Abstract
Multiple mechanisms underlie the surprising willingness of mothers to tolerate the semi-allogeneic fetal tissues during pregnancy. Chief among these is the expression of the HLA-G molecules that has been largely demonstrated to be responsible for reprogramming the local maternal immune response towards tolerance. We recently identified a subset of tolerogenic dendritic cells, DC-10 that secrete high amounts of IL-10 and express high levels of HLA-G and its ligand ILT4. DC-10 are present in the peripheral blood and are essential in inducing adaptive regulatory T cells. We investigated the presence of DC-10 and HLA-G-expressing CD4+ T cells in human decidua in the first trimester of pregnancy. Results showed that these cells are highly represented in human decidua as compared to the peripheral blood. This is the first report describing decidual DC-10 and CD4+HLA-G+ T cells, strongly suggesting that they may accumulate or be induced at the fetal maternal interface to promote tolerance.
1. Introduction
The fetus enjoys special privileges that minimize the risk of being rejected by the maternal immune system during pregnancy. The maternal immune system is alerted and responds actively to the fetal invasion, but the type of inflammation generated is not a milieu in which rejecting T cell responses are favored. At the fetal maternal interface, the decidua serves as an immunologically privileged tissue playing essential functions in pregnancy maintenance [1]. During the first trimester of pregnancy, the majority of leucocyte populations in the human decidua is composed of 70% natural killer (NK) cells, and 10–20% antigen presenting cells (APCs) [2], whereas T cells are sparse and B cells are virtually absent [2,3].
Dendritic cells (CD11chiDCs) are the key professional APCs representing 5–10% of all hematopoietic uterine cells [4]. DCs are not only essential for the induction of primary immune responses but also important for the establishment of immunological tolerance. The local microenvironment influences the functions and differentiation of DCs with tolerogenic activities that play a prominent role in dictating the quantity and quality of immune responses [2]. Two different myeloid DC subsets, BDCA-1+ and BDCA-3+, were detected in normal human first trimester decidua [5]. BDCA-1+ decidual cells express HLA-DR, CD80 and CD86 at low levels, consistent with the immature characteristics of myeloid DCs [6]. In addition, Kammerer et al. [3] have shown that early human pregnancy decidua harbors C-type lectin-expressing cells (DC-SIGN+) that show functional features of immature DCs.
During human pregnancy, non-classical HLA class I HLA-G proteins, specifically expressed in the trophoblasts, contribute to the establishment of immune tolerance [7]. Seven different isoforms of HLA-G exist, four of which are membrane-bound (HLA-G1 to -G4) and three are soluble forms (HLA-G5 to -G7). HLA-G locus is low polymorphic in the coding region, but polymorphisms that can regulate its expression are present at both 5′ Up-stream Regulatory Region (URR) and 3′ Un-translated Region (UTR) non-coding regions [8]. The immune-regulatory properties of HLA-G result from interactions with diverse inhibitory receptors: directly via Ig-like transcript (ILT)2 expressed on myeloid and lymphoid cells, ILT4 specifically expressed on APCs, including DCs, and KIR2DL4 on NK cells and cytotoxic T lymphocytes (CTL); indirectly via CD94/NKG2A on NK cells [9]. Myeloid APCs may express HLA-G [10] and its expression is greatly enhanced by interferon-γ, IL-10 and maturation stimuli [7].
The expression of membrane-bound HLA-G and the secretion of soluble HLA-G by myeloid APCs contribute to the generation of a tolerogenic microenvironment that may alter the functions of HLA-G-expressing myeloid APCs (HLA-G+ APCs) themselves, in a feedback loop. Thus, myeloid HLA-G+ APCs may be viewed as suppressor cells capable of inhibiting other effector cells and of generating regulatory cells, such as tolerogenic DCs and regulatory T cells (Tregs) [10]. Recently, a subset of IL-10-producing human DC (DC-10) has been characterized in the peripheral blood [11]. These cells secrete high levels of IL-10, express membrane-bound HLA-G, ILT2, ILT3, ILT4, and are potent inducers of adaptive IL-10-producing type 1 Tregs (Tr1) in vitro through the IL-10-dependent ILT4/HLA-G pathway [11].
CD4+ T cells constitutively expressing HLA-G have been shown to accumulate at sites of inflammation [12]. It has been demonstrated that CD4+HLA-G+ cells suppress T cell proliferation via a reversible non-contact IL-10- and soluble HLA-G5-dependent process that leads to regulation of tissue inflammation at the target organ [13].
In the present study we identify for the first time the presence of DC-10 and CD4+HLA-G+ T cells at the fetal maternal interface where they may contribute to the tolerance establishment and maintenance in the first trimester decidua.
2. Materials and methods
2.1. Subjects and tissue samples
First trimester decidua (n = 10) at 6–12 weeks of gestational age were obtained from Caucasian women with clinically normal pregnancies which were scheduled for elective abortion due to social or psychological reasons. Peripheral blood was collected soon before the aspiration procedure. Decidual tissues were taken through the cervix during dilatation and aspiration according to formal clinical procedures. Informed consent was obtained from all subjects, and the investigation was approved by the Ethical Committee of Fondazione IRCCS Ca’ Granda, Milan, Italy.
Human peripheral blood was obtained from healthy donors upon informed consent in accordance with local ethical committee approval (TIGET PERIBLOOD) and with the Helsinki Declaration. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation over Lymphoprep Ficoll gradients (Fresenius Kabi Norge AS, Halden, Norway).
This study was approved and monitored by the internal ethical committee of the San Raffaele Scientific Institute and the Ethical Committee of Fondazione IRCCS Ca’ Granda, Milan, Italy.
2.2. Isolation of mononuclear decidual cells
Samples were processed within 2 h after they have been collected. Decidual tissues were dissected into small pieces and enzymatically dissociated in RPMI 1640 medium containing collagenase type IV (1 mg/ml; Sigma Aldrich, Carlsbad, CA), and DNase I (final 0.01%, Invitrogen) for 90 min at 37 °C with gentle shaking [14]. The suspension was filtered through a 40-μm nylon mesh (Cell Strainer, BD Biosciences, San Jose, CA). Decidual mononuclear cells (DMNC) were separated by density gradient centrifugation over Lymphoprep Ficoll gradients (Fresenius Kabi Norge AS, Halden, Norway).
2.3. Flow cytometric analysis
DMNC and PBMC were initially incubated for 15 min at room temperature with FcR blocking reagent (Miltenyi Biotech, Germany) and stained for additional 30 min at room temperature with the following human monoclonal Antibodies (mAbs): CD1c (BDCA-1) and CD83 (Miltenyi Biotech, Germany), CD14, CD16, DC-SIGN, CD11c, CD4, CD8 (BD Bioscience, CA), CD45 (BioLegend, USA), ILT4 and CD56 (Beckman Coulter, France) and HLA-G (MEM-G9, Exbio, Praha, Czech Republic). Cells were identified using a multiparametric approach based on the combination of mAbs. Samples were acquired using a FACS Canto II flow cytometer (Becton Dickinson, Mountain View, CA), and data were analyzed with FCS express (De Novo Software). Quadrant markers were set accordingly to unstained controls.
2.4. Statistical analysis
Values are reported as Mean ± SEM. Mann–Whitney test was used to determine the statistical significance of the data. Significance was defined as ∗p ⩽ 0.05, ∗∗p ⩽ 0.005, ∗∗∗p ⩽ 0.0005, and ∗∗∗∗p < 0.0001. Statistical calculations were performed with the Prism program 5.0 (GraphPad Software, Inc. La Jolla, CA).
3. Results and discussion
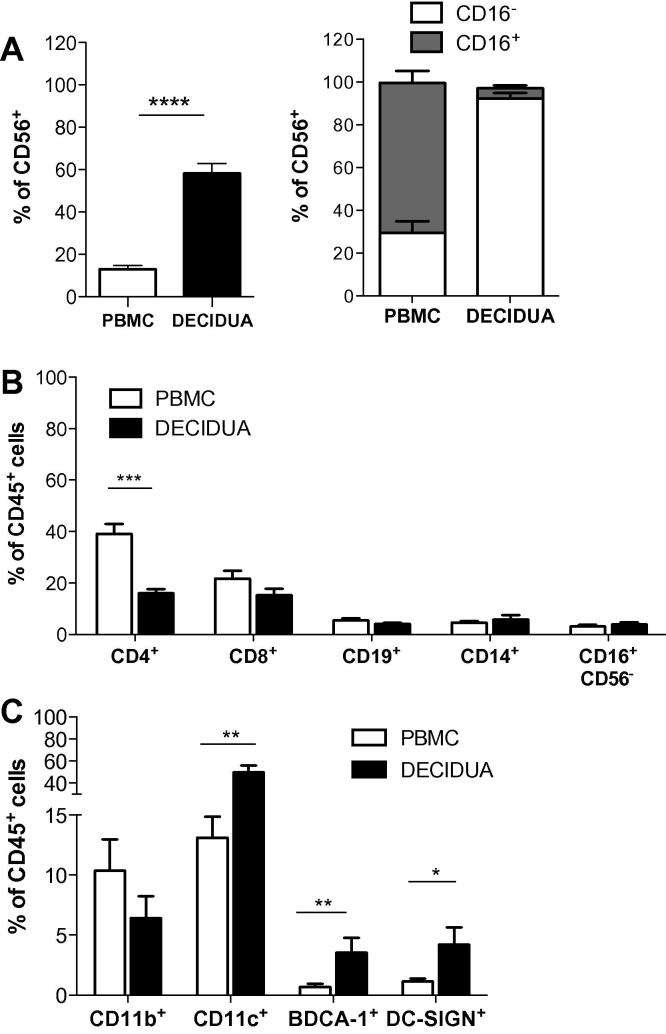
During pregnancy, subpopulations of leukocytes present at the fetal-maternal interface change participating in a coordinated manner to orchestrate sequential, reproductive events that result in the maintenance of pregnancy [15]. During the first trimester, NK cells represent the most abundant immune cells in the decidua [16]. These cells are CD56brightCD16−, are distinct from peripheral blood NK subsets [17], and are critical for the maintenance of pregnancy since their cytotoxicity is inhibited by the expression of HLA-E and HLA-G on trophoblasts [16]. As expected, 58.3 ± 4.6%, (mean ± SEM, n = 10) of the human decidual mononuclear cells were CD56+ NK cells, of which >90% were CD16- (Fig. 1A).
Fig. 1.
Analysis of immune cells infiltrating human decidua in the first trimester of pregnancy. Human decidua infiltrating cells were obtained by collagenase digestion followed by gradient centrifugation. Percentages of CD56+, CD56+CD16+, and CD56+CD16− cells (A), of CD4+, CD8+, CD19+, CD14+, and CD16+CD56− (B), CD11b+, CD11c+, BDCA-1+ (CD11c+CD1c+) and DC-SIGN+ cells (C) in the peripheral blood and human decidual leucocytes (gate on CD45+ cells) of pregnant women in the first trimester of pregnancy are presented. Percentages of positive cells (mean ± SEM) are shown. ∗p ⩽ 0.05, ∗∗p ⩽ 0.005, ∗∗∗p ⩽ 0.0005, and ∗∗∗∗p < 0.0001.
The remainder decidual leukocyte population included T and B lymphocytes, CD14+ monocytes, CD11b+ and CD11c+ myeloid cells. In comparison with peripheral blood lymphocytes, the proportion of CD4+ T cells infiltrating human decidua was significantly lower (16.01 ± 1.64%, mean ± SEM, n = 10, vs. 39.12 ± 3.83%, mean ± SEM, n = 9, p = 0.0009) (Fig. 1B). No differences in the percentages of CD8+ T cells, CD19+ B cells, and CD14+ monocytes were observed between the human decidua and the peripheral blood.
Human decidual leukocytes contained also 6.4 ± 1.8% (mean ± SEM, n = 8) of CD11b+ cells, 49.95 ± 6.07% (mean ± SEM, n = 9) of CD11c+ cells, and 3.54 ± 1.2%, (mean ± SEM, n = 9) of BDCA-1+ (CD11c+CD1c+) cells (Fig. 1C). The frequencies of decidual myeloid CD11c+ and BDCA-1+ cells were significantly higher than their counterpart in the peripheral blood of pregnant women (13.12 ± 1.74%, mean ± SEM n = 9, p = 0.0027, and 0.69 ± 0.27, mean ± SEM, n = 9, p = 0.0047, respectively) (Fig. 1C). Our data are in line with a previous report demonstrating the presence of several subsets of DCs, including BDCA-1+ (≈0.2%), in the human first trimester decidua [5]; however, we found a higher percentage of decidual BDCA-1+ cells (3.5%). This may be due to the method used for isolating decidua-infiltrating cells: Ban et al. [5] used a non-enzymatic method, while we isolated immune cells from decidual tissues by collagenase-mediated digestion [14]. In addition, in the previous study the percentage of BDCA-1+ cells was calculated based on total decidual cells, whereas we evaluated the frequency of BDCA-1+ cells by gating on CD45+ cells. In our setting, the percentage of CD45+ cells ranged from 10% to 70% of the total decidual cells.
Human decidua harbored a significant population of DC-SIGN+ cells that represents 6–10% of decidual cells [3,18]. Decidual DC-SIGN+ cells have been shown to be immature myeloid cells [3] and have been proposed to play a role in normal [3] and pathological pregnancy outcome [18]. In our setting, the percentage of decidual DC-SIGN+ cells was 2.9 ± 0.6% (mean ± SEM, n = 8), and their frequency was significantly higher than that observed in the peripheral blood of pregnant women (1.12 ± 0.23%, mean ± SEM n = 9, p = 0.01) (Fig. 1C).
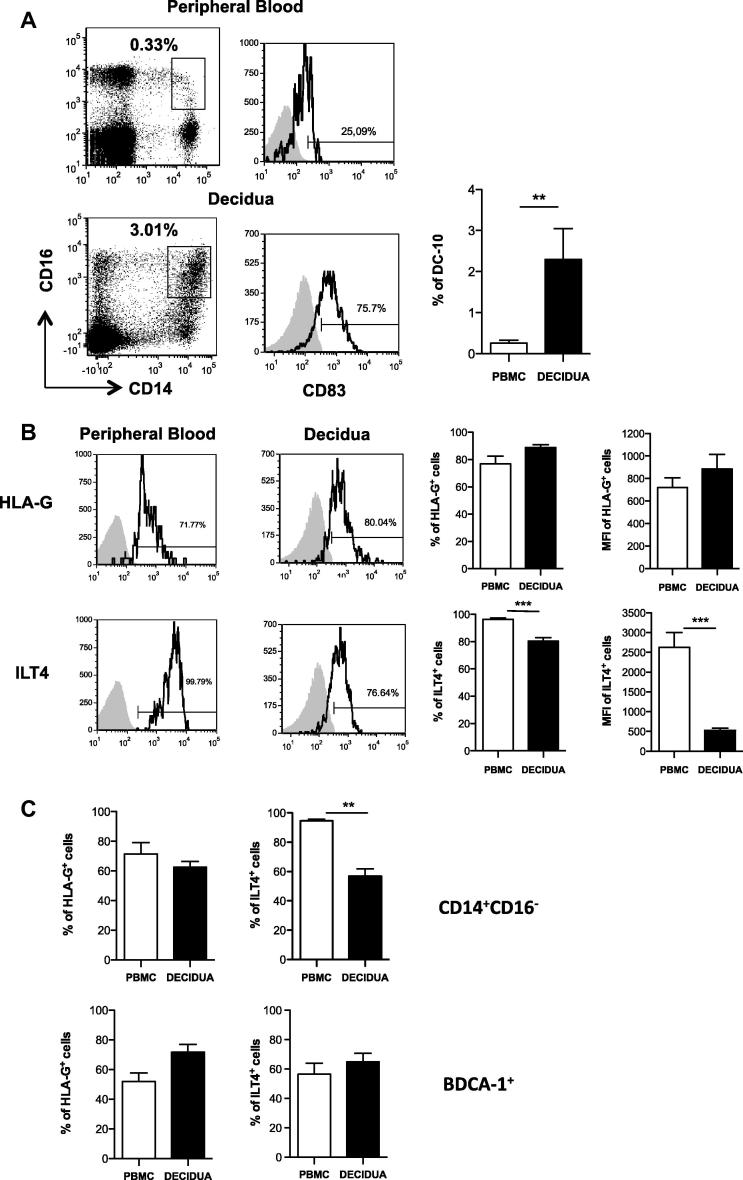
DC-10 are tolerogenic DCs characterized by their ability to secrete IL-10 and by the high expression of membrane-bound HLA-G [11]. Since DC-10 are highly potent in inducing Tr1 cells [11], we hypothesized that these cells might represent one of the major subsets of APCs present in the decidua involved in promoting and maintaining tolerance during pregnancy. DC-10 were identified by the concomitant expression of CD14, CD16 and CD83 on human decidual leucocytes (CD45+). Interestingly, a significantly higher percentage of DC-10 was present in the human decidua than in the peripheral blood of pregnant women (2.29 ± 0.75%, mean ± SEM, n = 10 vs. 0.25 ± 0.07%, mean ± SEM, n = 8, p = 0.0014) (Fig. 2A). Human decidual DC-10 expressed similar levels of HLA-G, but significantly lower levels of ILT4 (80.38 ± 2.45%, mean ± SEM, n = 10 vs. 96.38 ± 1.01%, mean ± SEM, n = 8; p = 0.0003) as compared to circulating DC-10 (Fig. 2B). Similarly, inflammatory monocytes (CD14+CD16−) present in the human decidua expressed similar levels of HLA-G but lower amounts of ILT4 as compared to their circulating counterpart (56.86 ± 4.89%, mean ± SEM, n = 7 vs. 94.57 ± 1.23%, mean ± SEM, n = 9; p = 0.001, Fig. 2C). The expression of HLA-G and ILT4 on decidual BDCA-1+ cells was comparable to that observed in their peripheral blood counterpart (Fig. 2C).
Fig. 2.
Tolerogenic DC-10 are present at high frequency in the human decidua in the first trimester of pregnancy. Human decidua infiltrating cells were obtained by collagenase digestion followed by gradient centrifugation. (A) DC-10 were identified in the peripheral blood or in the human decidua according to CD14, CD16, and CD83 expression. Dot plots and histograms from one representative donor out of ten donors analyzed and the mean ± SEM of the percentages of positive cells are presented. ∗∗p ⩽ 0.005. (B–C) Circulating and decidual DC-10, inflammatory monocytes, and BDCA-1+ cells expressing the tolerogenic markers HLA-G and ILT4. Peripheral blood and human decidual cells were analyzed by flow cytometry to determine levels of HLA-G and ILT4 expression. Analyses were performed on gated CD14+CD16+ cells in the peripheral blood and CD45+CD14+CD16+ cells in the human decidua (B), gated CD14+CD16− cells (inflammatory monocytes) and CD1c+CD11c+ (BDCA-1+) cells in the peripheral blood and CD45+CD14+CD16− cells (inflammatory monocytes) and CD45+CD1c+CD11c+ (BDCA-1+) cells in the human decidua (C). Dot plots from one representative donor out of ten donors analyzed and the mean ± SEM of the percentages of inflammatory monocytes, and BDCA-1+ cells detected in all donors analyzed are presented. ∗∗p ⩽ 0.005 and ∗∗∗p ⩽ 0.0005.
The increased frequency of DC-10 observed in the decidua may be dependent on several mechanisms: DC-10 can be recruited from the peripheral blood, resident decidual DCs can be converted into DC-10, or the decidual microenvironment can promote the de novo induction of DC-10. The decidual microenvironment is enriched of several chemokines, including CCL2 [19] and CX3CL1 [20] that have a role in tissue remodeling and in the recruitment of immune cells. Peripheral blood DC-10 express CCR2 and CX3CR1 [11], thus it can be hypothesized that they are attracted and accumulated in the decidua. Several cytokines including IL-4, IL-10, and GM-CSF as well as growth factors and hormones with anti-inflammatory properties are present at the decidual level (reviewed in [21]). This pro-tolerogenic microenvironment is known to promote alternatively activated macrophages [22] and tolerogenic DCs [23]. In particular, the high levels of IL-10 may promote the up-regulation of HLA-G, ILT2 and ILT4 on resident decidual immature DCs converting them into DC-10. Alternatively, the presence of GM-CSF, IL-4 and IL-10 can allow, through the IL-10 dependent ILT4/HLA-G pathway, the de novo induction of tolerogenic DC-10. Further functional studies are required to better define the origin and the role of DC-10 in the decidua.
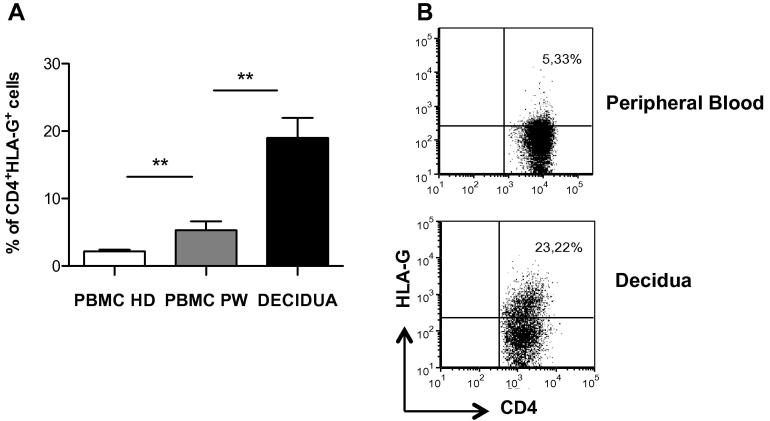
In addition to DCs, CD4+CD25+FOXP3+ Tregs are enriched at the fetal-maternal interface [24,25] and are expanded accordingly to gestational stages [26]. We confirmed that CD4+CD25+FOXP3+ Tregs cells are present in the human decidua, and no differences in the percentages of these cells were observed between human decidua and peripheral blood in our cohort of pregnant women (data not shown). In addition to CD4+CD25+FOXP3+ Tregs, other Tregs either naturally occurring or induced have been described [27]. Among them, we focused our attention on a new type of naturally occurring CD4+ and CD8+ Tregs that constitutively express HLA-G. CD4+HLA-G+ and CD8+HLA-G+ Tregs are present in the peripheral blood [12]. Moreover, CD4+HLA-G+ Tregs have been shown to accumulate at sites of inflammation, and are potent suppressors of T cell proliferation in vitro [13]. To date, there is no evidence of the presence of HLA-G-expressing CD4+ or CD8+ T cells in the peripheral blood and the decidua during pregnancy. We first investigated the frequency of circulating CD4+HLA-G+ T cells in pregnant women as compared to healthy donors. Results showed a significantly higher frequency of HLA-G expressing CD4+ T cells in the peripheral blood of pregnant women (PBMC PW) compared to that of healthy donors (PBMC HD) (5.29 ± 1.3%, mean ± SEM, n = 8 vs. 2.13 ± 0.28%, mean ± SEM, n = 53, respectively, p = 0.0053) (Fig. 3A). Interestingly, a distinct population of CD4+ T cells expressing HLA-G was detected in the human decidua (18.97 ± 2.98% mean ± SEM, n = 9) (Fig. 3A and B). The frequency of CD8+HLA-G+ T cells in the human decidua was comparable to that observed in the peripheral blood of pregnant women (data not shown).
Fig. 3.
High frequency of CD4+HLA-G+ T cells are present in the human decidua in the first trimester of pregnancy. Human decidua infiltrating cells were obtained by collagenase digestion followed by gradient centrifugation. Peripheral blood of healthy donors (PBMC HD) and of pregnant women (PBMC PW) as well as human decidua infiltrating cells were analyzed by flow cytometry to determine levels of HLA-G expression. Analyses were performed on gated CD4+ T cells in the peripheral blood and CD45+CD4+ T cells in the human decidua, respectively. The mean ± SEM of the percentages of CD4+HLA-G+ T cells in all donors analyzed, ∗∗p ⩽ 0.005 (A) and dot plots from one representative donor out of ten donors analyzed are presented (B).
Similarly to what has been described in the central nervous system [13], CD4+HLA-G+ T cells can be recruited to the human decidua in the first phases of embryo implantation to control immune responses against the semi-allogeneic fetal antigens. It still remains to be defined whether decidual CD4+HLA-G+ T cells express CCR5 or other chemokine receptors, which may allow their recruitment into the decidua, in analogy to what has been described for the same cells during the active inflammatory phase of multiple sclerosis [13]. We cannot exclude that CD4+HLA-G+ T cells could be directly induced in the decidua by local IL-10 as it is known that IL-10 promotes HLA-G expression in CD4+ T cells [28]. Moreover, we previously demonstrated that during Tr1 cell induction via DC-10, IL-10-derived from DC-10 induces HLA-G expression on CD4+ T cells that become anergic T cells, and subsequently Tr1 cells [11]. In this scenario, it can be speculated that naive CD4+ T cells, once recruited in the decidua, can be activated via DC-10, up-regulate HLA-G expression and then, in the continuous presence of IL-10 and chronic allo-specific stimulation, they become allo-specific Tr1 cells. It still remains to define whether CD4+HLA-G+ T cells are precursors of Tr1 cells or represent a distinct subset of Tregs, which co-operate with other Tregs, including CD4+CD25+FOXP3+ Tregs, in promoting and maintaining fetal-maternal tolerance.
4. Future perspectives
In addition to an overall improvement of the knowledge on the biological mechanisms underlying tolerance associated with HLA-G expression, our findings may have different potential clinical implications. The ultimate goal of this study is the development of a risk assessment of women with a history of recurrent implantation failure who undergo in vitro fertilization (IVF). From a diagnostic point of view, the tolerance status of women with recurrent embryo implantation failure after IVF can be evaluated. The analysis of DC-10 and HLA-G+ Tregs and their potential association with HLA-G polymorphisms will allow the identification of specific HLA-G genotypes that could be included among the pre-IVF screening tests. From a therapeutic point of view, the study will open new perspectives for the modulation of immune responses in the early phases of pregnancy with the aim of minimizing the negative effects on embryo implantation associated with a reduced maternal tolerance status.
Acknowledgments
This work was supported by Telethon Foundation “Comitato Telethon Fondazione Onlus” Core Grant OSR-TIGET project E2 (Rome), the Italian Ministry of Health, and Fondazione Giorgio Pardi, Milan. Dr. Giada Amodio conducted this study as partial fulfillment of her PhD. in Molecular Medicine, Program in Basic and Applied Immunology, San Raffaele University, Milan, Italy.
Author’s roles: G.A., A.M., and A.S. performed the experiments and the analysis of the data and contributed to the preparation of the manuscript. M.C., E.S. and P.V. provided the clinical samples. M.G.R. contributed to the scientific supervision. S.G. and P.P.-B. conceived the scientific idea, supervised the project and prepared the manuscript.
Contributor Information
Paola Panina-Bordignon, Email: panina.paola@hsr.it.
Silvia Gregori, Email: gregori.silvia@hsr.it.
References
- 1.Loke Y.W., King A., Burrows T.D. Decidua in human implantation. Hum Reprod. 1995;10(Suppl. 2):14–21. doi: 10.1093/humrep/10.suppl_2.14. [DOI] [PubMed] [Google Scholar]
- 2.Plaks V., Birnberg T., Berkutzki T., Sela S., BenYashar A. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kammerer U., Eggert A.O., Kapp M., McLellan A.D., Geijtenbeek T.B. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blois S.M., Alba Soto C.D., Tometten M., Klapp B.F., Margni R.A. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70:1018–1023. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- 5.Ban Y.L., Kong B.H., Qu X., Yang Q.F., Ma Y.Y. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin Exp Immunol. 2008;151:399–406. doi: 10.1111/j.1365-2249.2007.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner L., Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P., Adrian-Cabestre F., Menier C., Guiard V., Gourand L. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–811. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 8.Donadi E.A., Castelli E.C., Arnaiz-Villena A., Roger M., Rey D. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci CMLS. 2011;68:369–395. doi: 10.1007/s00018-010-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Villar J.J., Melero I., Navarro F., Carretero M., Bellon T. The CD94/NKG2-A inhibitory receptor complex is involved in natural killer cell-mediated recognition of cells expressing HLA-G1. J Immunol. 1997;158:5736–5743. [PubMed] [Google Scholar]
- 10.Carosella E.D., Gregori S. LeMaoult J The tolerogenic interplay(s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011;118:6499–6505. doi: 10.1182/blood-2011-07-370742. [DOI] [PubMed] [Google Scholar]
- 11.Gregori S., Tomasoni D., Pacciani V., Scirpoli M., Battaglia M. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 12.Feger U., Tolosa E., Huang Y.H., Waschbisch A., Biedermann T. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–577. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y.H., Zozulya A.L., Weidenfeller C., Metz I., Buck D. Specific central nervous system recruitment of HLA-G(+) regulatory T cells in multiple sclerosis. Ann Neurol. 2009;66:171–183. doi: 10.1002/ana.21705. [DOI] [PubMed] [Google Scholar]
- 14.Vigano P., Gaffuri B., Somigliana E., Infantino M., Vignali M. Interleukin-10 is produced by human uterine natural killer cells but does not affect their production of interferon-gamma. Mol Hum Reprod. 2001;7:971–977. doi: 10.1093/molehr/7.10.971. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lopez N., Guilbert L.J., Olson D.M. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukocyte Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 16.Manaster I., Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 17.Koopman L.A., Kopcow H.D., Rybalov B., Boyson J.E., Orange J.S. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tirado-Gonzalez I., Munoz-Fernandez R., Blanco O., Leno-Duran E., Abadia-Molina A.C. Reduced proportion of decidual DC-SIGN+ cells in human spontaneous abortion. Placenta. 2010;31:1019–1022. doi: 10.1016/j.placenta.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 19.He Y.Y., Du M.R., Guo P.F., He X.J., Zhou W.H. Regulation of C–C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- 20.Hannan N.J., Salamonsen L.A. CX3CL1 and CCL14 regulate extracellular matrix and adhesion molecules in the trophoblast: potential roles in human embryo implantation. Biol Reprod. 2008;79:58–65. doi: 10.1095/biolreprod.107.066480. [DOI] [PubMed] [Google Scholar]
- 21.Shakhawat A., Shaikly V., Elzatma E., Mavrakos E., Jabeen A. Interaction between HLA-G and monocyte/macrophages in human pregnancy. J Reprod Immunol. 2010;85:40–46. doi: 10.1016/j.jri.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Cupurdija K., Azzola D., Hainz U., Gratchev A., Heitger A. Macrophages of human first trimester decidua express markers associated to alternative activation. Am J Reprod Immunol. 2004;51:117–122. doi: 10.1046/j.8755-8920.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 23.Tagliani E., Erlebacher A. Dendritic cell function at the maternal-fetal interface. Exp Rev Clin Immunol. 2011;7:593–602. doi: 10.1586/eci.11.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki Y., Sakai M., Miyazaki S., Higuma S., Shiozaki A. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 25.Mjosberg J., Berg G., Jenmalm M.C., Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010;82:698–705. doi: 10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- 26.Somerset D.A., Zheng Y., Kilby M.D., Sansom D.M., Drayson M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregori S., Goudy K.S., Roncarolo M.G. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol. 2012;3:30. doi: 10.3389/fimmu.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andolfi G., Fousteri G., Rossetti M., Magnani C.F., Jofra T. Enforced IL-10 Expression Confers Type 1 Regulatory T Cell (Tr1) Phenotype and Function to Human CD4(+) T Cells. Mol Ther: J Am Soc Gene Ther. 2012;20(9):1778–1790. doi: 10.1038/mt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]