Highlights
► Peripheral LPS induces cholesterol 25-hydroxylase expression in mouse brain. ► 25-Hydroxycholesterol upregulates LXR target genes in a mouse neuronal cell line. ► 25-Hydroxycholesterol downregulates neuronal SREBP2-dependent transcription. ► 25-Hydroxycholesterol attenuates neuronal cholesterol biosynthesis.
Keywords: 25-Hydroxycholesterol, Cholesterol 25-hydroxylase, Oxysterol, Endotoxin, Neuroinflammation, Neurons
Abstract
Aberrant oxysterol biosynthesis is implicated in the pathogenesis of neurodegenerative diseases. During the present study we have investigated the effects of exogenously added 25-hydroxycholesterol (25-HC) on transcription of cholesterol biosynthetic genes, sterol-regulatory element binding protein (SREBP) processing and cholesterol biosynthesis in the murine CATH.a neuronal cell line. A single i.p. injection of lipopolysaccharide resulted in robust induction of cholesterol 25-hydroxylase mRNA and protein levels in brains of treated mice. In vitro, 25-HC upregulated the transcription of ATP-binding cassette transporter A1 (ABCA1) and (to a lesser extent) apolipoprotein E (apoE) in CATH.a neurons. Cholesterol biosynthetic gene expression (squalene synthase, HMG-CoA synthase, HMG-CoA reductase, and SREBP2) was downregulated by 25-HC. 25-HC also significantly attenuated proteolytic processing of SREBP2. Finally, 25-HC downregulated cholesterol biosynthesis in CATH.a neurons. Our results demonstrate that 25-HC is a potent effector oxysterol of neuronal cholesterol homeostasis.
1. Introduction
The brain contains approx. 25% of total body cholesterol and the majority of cholesterol is produced by endogenous synthesis [4]. Brain cholesterol biosynthesis is highest during development and requires intact neuronal cholesterol biosynthetic pathways [10]. When myelination is completed cholesterol biosynthesis decreases and glial cells synthesize and secrete apolipoprotein(apo)E-containing lipoprotein particles, which are utilized by neurons as cholesterol source [31]. However, in the hippocampus neurons express cholesterol biosynthetic genes suggesting that also mature neurons retain the ability of cholesterol biosynthesis [40]. Part of excess neuronal cholesterol can be converted by acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) to cholesteryl esters (CE), which are stored intracellularly. Besides storage, neurons convert excess cholesterol to 24(S)-hydroxycholesterol (24-HC; [4]) which plays a dual role: First, 24-HC is a specific brain cholesterol metabolite that is secreted via the blood–brain barrier into the circulation where it is transported in association with lipoproteins and further metabolized by the liver [4]. Second, 24-HC is a bioactive oxysterol that regulates the expression of enzymes centrally involved in brain cholesterol homeostasis [41]. Apart from 24-HC the brain is capable of synthesizing 25-HC and 27-HC via cholesterol 25-hydroxylase (CH25H) and cholesterol 27-hydroxylase (CYP27A1) [35]. Aberrant oxysterol synthesis is implicated in neurodegenerative diseases such as Alzheimer's disease [25], multiple sclerosis [21], or cerebrotendinous xanthomatosis [28].
Proinflammatory stimuli like lipopolysaccharide (LPS) lead to induction of CH25H, the enzyme responsible for the formation of 25-HC [22]. Recent work identified both, CH25H and 25-HC as modulators of the immune response and inflammation [1,9,29]. Bauman et al. [1] revealed a central role for 25-HC as regulator of the immune system where this oxysterol is able to suppress Ig class switch recombination from IgM to IgA. CH25H in macrophages is rapidly induced upon treatment with LPS and low dose application of LPS in healthy volunteers led to an increase in plasma 25-HC levels via Toll-like receptor (TLR)4-dependent pathways [9]. An alternative pathway for suppression of TLR-mediated innate immune response by 25-HC is provided by activation of liverXreceptor (LXR)-dependent pathways [6,18]. 25-HC is a potent regulator of LXR-mediated pathways, that impact on brain lipid homeostasis [34], expression of the cholesterol efflux pumps ATP-binding cassette transporter (ABC) A1 and ABCG1, and apoE expression [5,20,38]. 25-HC is also able to stimulate LXR-independent oligodendrocyte apoptosis and suppresses myelin gene expression in peripheral nerves via LXR/Wnt/β-catenin-mediated pathways [26]. 25-HC can also act as a negative regulator of sterol regulatory element binding protein (SREBP)-dependent pathways by binding to insulin-induced gene 1 and 2 anchor proteins (Insig1 and -2) thereby inhibiting proteolytic activation of SREBPs [15].
Based on previous evidence the first part of the study aimed at investigating the consequences of a peripherally induced inflammation on CH25H mRNA and protein expression in mouse brain under inflammatory conditions. In the second part we sought to clarify effects of exogenously added 25-HC on transcriptional regulation of cholesterol biosynthetic genes, SREBP processing, and cholesterol biosynthesis in the murine CATH.a neuronal cell line.
2. Materials and methods
A detailed Materials and Methods section describing animal experiments [39], cell culture of CATH.a neurons [37], Western blotting, GC–MS analysis, quantitative Real Time qPCR [30] (primers in Supplemental Table I), measurement of SREBP2 processing and cholesterol biosynthesis [8], and statistical procedures are given in the Supplementary Information.
3. Results and discussion
3.1. Peripheral LPS induces CH25H mRNA and protein in murine brain
In the first set of experiments two different concentrations of LPS were used to induce inflammation. To confirm a neuroinflammatory response, brain TNFα mRNA induction was analyzed by qPCR. These results revealed that TNFα gene expression was elevated by a maximum of 33- (1.6 mg/kg LPS; 3 h) and 64-fold (8.3 mg/kg LPS; 24 h) in comparison to control mice (PBS injected) (Fig. 1A). This is in line with a comparable mouse model where brain TNFα levels remained elevated for several months [33]. Under our experimental conditions a maximum induction of CH25H mRNA expression of 7- (1.6 mg/kg LPS; 3 h) and 10-fold (8.3 mg/kg LPS; 24 h) was observed (Fig. 1B). Bauman et al. reported a 25-fold induction of CH25H mRNA in brains of mice exposed to the TLR agonist KDO [1].
Fig. 1.
CH25H mRNA and protein expression in brain of LPS-treated mice. Mice received a single i.p. injection of LPS (1.6 or 8.3 220 mg/kg) or PBS (vehicle, ‘v’). Animals were killed by cervical dislocation, brains were removed, RNA was isolated and TNF (A) and CH25H (B) mRNA expression was analyzed by qPCR at the indicated time points. cDNA levels were normalized to GAPDH as a reference gene and expressed relative to gene expression in vehicle controls. CH25H protein (C) was analyzed by Western blotting (densitometric evaluation of immunoreactive bands is shown). 25-HC concentrations (D) in brains of vehicle- and LPS-treated mice (8.3 mg/kg body weight; 48 h) were quantitated by GC–MS analysis. Expression analysis of selected gene products (E) was performed by qPCR at the indicated time points post LPS (8.3 mg/kg body weight). Results shown in (A), (B), (D), and (E) represent means ± SEM of three animals per time point measured in triplicates. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. n.s. = not significant.
Western blot analysis of CH25H in brain homogenates revealed an apparent molecular mass of 36 kDa consistent with other reports [16,22]. After 2, 4 and 8 days post LPS application an induction of CH25H protein (up to 3-fold higher as compared to controls) was found (Fig. 1C). Compatibly, brain 25-HC concentrations increased significantly from 4.3 (vehicle) to 11.2 (LPS; p < 0.05) ng/mg wet tissue (Fig. 1D). These data are in line with a report by Lütjohann et al. demonstrating that 25-HC concentrations are below 3% of 24-HC concentrations in human brain [24]. The expression levels of selected genes involved in brain cholesterol homeostasis were also altered in response to LPS (Fig. 1E). LPS treatment induced a (statistically not significant) decrease of 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS1; responsible for the formation of HMG-CoA from acetyl-CoA and acetoacetyl-CoA) and HMG-CoA reductase (HMGCR; catalyzing the rate-limiting step of cholesterol biosynthesis from HMG-CoA to mevalonate; [14]). In contrast, ABCA1 was significantly upregulated at all time points investigated, while apoE induction reached statistical significance only at 3 h post LPS (Fig. 1E).
3.2. 25-HC as transcriptional regulator of cholesterol homeostasis in murine CATH.a neurons
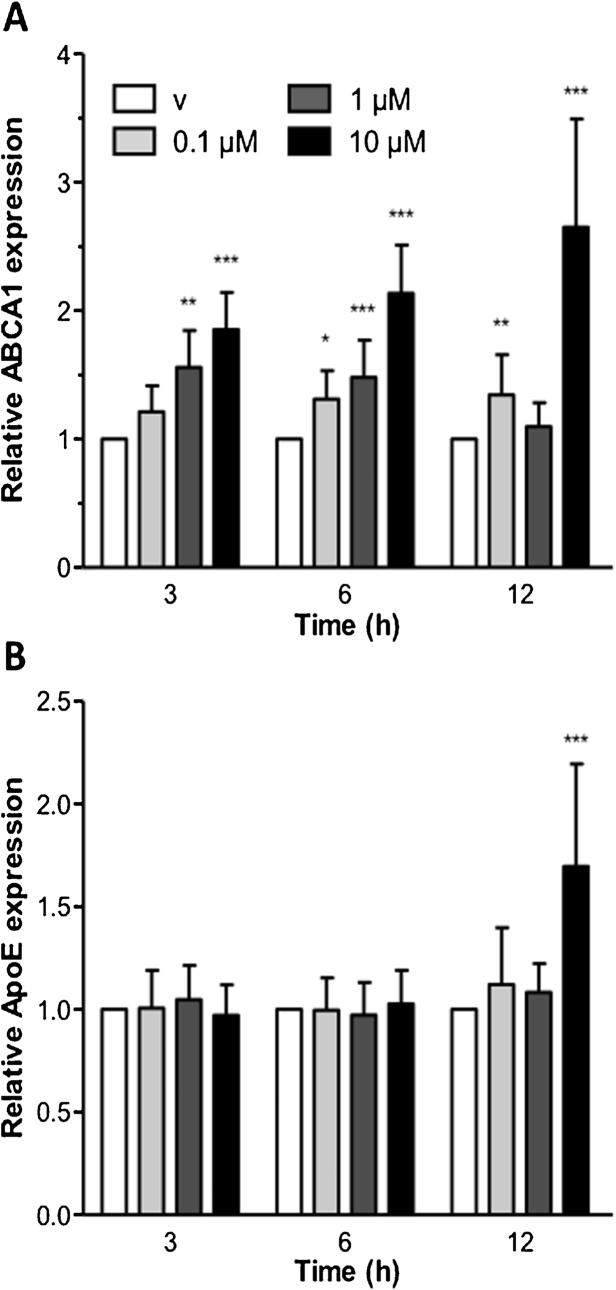
In vitro, 25-HC upregulated mRNA levels of ABCA1 (maximum of 2.6-fold at 12 h; Fig. 2A) and to a lesser extent also of apoE (1.7-fold) at high 25-HC concentrations and later (12 h) time points (Fig. 2B).
Fig. 2.
25-HC induces ABCA1 and ApoE transcription in CATH.a cells. CATH.a cells were incubated in the presence of increasing 25-HC concentrations. At the indicated time points mRNA was isolated and reverse transcribed. cDNA levels of ABCA1 (A) and ApoE (B) were normalized to β-actin as a reference gene and expressed relative to gene expression in vehicle (‘v’) controls (ethanol, 0.05%). Results represent means of triplicates ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Next transcriptional changes in genes involved in CATH.a cholesterol biosynthesis were studied. 25-HC induced a pronounced downregulation (1.8- to 2.6-fold) of farnesyl-diphosphate farnesyltransferase (FDFT1; the enzyme responsible for squalene synthesis from farnesyl diphosphate) transcription (Fig. 3A). Treatment of cells with 25-HC led to a robust downregulation of HMGCS1 in neurons in a concentration-dependent manner (Fig. 3B). 25-HC significantly decreased expression of HMGCR by a maximum of 60% at 12 h (Fig. 3C). Finally, 25-HC induced a pronounced decrease in transcription of SREBP2, where reduction was concentration-dependent reaching levels of approx. 50% as compared to baseline levels (Fig. 3D). Immunoblot analyses (Fig. 4) revealed that proteolytic processing of SREBP2 protein is significantly attenuated at 100 nM 25-HC with almost no mature SREBP2 detectable at 1 μM 25-HC. In healthy human volunteers, plasma 25-HC levels are approx. 5 nM [12] but can reach concentrations of about 500 nM in patients that carry mutations in the gene coding for the oxysterol 7 alpha hydroxylase (CYP7B1) [36]. These are 25-HC concentrations that potently affect SREBP processing in vitro (Fig. 4).
Fig. 3.
25-HC impairs cholesterol biosynthetic gene transcription. CATH.a cells were incubated in the presence of increasing 25-HC concentrations. At the indicated time points mRNA was isolated and reverse transcribed. cDNA levels of FDFT1 (A), HMGCS (B), HMGCR (C), and SREBP2 (D) were normalized to β-actin as a reference gene and expressed relative to gene expression in vehicle (‘v’) controls (ethanol, 0.05%). Results represent means of triplicates ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4.

25-HC inhibits SREBP2 activation. CATH.a cells were serum-starved (5% LPDS) for 4 h in the presence of 50 μM mevalonate, 10 μM compactin and 25-HC at the indicated concentrations. Total cell protein was isolated and mature SREBP2 (mSREBP2) was detected by Western blotting (A). β-actin was used as loading control. Graphical representation of relative optical densities of immunoreactive SREBP2 normalized to those of β-actin is shown in (B).
3.3. 25-HC impairs cholesterol biosynthesis
CATH.a cells were pre-incubated with 25-HC and [14C]acetate (2 μCi/well) as cholesterol precursor. At the indicated time points lipids were extracted, separated by TLC and bands co-migrating with free cholesterol and cholesteryl ester (CE) standards were cut out and subjected to scintillation counting. These experiments demonstrated that at 2.5 and 5 μM 25-HC cholesterol biosynthesis is significantly inhibited by 30 and 55%, respectively (Fig. 5A). The radioactivity recovered from the CE fraction was approx. 8% of total radioactivity under control conditions, indicating low neuronal CE synthesis (Fig. 5B). 25-HC treatment significantly increased the percentage radioactivity in the CE fraction to 11 and 21% of total (FC + CE) radioactivity (2.5 and 5 μM 25-HC) indicating ACAT activation in response to 25-HC. This is in line with previous reports where 25-HC activates ACAT-mediated cholesterol esterification in CHO cells [11] and enterocytes [13]. This might be of importance for neuronal function since ACAT antagonism alters amyloid β processing in vitro [32] and ameliorates amyloid pathology in a mouse model of Alzheimer's disease [17].
Fig. 5.
25-HC impairs cholesterol biosynthesis. Cells received medium containing the indicated concentrations of 25-HC and [14C]acetate (2 μCi/well). After 6 h cellular and medium lipids were extracted, separated by TLC and the bands co-migrating with unlabeled cholesterol (FC) and CE (16:0) standards were cut out from the plates. Radioactivity was counted on a β-counter. ‘v’ = vehicle control (ethanol). Results shown represent means ± SEM of three independent experiments per group. Significances were calculated with 1-way ANOVA followed by a Bonferroni post-test. *p≤0.05, **p≤0.01, ***p≤0.001.
Radioactivity recovered from the cholesterol fraction in the medium under control conditions was relatively high (40% of cell-associated activity) indicating efficient cholesterol efflux by CATH.a cells. In response to 2.5 and 5 μM 25-HC, cholesterol radioactivity in the medium decreased to approx. 50 and 30% of controls (Fig. 5C), comparable to what was observed for the cellular cholesterol pool. In contrast, the contribution of medium CE radioactivity is rather low (approx. 6% of total CE radioactivity; Fig. 5D).
Side chain-oxidized cholesterol metabolites have the unique ability to limit cellular cholesterol levels in vitro [15]. A major question arising is the ability of oxysterols to interfere with cholesterol homeostasis in vivo. This is due to the fact that a 100- to 500-fold excess of cholesterol will compete for oxysterol binding to LXR [3]. Currently murine knockout models are available for the three major cholesterol hydroxylases contributing to oxysterol synthesis in the brain. Deletion of CYP46A1 (CH24H) leads to significantly reduced CNS cholesterol synthesis and excretion. This might be a reflection of cholesterol turnover in a metabolically active subset of neurons where the majority of CH24H is expressed [23]. In addition CH24H−/− mice exhibit severely impaired learning abilities [19]. Deletion of CYP27A1 (CH27H) in mice leads to increased cholestanol levels in the brain [2], a condition reminiscent of what is observed in patients with cerebrotendinous xanthomatosis who carry mutations in the CYP27A1 gene [28]. In the CYP27A1−/− mice increased lathosterol concentrations in the cerebrum were suggested to be indicative for increased cholesterol biosynthesis [2]. A recent report identified CYP27A1 deletion in the mouse as a causative factor for defective retinal cholesterol homeostasis [27]. In addition, abnormal neovascularization in the retina of CYP27A1−/− mice reproduced some features observed in patients with age-related macular degeneration [27]. CH25H knockout leads to decreased levels of 25-HC in the lung and serum of knockout mice and severely impacts on serum IgA levels [1]. Finally a triple knockout of CYP46A1, CYP27A1, and CH25H failed to induce transcription of LXR target genes in response to a high cholesterol diet [7]. These reports strongly suggest that oxysterols are in vivo regulators of cholesterol homeostasis in different organs including the brain. Data of the present study indicate that CH25H is upregulated under neuroinflammatory conditions in vivo and that 25-HC is a potent regulator of neuronal cholesterol homeostasis in vitro.
Conflict Of Interest
None.
Acknowledgements
Financial support was provided by the Austrian Science Fund (FWF; grant No. F3007 and W1226-B18), the Austrian Nationalbank (P14534), and the Austrian Research Promotion Agency (FFG; grant No. Bridge P820107). S.W. was funded by the FWF within the PhD program Molecular Medicine of the Medical University of Graz.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2013.01.014.
Appendix A. Supplementary data
References
- 1.Bauman D.R., Bitmansour A.D., McDonald J.G., Thompson B.M., Liang G., Russell D.W. 25-Hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin A production. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavner A., Shafaati M., Hansson M., Olin M., Shpitzen S., Meiner V., Leitersdorf E., Bjorkhem I. On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. Journal of Lipid Research. 2010;51:2722–2730. doi: 10.1194/jlr.M008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorkhem I. Do oxysterols control cholesterol homeostasis? Journal of Clinical Investigation. 2002;110:725–730. doi: 10.1172/JCI16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 5.Brahimi F., Bertrand P., Starck M., Galteau M.M., Siest G. Control of apolipoprotein E secretion in the human hepatoma cell line KYN-2. Cell Biochemistry and Function. 2001;19:51–58. doi: 10.1002/cbf.899. [DOI] [PubMed] [Google Scholar]
- 6.Castrillo A., Joseph S.B., Marathe C., Mangelsdorf D.J., Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. Journal of Biological Chemistry. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 7.Chen W., Chen G., Head D.L., Mangelsdorf D.J., Russell D.W. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metabolism. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBose-Boyd R.A., Ou J., Goldstein J.L., Brown M.S. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1477–1482. doi: 10.1073/pnas.98.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diczfalusy U., Olofsson K.E., Carlsson A.M., Gong M., Golenbock D.T., Rooyackers O., Flaring U., Bjorkbacka H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. Journal of Lipid Research. 2009;50:2258–2264. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietschy J.M., Turley S.D. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. Journal of Lipid Research. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Du X., Pham Y.H., Brown A.J. Effects of 25-hydroxycholesterol on cholesterol esterification and sterol regulatory element-binding protein processing are dissociable: implications for cholesterol movement to the regulatory pool in the endoplasmic reticulum. Journal of Biological Chemistry. 2004;279:47010–47016. doi: 10.1074/jbc.M408690200. [DOI] [PubMed] [Google Scholar]
- 12.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Analytical Biochemistry. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 13.Field F.J., Mathur S.N. Regulation of acyl CoA:cholesterol acyltransferase by 25-hydroxycholesterol in rabbit intestinal microsomes and absorptive cells. Journal of Lipid Research. 1983;24:1049–1059. [PubMed] [Google Scholar]
- 14.Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein J.L., DeBose-Boyd R.A., Brown M.S. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Honda A., Miyazaki T., Ikegami T., Iwamoto J., Maeda T., Hirayama T., Saito Y., Teramoto T., Matsuzaki Y. Cholesterol 25-hydroxylation activity of CYP3A. Journal of Lipid Research. 2011;52:1509–1516. doi: 10.1194/jlr.M014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutter-Paier B., Huttunen H.J., Puglielli L., Eckman C.B., Kim D.Y., Hofmeister A., Moir R.D., Domnitz S.B., Frosch M.P., Windisch M., Kovacs D.M. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 19.Kotti T.J., Ramirez D.M., Pfeiffer B.E., Huber K.M., Russell D.W. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3869–3874. doi: 10.1073/pnas.0600316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffitte B.A., Repa J.J., Joseph S.B., Wilpitz D.C., Kast H.R., Mangelsdorf D.J., Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leoni V., Masterman T., Diczfalusy U., De Luca G., Hillert J., Bjorkhem I. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neuroscience Letters. 2002;331:163–166. doi: 10.1016/s0304-3940(02)00887-x. [DOI] [PubMed] [Google Scholar]
- 22.Lund E.G., Kerr T.A., Sakai J., Li W.P., Russell D.W. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. Journal of Biological Chemistry. 1998;273:34316–34327. doi: 10.1074/jbc.273.51.34316. [DOI] [PubMed] [Google Scholar]
- 23.Lund E.G., Xie C., Kotti T., Turley S.D., Dietschy J.M., Russell D.W. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. Journal of Biological Chemistry. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 24.Lutjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Bjorkhem I. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutjohann D., Papassotiropoulos A., Bjorkhem I., Locatelli S., Bagli M., Oehring R.D., Schlegel U., Jessen F., Rao M.L., von Bergmann K., Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. Journal of Lipid Research. 2000;41:195–198. [PubMed] [Google Scholar]
- 26.Makoukji J., Shackleford G., Meffre D., Grenier J., Liere P., Lobaccaro J.M., Schumacher M., Massaad C. Interplay between LXR and Wnt/beta-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. Journal of Neuroscience. 2011;31:9620–9629. doi: 10.1523/JNEUROSCI.0761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omarova S., Charvet C.D., Reem R.E., Mast N., Zheng W., Huang S., Peachey N.S., Pikuleva I.A. Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. Journal of Clinical Investigation. 2012;122:3012–3023. doi: 10.1172/JCI63816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panzenboeck U., Andersson U., Hansson M., Sattler W., Meaney S., Bjorkhem I. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendinous xanthomatosis. Journal of Lipid Research. 2007;48:1167–1174. doi: 10.1194/jlr.M700027-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Park K., Scott A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. Journal of Leukocyte Biology. 2010;88:1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfrieger F.W., Ungerer N. Cholesterol metabolism in neurons and astrocytes. Progress in Lipid Research. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Puglielli L., Konopka G., Pack-Chung E., Ingano L.A., Berezovska O., Hyman B.T., Chang T.Y., Tanzi R.E., Kovacs D.M. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nature Cell Biology. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 33.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repa J.J., Li H., Frank-Cannon T.C., Valasek M.A., Turley S.D., Tansey M.G., Dietschy J.M. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. Journal of Neuroscience. 2007;27:14470–14480. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell D.W. Oxysterol biosynthetic enzymes. Biochimica et Biophysica Acta. 2000;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 36.Schule R., Siddique T., Deng H.X., Yang Y., Donkervoort S., Hansson M., Madrid R.E., Siddique N., Schols L., Bjorkhem I. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. Journal of Lipid Research. 2010;51:819–823. doi: 10.1194/jlr.M002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son H.J., Lee J.A., Shin N., Choi J.H., Seo J.W., Chi D.Y., Lee C.S., Kim E.M., Choe H., Hwang O. A novel compound PTIQ protects the nigral dopaminergic neurones in an animal model of Parkinson's disease induced by MPTP. British Journal of Pharmacology. 2012;165:2213–2227. doi: 10.1111/j.1476-5381.2011.01692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam S.P., Ramharack R. The effect of 25-hydroxycholesterol on the regulation of apolipoprotein E mRNA levels and secretion in the human hepatoma HepG2. Atherosclerosis. 1992;95:137–146. doi: 10.1016/0021-9150(92)90017-b. [DOI] [PubMed] [Google Scholar]
- 39.Ullen A., Fauler G., Kofeler H., Waltl S., Nusshold C., Bernhart E., Reicher H., Leis H.J., Wintersperger A., Malle E., Sattler W. Mouse brain plasmalogens are targets for hypochlorous acid-mediated modification in vitro and in vivo. Free Radical Biology and Medicine. 2010;49:1655–1665. doi: 10.1016/j.freeradbiomed.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdez C.M., Smith M.A., Perry G., Phelix C.F., Santamaria F. Cholesterol homeostasis markers are localized to mouse hippocampal pyramidal and granule layers. Hippocampus. 2010;20:902–905. doi: 10.1002/hipo.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Muneton S., Sjovall J., Jovanovic J.N., Griffiths W.J. The effect of 24S-hydroxycholesterol on cholesterol homeostasis in neurons: quantitative changes to the cortical neuron proteome. Journal of Proteome Research. 2008;7:1606–1614. doi: 10.1021/pr7006076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.