men and women.
The present study has limitations. First, study participants underwent coronary angiography for stable angina or ACS. This might restrict extrapolation of our findings to a more healthy general population. Second, this was not a lesion-specific analysis; rather, LAD, CX and/or RCA were imaged in a proximal 4 cm segment. However, it has been shown that vessel-based measurements correlate well with lesion-specific analysis[26]. Third, the number of culprit vessels available for analysis in women was too low to exclude type II error. Fourth, in culprit vessels it may be difficult to differentiate plaque from thrombi by 20 MHz IVUS. Therefore, plaque composition in culprit vessels may be affected by thrombi. Fifth, hormone replacement therapy was not assessed in women.
In conclusion, the present study has revealed that age- and gender-related differences in plaque burden and plaque composition significantly depend upon whether the vessel contained a non-culprit or culprit lesion. More research is needed to understand the pathophysiology of plaque morphology changes during aging in women and men.
COMMENTS
Background
Only few studies so far characterized age- and gender-related differences in coronary plaques in vivo with the use of virtual histology (VH) intravascular ultrasound (IVUS). In these studies the compositional differences of non-culprit and culprit vessels were not distinguished. The present study therefore examines age- and gender-related differences in non-culprit versus culprit coronary artery vessels assessed with VH-IVUS.
Research frontiers
Differences in the plaque composition of non-culprit and culprit vessels according to age and gender have not been described so far.
Innovations and breakthroughs
The present study shows that age- and gender-related differences in plaque burden and plaque composition significantly depend on whether the vessel contained a non-culprit or culprit lesion. More research is needed to understand the pathophysiology of plaque morphology changes during aging in women and men.
Applications
The present study has research implications. More research is needed to understand the pathophysiology of plaque morphology changes during aging in women and men.
Terminology
Vessel containing a culprit lesion: To classify as vessel containing a culprit lesion, the patient had to present with an acute coronary syndrome, and the VH-IVUS had to be performed in a vessel segment containing the culprit lesion according to conventional coronary angiography.
Peer review
The authors showed the characteristics of coronary disease according to gender and age and different patterns of plaque composition between culprit and non-culprit vessels. However, several issues should be considered and clarified more (e.g., lesion inclusion criteria and the definition of culprit/non-culprit vessels should be clarified).
Footnotes
Supported by Swiss Heart Foundation, Bern, Switzerland; the Swiss National Science Foundation, No. 310000-118468/1, Bern, Switzerland; and the Kamillo-Eisner Foundation, Hergiswil, Switzerland
P- Reviewer Park SJ S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Arias E, Anderson RN, Kung HC, Murphy SL, Kochanek KD. Deaths: final data for 2001. Natl Vital Stat Rep. 2003;52:1–115. [PubMed] [Google Scholar]
- 2.Pearte CA, Furberg CD, O'Meara ES, Psaty BM, Kuller L, Powe NR, Manolio T. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: the Cardiovascular Health Study. Circulation. 2006;113:2177–2185. doi: 10.1161/CIRCULATIONAHA.105.610352. [DOI] [PubMed] [Google Scholar]
- 3.Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Niedermaier G, Keller PF, Gutzwiller F, Erne P. Acute coronary syndromes in young patients: presentation, treatment and outcome. Int J Cardiol. 2011;148:300–304. doi: 10.1016/j.ijcard.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Eberli FR, Stuck AE, Gutzwiller F, Erne P. Age-related differences in the use of guideline-recommended medical and interventional therapies for acute coronary syndromes: a cohort study. J Am Geriatr Soc. 2008;56:510–516. doi: 10.1111/j.1532-5415.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- 5.Radovanovic D, Urban P, Simon R, Schmidli M, Maggiorini M, Rickli H, Stauffer JC, Seifert B, Gutzwiller F, Erne P. Outcome of patients with acute coronary syndrome in hospitals of different sizes. A report from the AMIS Plus Registry. Swiss Med Wkly. 2010;140:314–322. doi: 10.4414/smw.2010.12986. [DOI] [PubMed] [Google Scholar]
- 6.Dollar AL, Kragel AH, Fernicola DJ, Waclawiw MA, Roberts WC. Composition of atherosclerotic plaques in coronary arteries in women less than 40 years of age with fatal coronary artery disease and implications for plaque reversibility. Am J Cardiol. 1991;67:1223–1227. doi: 10.1016/0002-9149(91)90931-a. [DOI] [PubMed] [Google Scholar]
- 7.Mautner SL, Lin F, Mautner GC, Roberts WC. Comparison in women versus men of composition of atherosclerotic plaques in native coronary arteries and in saphenous veins used as aortocoronary conduits. J Am Coll Cardiol. 1993;21:1312–1318. doi: 10.1016/0735-1097(93)90302-h. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 9.Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001;141:S58–S62. doi: 10.1067/mhj.2001.109946. [DOI] [PubMed] [Google Scholar]
- 10.Qian J, Maehara A, Mintz GS, Margolis MP, Lerman A, Rogers J, Banai S, Kazziha S, Castellanos C, Dani L, et al. Impact of gender and age on in vivo virtual histology-intravascular ultrasound imaging plaque characterization (from the global Virtual Histology Intravascular Ultrasound [VH-IVUS] registry) Am J Cardiol. 2009;103:1210–1214. doi: 10.1016/j.amjcard.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Pundziute G, Schuijf JD, van Velzen JE, Jukema JW, van Werkhoven JM, Nucifora G, van der Kley F, Kroft LJ, de Roos A, Boersma E, et al. Assessment with multi-slice computed tomography and gray-scale and virtual histology intravascular ultrasound of gender-specific differences in extent and composition of coronary atherosclerotic plaques in relation to age. Am J Cardiol. 2010;105:480–486. doi: 10.1016/j.amjcard.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.Saguner AM, Dür S, Perrig M, Schiemann U, Stuck AE, Bürgi U, Erne P, Schoenenberger AW. Risk factors promoting hypertensive crises: evidence from a longitudinal study. Am J Hypertens. 2010;23:775–780. doi: 10.1038/ajh.2010.71. [DOI] [PubMed] [Google Scholar]
- 14.Schoenenberger AW, Schoenenberger-Berzins R, Suter PM, Zuber M, Erne P. Effect of moderate weight reduction on resting and exercise blood pressure in overweight subjects. J Hum Hypertens. 2007;21:683–685. doi: 10.1038/sj.jhh.1002204. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 17.Schoenenberger AW, Kobza R, Jamshidi P, Zuber M, Abbate A, Stuck AE, Pfisterer M, Erne P. Sudden cardiac death in patients with silent myocardial ischemia after myocardial infarction (from the Swiss Interventional Study on Silent Ischemia Type II [SWISSI II]) Am J Cardiol. 2009;104:158–163. doi: 10.1016/j.amjcard.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Schoenenberger AW, Jamshidi P, Kobza R, Zuber M, Stuck AE, Pfisterer M, Erne P. Progression of coronary artery disease during long-term follow-up of the Swiss Interventional Study on Silent Ischemia Type II (SWISSI II) Clin Cardiol. 2010;33:289–295. doi: 10.1002/clc.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 20.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, et al. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/ Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 21.Toggweiler S, Urbanek N, Schoenenberger AW, Erne P. Analysis of coronary bifurcations by intravascular ultrasound and virtual histology. Atherosclerosis. 2010;212:524–527. doi: 10.1016/j.atherosclerosis.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Toggweiler S, Schoenenberger A, Urbanek N, Erne P. The prevalence of endothelial dysfunction in patients with and without coronary artery disease. Clin Cardiol. 2010;33:746–752. doi: 10.1002/clc.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenenberger AW, Urbanek N, Toggweiler S, Seelos R, Jamshidi P, Resink TJ, Erne P. Deviation from Murray's law is associated with a higher degree of calcification in coronary bifurcations. Atherosclerosis. 2012;221:124–130. doi: 10.1016/j.atherosclerosis.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Schoenenberger AW, Urbanek N, Bergner M, Toggweiler S, Resink TJ, Erne P. Associations of reactive hyperemia index and intravascular ultrasound-assessed coronary plaque morphology in patients with coronary artery disease. Am J Cardiol. 2012;109:1711–1716. doi: 10.1016/j.amjcard.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007;3:113–120. [PubMed] [Google Scholar]
- 26.Shin ES, Garcia-Garcia HM, Garg S, Serruys PW. A comparison between plaque-based and vessel-based measurement for plaque component using volumetric intravascular ultrasound radiofrequency data analysis. Int J Cardiovasc Imaging. 2011;27:491–497. doi: 10.1007/s10554-010-9698-9. [DOI] [PubMed] [Google Scholar]


