Abstract
Motoneurons innervating laryngeal muscles are located in the nucleus ambiguus (Amb), but there is no general agreement on the somatotopic representation and even less is known on how an injury in the recurrent laryngeal nerve (RLN) affects this pattern. This study analyzes the normal somatotopy of those motoneurons and describes its changes over time after a crush injury to the RLN. In the control group (control group 1, n = 9 rats), the posterior cricoarytenoid (PCA) and thyroarytenoid (TA) muscles were injected with cholera toxin-B. In the experimental groups the left RLN of each animal was crushed with a fine tip forceps and, after several survival periods (1, 2, 4, 8, 12 weeks; minimum six rats per time), the PCA and TA muscles were injected as described above. After each surgery, the motility of the vocal folds was evaluated. Additional control experiments were performed; the second control experiment (control group 2, n = 6 rats) was performed labeling the TA and PCA immediately prior to the section of the superior laryngeal nerve (SLN), in order to eliminate the possibility of accidental labeling of the cricothyroid (CT) muscle by spread from the injection site. The third control group (control group 3, n = 5 rats) was included to determine if there is some sprouting from the SLN into the territories of the RLN after a crush of this last nerve. One week after the crush injury of the RLN, the PCA and TA muscles were injected immediately before the section of the SLN. The results show that a single population of neurons represents each muscle with the PCA in the most rostral position followed caudalwards by the TA. One week post-RLN injury, both the somatotopy and the number of labeled motoneurons changed, where the labeled neurons were distributed randomly; in addition, an area of topographical overlap of the two populations was observed and vocal fold mobility was lost. In the rest of the survival periods, the overlapping area is larger, but the movement of the vocal folds tends to recover. After 12 weeks of survival, the disorganization within the Amb is the largest, but the number of motoneurons is similar to control, and all animals recovered the movement of the left vocal fold. Our additional controls indicate that no tracer spread to the CT muscle occurred, and that many of the labeled motoneurons from the PCA after 1 week post-RLN injury correspond to motoneurons whose axons travel in the SLN. Therefore, it seems that after RLN injury there is a collateral sprouting and collateral innervation. Although the somatotopic organization of the Amb is lost after a crush injury of the RLN and does not recover in the times studied here, the movement of the vocal folds as well as the number of neurons that supply the TA and the PCA muscles recovered within 8 weeks, indicating that the central nervous system of the rat has a great capacity of plasticity.
Keywords: larynx, nucleus ambiguus, peripheral nerve injury, somatotopy
Introduction
The nucleus ambiguus (Amb) is as a column of motoneurons within the medulla oblongata, containing special visceral efferent motoneurons innervating the muscles of the larynx, pharynx and esophagus (Ramón y Cajal, 1909; Lawn, 1966a,b; Kalia & Mesulam, 1980a,b; Hinrichsen & Ryan, 1981; Bieger & Hopkins, 1987). It includes three serially arranged subdivisions; the most rostral is the compact formation that projects to all levels of the esophagus; an intermediate subdivision, the semicompact formation, which contains motoneurons for the pharyngeal constrictors and also the cricothyroid (CT) muscle; and the caudal-most subdivision, the loose formation, which projects to the intrinsic laryngeal musculature with the exception of the CT muscle (Bieger & Hopkins, 1987).
Previous studies using retrograde tracing methods in rats (Wetzel et al. 1980; Hinrichsen & Ryan, 1981; Lobera et al. 1981; Pásaro et al. 1981; Portillo & Pásaro, 1988; Pascual-Font et al. 2011; Hernández-Morato et al. 2013) or cats (Gacek, 1975; Lobera et al. 1981; Yoshida et al. 1982; Pásaro et al. 1983; Davis & Nail, 1984) have localized the somata of motoneurons innervating laryngeal muscles within the ipsilateral Amb. These studies all agreed in locating the somata of the motoneurons innervating the CT muscle in the rostral half of the Amb, but with variable degrees of rostrocaudal overlap with the motoneurons innervating the remaining intrinsic muscles. The motoneurons innervating the posterior cricoarytenoid (PCA) muscle were reported located caudally to those of the CT muscle, and the motoneurons innervating the lateral cricoarytenoid (LCA) and thyroarytenoid (TA) were consistently reported as being located the most caudally in the Amb. Some authors report a single column of motoneurons innervating laryngeal intrinsic muscles (Szentágothai, 1943; Kalia & Mesulam, 1980a,b; Hinrichsen & Ryan, 1981; Schweizer et al. 1981; Yoshida et al. 1982; Portillo & Pásaro, 1988; Hernández-Morato et al. 2013), while other authors suggest that there are two distinct columns (Gacek, 1975; Wetzel et al. 1980; Bieger & Hopkins, 1987; Patrickson et al. 1991), but these reports are performed on different species.
During the regeneration of peripheral nerves after injury, it is often the case that specificity is not well maintained between the regenerating motoneurons and their muscle targets (Brushart & Mesulam, 1980). In the case of motor nerves, this may result in functional problems, as the uninjured synergists of the denervated muscles may still be functionally active and thus may be able to compensate for the altered actions of the reinnervated muscles. This is not the case though for the intrinsic muscles of the larynx where each of the muscles has a unique prime action that is distinct from the other muscles, and though those actions partially overlap there is no effective synergist that can compensate fully for the action of a muscle that becomes partly or totally denervated (Crumley, 1979). Instead, what is frequently observed following recurrent laryngeal nerve (RLN) injury and subsequent reinnervation of the paralyzed larynx is synkinesis, in which there is synchronous contraction of muscles that would normally not contract together, which results in an immobile vocal fold (Crumley, 1979; Rice, 1982). It has been hypothesized that laryngeal synkinesis is the consequence of misdirected axonal regeneration as the injured recurrent laryngeal motor axons regrow and the subsequently inappropriate reinnervation of abductor laryngeal muscles by fibers of adductor motoneurons and vice versa (Crumley, 1979).
Non-selective reinnervation has been reported in hypoglossal, facial, oculomotor and sciatic nerves (Mizuno et al. 1980; Thomander, 1984; Fernández et al. 1985, 1992; Aldskogius & Thomander, 1986; Scherer, 1986; English, 2005). In the case of the larynx, only two previous studies have investigated the effects of RLN injury on the organization of the different pools of motoneurons of the Amb (Nahm et al. 1990; Flint et al. 1991). The work of Nahm et al. (1990) used a freezing injury, which is comparable in effect to a crush injury, as the endoneurial tubes are not disrupted, but only described the effect of reinnervation on the PCA motoneuron pool. They showed that following axonal injury the motoneurons innervating the PCA were located along the entire length of the RLN distribution in the Amb, instead of being confined solely to the middle third. Thus, the area occupied by the motoneurons innervating the PCA expanded following reinnervation, but remained confined to the region that normally is occupied by the neurons whose axons are located within the RLN. Flint et al. (1991) showed that after complete section of the RLN and then suturing of the cut ends of the nerve, the functional organization within the Amb was altered, as the motoneurons reinnervating the PCA and TA/LCA muscles were found located in the position occupied only by TA /LCA motoneurons in control animals.
These latter two studies either studied a single muscle motoneuron pool (Nahm et al. 1990) or restricted the study to a small number of animals and time points (Flint et al. 1991). The present study was undertaken to provide a more comprehensive study of the topographic reorganization of motoneurons innervating the intrinsic muscles of the larynx following RLN injury. In the present study the contrasting effects of a crush nerve injury were studied in two muscle motoneuron pools, those of PCA (which is the intrinsic laryngeal abductor muscle) and TA (one of the adductor muscles in the larynx), and over a range of survival times, to investigate the detailed time course of recovery, both morphologically and functionally.
Materials and methods
Animal preparation
The present research was undertaken in accordance with the laws and regulations for the care and handling of animals in research, both of the European Union (2010/63/EU) and of Spain (Royal Decree 223/1998), and was approved by the Committee of Animal Experimentation of the Complutense University of Madrid. Experiments were carried out on 52 adult male Sprague–Dawley rats (Rattus norvegicus) of 250–350 g body weight. Animals were maintained in the central facilities of the Complutense University, and all the surgical procedures were performed in the animal operating room of that unit. During the first 2 days following surgery, the animals were treated with a standard analgesic protocol consisting of a dose of buprenorphine (0.05 mg kg−1) plus meloxicam (1.0 mg kg−1) administered every 8 h.
Surgical procedure: crush injury
Animals were anesthetized with an intraperitoneal injection (xylazine; Rompun, Bayer, Spain; 8 mg kg−1; plus ketamine; Imalgene, Merial, France; 90 mg kg−1) and maintained on a heated pad at 37 °C during the procedure.
A midline skin incision was made ventrally in the neck to expose the larynx. The salivary glands and infrahyoid muscles were then reflected laterally with a wire retractor in order to expose the related laryngeal, vascular and nervous structures. Once the RLN nerve was exposed and stripped from its fascial sheet, it was isolated from the surrounding tissue at a level corresponding to the transverse plane of the 7th tracheal ring, and a 1-mm-long crush injury was produced by applying a steady force for 10 s with smooth-tip forceps. This procedure was repeated twice more, in the same location, but with forceps oriented at an angle of 90 ° to the long axis of the nerve in relation to the first crush to ensure as complete a crush injury as possible. Then the surgical wound was closed in layers. Immediately after surgery and before the animal recovered consciousness the completeness of the injury was confirmed in each animal using a laryngoscope to assess the extent to which the left vocal fold had been immobilized (see below for details of the method used); if the left vocal fold displayed any movement the animal was excluded from further analysis. Following nerve injury animals were allowed to survive for periods of 1 (n = 6), 2 (n = 6), 4 (n = 6), 8 (n = 8) and 12 (n = 6) weeks (wpi, weeks post-injury).
Surgical procedure: muscle tracing
At the end of each time interval, animals were anesthetized once more as described above, and vocal fold motion was assessed by direct visualization with a laryngoscope. Then the surgical field was exposed as described previously in order to inject the tracer into the PCA and TA muscles. In half of the experiments, the first muscle to be injected was the PCA and in the remaining half it was the second muscle to be injected. Two different tracers were employed, one for each muscle: subunit B of cholera toxin (CtB) conjugated to Alexa Fluor 488 (green fluorescence) and CtB conjugated to Alexa Fluor 594 (red fluorescence; Invitrogen, CA, USA). Both muscles were injected with one or the other tracer one after the other. In each muscle a volume of 0.5 μL of 10 μg mL−1 (1%) CtB was injected, using a 1-mL Hamilton syringe 30 μm diameter.
The inferior constrictor muscle was laterally reflected and the larynx slightly rotated in order to expose the PCA muscle. To prevent spread of the tracer to the muscles of the esophagus, whose motoneurons are located close to those of the larynx, a piece of parafilm was passed beneath the larynx. The TA muscle was exposed by opening a window through the thyroid cartilage. To prevent spread of the tracer, once the tracer was injected into the muscle, the needle was left inside the muscle for a minimum of 2 min. At the end of the procedure the surgical field was cleaned with saline and the wound closed in layers. Previous experiments by our laboratory (Pascual-Font et al. 2011) have shown that tracer leakage under these circumstances appears to be minimal. Delivery of CtB tracer into the area around the larynx in an attempt to simulate leakage failed to produce any labeling of motoneurons. In order to determine the normal distribution of the PCA and TA motoneurons within the Amb, nine animals in which the laryngeal nerves had been left intact were injected with tracer in the PCA (n = 2), the TA (n = 2) or both (n = 5; control group 1, total n = 9). Two further sets of control experiments were undertaken. In the second control group (control group 2, n = 6), the PCA (n = 2), the TA (n = 2) or both muscles (n = 2) were injected immediately prior to the section of the superior laryngeal nerve (SLN), in order to eliminate the possibility of labeling of the CT muscle by spread from the injection site. In the third control group (control group 3, n = 5), 1 week after the crush injury of the RLN, the PCA and TA muscles were injected immediately before the section of the SLN, in order to determine if there was some sprouting from the SLN into the territories of the RLN following the crush injury. In control groups 2 and 3 the section of the SLN was made in the main trunk of the nerve, just before the division of the nerve in its branches.
Functional evaluation
Three laryngoscopic examinations were carried out in each anesthetized animal, one prior to the crush injury to the RLN, one immediately after the crush injury and one prior to death. The laryngoscope (Karl Storz GmbH, KG, Tuttilgen, Germany) was inserted orally until the vocal folds were visible. The laryngoscopy was video-recorded by means of a camera connected to the laryngoscope and to an endoscopic video recording system (Telecam SL NLSC 20212120; Karl Storz Endoskope). The protocol for the evaluation of the motion of the vocal folds was performed as previously described (Inagi et al. 1997). Briefly, for each videolaryngoscopy two glottal frames were selected, consisting of images of maximal adduction and maximal abduction (Fig. 1). The angles between the vocal folds were calculated by means of Image J software (National Institutes of Health, Bethesda, Maryland, USA). A functional score was assigned to the left vocal fold movements observed, with a score of 0 being assigned to a paralyzed vocal fold; 1 for a slight movement being visible; 2 for normal or near to normal movement. Work by previous authors has shown that the effects of the anesthetic used upon laryngeal fold mobility appear to be minimal (Aoyama et al. 2010; Pitman et al. 2011).
Fig. 1.
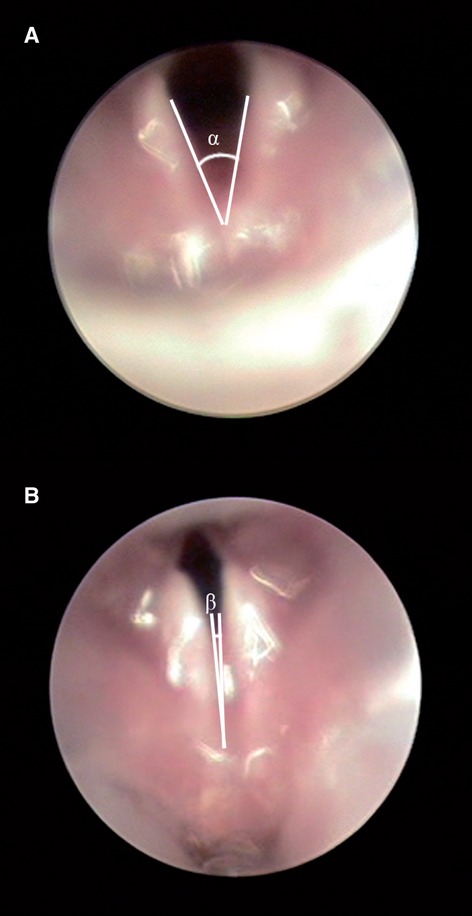
Images of the laryngeal folds in a normal rat viewed by videolaryngoscopy at maximal abduction (A) and maximal adduction (B).
Histological procedures
Three days after the application of the tracer, the rats were killed (Vercelli et al. 2000). A lethal dose of pentobarbital (200 mg kg−1) was administered and the animals were perfused through the left ventricle with saline (200 mL, 37 °C) followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4 (250 mL, 4 °C). Immediately after the perfusion, the brainstems were removed from the cranium and post-fixed for 2 h in the same fixative, rinsed in PB, and cryoprotected by immersion overnight in 15% w.v. sucrose in PB, and then in 30% w.v. sucrose in PB until they sank (2–3 days). Using a cryostat, serial 50-μm transverse sections from pyramidal decussation to the facial nucleus (about 6 mm) were obtained and collected in individual wells containing PB. The sections were incubated for 10 min with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 1 : 1000; Roche, Basel, Switzerland) for the fluorescent identification of the nuclei that are stained blue following this procedure. Brainstem sections were mounted onto poly-l-lysine-coated slides, air-dried and cover-slipped following the standard histological procedures in order to be observed and photographed with a fluorescent microscopy (Nikon Eclipse 800M).
Neuron counting, localization within the brainstem and morphometric analysis
The neurons in all the fluorescent-stained serial sections were counted by two independent observers. The total number of labeled neurons in each animal was counted and the mean number (± SE or ± SD) for each nerve group was calculated. The sections were examined at high power (40 ×) on a Nikon Eclipse 800 photomicroscope with the appropriate fluorescent filter either for Alexa Fluor 488, Alexa Fluor 594 or DAPI. The neuron counting was done as previously described (McHanwell & Biscoe, 1981a,b; Pascual-Font et al. 2011; Hernández-Morato et al. 2013).
The rostrocaudal location of the labeled neurons was measured and registered, without correction for shrinkage, with reference to specific brainstem landmarks. Landmarks were selected to be both close to the dorsal surface and the midline (the latter in order to minimize variance due to asymmetrical sectioning). The landmarks chosen were the caudal-most extent of the dorsal cochlear nucleus, the rostral limit of the area postrema (AP), and the obex – in the rat this corresponds to the caudal end of the AP (Hamilton & Norgren, 1984; Paxinos & Watson, 2005). All the positional data presented in this study are in micrometers and indicate a rostrocaudal measurement relative to the obex.
The morphometric analysis of the labeled neurons was performed on photographs of the sections taken at 40 × magnification. The area, and major and minor axis of labeled neuronal soma were measured by Image J software. All the data are expressed as mean ± SD.
Results
Control group 1
The number of labeled motoneurons in the Amb following the injection of the CtB into the PCA ranged between 20 and 56 (mean 41 ± 7), and following injection of the TA between 20 and 51 (mean 31 ± 4; Fig. 2). All the motoneurons exhibited a multipolar morphology.
Fig. 2.
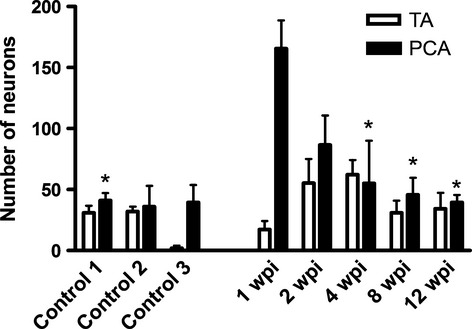
Histogram showing the mean number of labeled motoneurons present in control animals and in animals allowed to survive for different times following laryngeal nerve crush injury (wpi, weeks post-injury). Error bars: standard error of mean. *Statistically significant differences to 1 wpi (P < 0.01). PCA, posterior cricoarytenoid; TA, thyroarytenoid.
Neuronal labeling was restricted to the side of the brainstem ipsilateral to that of the injected muscle. The motoneurons innervating the PCA muscle were located rostrally to those innervating the TA muscle. The motoneuron pools innervating the PCA and the TA muscles were present in the Amb as two discrete columns. The PCA muscle motoneuron pool was located between 907 (± 48) and 1985 (± 87) μm rostral to the obex, while the TA pool was between 335 (± 67) μm caudal to the obex and 720 (± 56) μm rostral to it. Thus, there was no overlap between the two motoneuron pools rostrocaudally (Fig. 3).
Fig. 3.
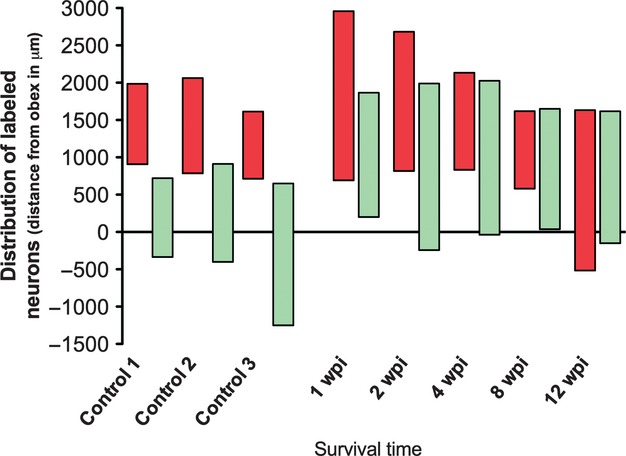
Histogram showing the rostrocaudal position of labeled motoneurons present in control animals and in experimental animals allowed to survive for different times following laryngeal nerve crush injury. Labeled motoneurons after the injection of tracer in the PCA muscle appear in red, and in green those of the TA muscle. The 0 represents the obex, the positive values represent the rostral distance from the obex, and the negative values the caudal distance from the obex (wpi, weeks post-injury).
Control group 2
The number of labeled motoneurons in the Amb following the injection of the CtB into the PCA, prior to the section of the SLN, was found to be 40 (± 9), and 35 (± 4) for the TA. The number and location of labeled neurons after the injection of tracer into the PCA and TA muscles in control group 1 was similar to that of control group 2, in which the SLN was cut immediately after the tracer injection, showing that during the injection procedure there was no spread to the CT muscle or any structure supplied by the SLN (Figs 2 and 3).
Experimental group
Functional evaluation of the vocal folds
The recovery of vocal fold movement, as assessed by laryngoscopy, increased with survival time (Fig. 4; Table 1). At 1 wpi all the animals were seen to have an ipsilateral paralysis and thus complete immobility (score 0) of the vocal folds (6/6). At 2 wpi the vocal folds of 33% of the animals were found to be paralyzed (score 0), 33% were found to have some mobility (score 1), while 33% showed normal mobility (score 2). At 4 wpi only one of the animals examined did not exhibit any recovery of vocal fold mobility (score 0), one animal exhibited restricted mobility (score 1), two animals exhibited normal movements (score 2) and the remaining two animals could not be examined due to sudden death during the laryngoscopy (Table 1). At 8 wpi the left vocal fold was immobile (score 0) only in one examined animal (8%), while in 75% of animals the vocal fold movement appeared normal (score 2; again in two animals laryngoscopic examination was not possible). At 12 wpi, 80% of animals exhibited normal vocal fold movement (score 2) and 17% (one animal) had the left vocal fold remained paralyzed (in one animal a laryngoscopic examination was not possible again due to death during the laryngoscopy; Fig. 4; Table 1).
Fig. 4.
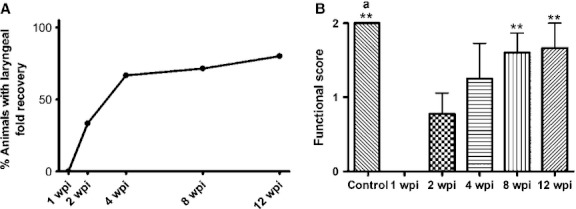
Functional evaluation. Videolaryngoscopic analysis showing the time course of functional recovery with increasing survival time after injury. (A) Percentage of animals that have recovered the movement of laryngeal folds. (B) Functional score. Means of control, 8 and 12 wpi groups are statistically different from that of 1 wpi (**). The mean of the control group is statistically different from that of 2 wpi (a). Data are presented as mean ± SEM. Statistical evaluations were based on one-way anova test (**P < 0.001; aP < 0.05). wpi, weeks post-injury.
Table 1.
Movement of the left vocal fold measured per animal by videolaryngoscopy
| Group (number of animals) | Control (6) | 1 wpi (6) | 2 wpi (6) | 4 wpi (6) | 8 wpi (8) | 12 wpi (6) | |
|---|---|---|---|---|---|---|---|
| Vocal folds motion (number of animals) | Paralyzed | 0 | 6 | 2 | 1 | 1 | 1 |
| Slight motion | 0 | 0 | 2 | 1 | 0 | 0 | |
| Normal | 6 | 0 | 2 | 2 | 5 | 4 | |
wpi, weeks post-injury.
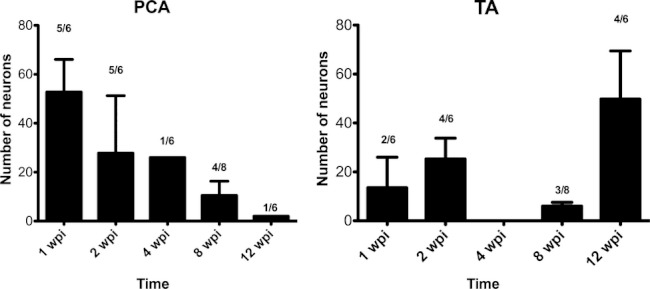
Number of motoneurons
At 1 wpi, the number of labeled neurons from the PCA notably increased (165 ± 23). From this time, the number of labeled neurons gradually decreased, until 12 wpi, when the labeled neurons reached a number comparable to that of the control (40 ± 6; Fig. 2). Differences were statistically significant between the group at 1 wpi, and the control group 1, 4, 8 and 12 wpi. Regarding the number of labeled neurons from the TA, at 1 wpi the number was markedly decreased, although this number increased with increasing survival time, reaching the maximum at 4 wpi (75 ± 3). In the next two groups (8 and 12 wpi), the number of labeled neurons from TA is similar to the control group (31 ± 10, 34 ± 13, respectively).
In control group 3, there were no labeled motoneurons from the TA in all the rats but one, in which there were six labeled motoneurons. The mean number of labeled motoneurons from PCA was 25 (± 12; Fig. 2). This may indicate that some tracing through the SLN after RLN injury may take place. All the data are shown in Table 2, in which we include also the data expressed as mean ± SD.
Table 2.
Number of labeled motoneurons after the injection of retrotracer into the PCA and TA muscles
| Group | Control 1 | Control 2 | Control 3 | 1 wpi | 2 wpi | 4 wpi | 8 wpi | 12 wpi | |
|---|---|---|---|---|---|---|---|---|---|
| No. of neurons | PCA | 41 ± 8 (± 12) | 40 ± 11 (± 20) | 25 ± 12 (± 23) | 165 ± 23 (± 56) | 87 ± 24 (± 58) | 35 ± 22 (± 38) | 46 ± 14 (± 38) | 40 ± 6 (± 14) |
| TA | 31 ± 4 (± 11) | 35 ± 5 (± 9) | 1.5 ± 1.5 (± 3) | 14 ± 7 (± 16) | 55 ± 20 (± 48) | 75 ± 3 (± 6) | 31 ± 10 (± 30) | 34 ± 13 (± 31) | |
Data are expressed as mean ± SE; in parentheses are SD values.
PCA, posterior cricoarytenoid; TA, thyroarytenoid; wpi, weeks post-injury.
Location of the neurons
Labeled motoneurons could be observed ipsilaterally to the injected muscle as early as 1 wpi following CtB injection into either the PCA muscle or the TA muscle. However, the rostrocaudal distribution of the labeled motoneurons in animals at all survival periods showed marked changes in comparison to the control group. In all the experimental groups, there was a degree of overlap between the pools of motoneurons supplying both muscles. The rostrocaudal length of the overlap between the two motoneuron pools varied in extent between 600 and 1200 μm in all experimental groups, except in the 2-week survival group, in which the rostrocaudal length of the overlap was 1830 μm (Figs 3 and 5; Table 3).
Fig. 5.
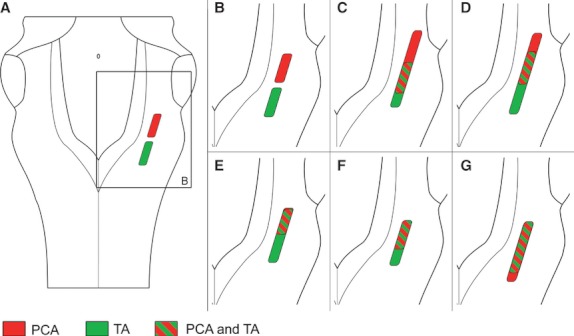
Schematic diagrams of the medulla oblongata viewed from its dorsal aspect, showing the distribution of the labeled populations of motoneurons after the injection of tracer into the thyroarytenoid muscle (TA, in green) and into the posterior cricoarytenoid muscle (PCA in red) in the control group (A, B), and 1 (C), 2 (D), 4 (E), 8 (F) and 12 (G) weeks after the RLN crush injury. Note the rostrocaudal overlap region between the two populations (oblique lines).
Table 3.
Degree of overlap between the pools of motoneurons supplying the PCA and TA muscles
| Group | Control | 1 wpi | 2 wpi | 4 wpi | 8 wpi | 12 wpi |
|---|---|---|---|---|---|---|
| Region of overlap (μm) | 0 | 1137 ± 132 | 1833 ± 116 | 675 ± 305 | 941 ± 90 | 1241 ± 175 |
The rostrocaudal length of the overlap between the two motoneuron pools varied in extent between 675 and 1830 μm in all experimental groups, except in the control group, in which there is not any degree of overlap. Data are expressed as mean ± SEM.
wpi, weeks post-injury.
At 1, 2 and 4 wpi, the motoneuron pool of the PCA muscle was expanded both caudally and rostrally when compared with the normal PCA motoneuron pool, being located not only in the loose formation but also in the semicompact formation of the NA. At 8 wpi, the location of this motoneuron pool of the PCA was comparable to that of the control group, although at 12 wpi it was found to be caudally displaced, being even more caudal than the motoneuron pool of the TA muscle (Figs 3 and 5).
The extent of the location of the motoneuron pool of the TA was greater than that of the control group in all the experimental groups, and it was extended rostrally, except at 2 wpi, when it was extended not only rostrally but also caudally (Figs 3 and 5). No double-labeled motoneurons were ever found.
Surprisingly, in all the experimental groups there were some animals in which small numbers of labeled motoneurons were found to be located in the contralateral Amb. Following injection of PCA muscle, both the number of animals with contralateral labeled motoneurons and the total number of labeled contralateral neurons declined after longer survival times; at 12 wpi contralateral labeled motoneurons were found only in one animal, where only seven motoneurons were labeled. Following the injection of tracer into the TA muscle, contralateral labeled motoneurons were observed in less than half of the animals of each experimental group, except in the animals allowed to survive until 12 wpi, in which four of the seven animals showed contralateral labeling (Fig. 6).
Fig. 6.
Number of motoneurons labeled on the contralateral side to the injection site. The numbers on the bars represent the ratio of animals that present contralateral labeled motoneurons in each group. Error bars: standard error of mean. PCA, posterior cricoarytenoid; TA, thyroarytenoid; wpi, weeks post-injury.
Morphometry of neurons
Morphometric data regarding area, major and minor axis of the somata of the labeled motoneurons are summarized in Fig. 7 and Table 4. In general, the size of the labeled motoneurons after the injection of the PCA muscle increased until 4 wpi, and after that time it decreased and reached the control size. On the other hand, the size of the labeled motoneurons after the injection of the TA muscle increased in the first week after the crush injury and in the next survival times it decreased, being smaller than control at 12 wpi. The typical morphology of the neurons is shown in Fig. 8.
Fig. 7.
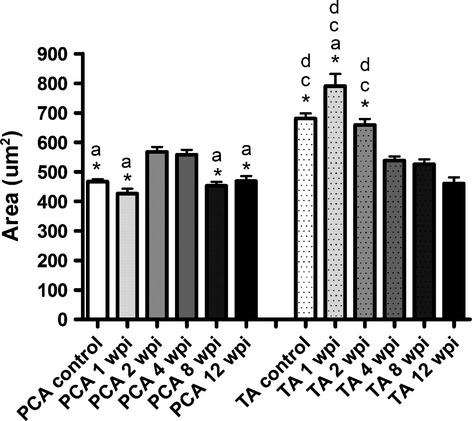
Area of the soma of the labeled motoneurons present in control animals and in animals allowed to survive for different times following laryngeal nerve crush injury after the injection of tracer in the posterior cricoarytenoid (PCA) muscle and in the thyroarytenoid (TA) muscle. Data are presented as mean ± SEM. Statistical evaluations were based on one-way anova test (P < 0.001; except differences between TA area of 1 wpi and 2 wpi groups: P < 0.05). (Statistically significant differences with: a, 2 wpi; *, 4 wpi; c, 8 wpi; d, 12 wpi). wpi, weeks post-injury.
Table 4.
Morphometric data of the soma of the motoneurons that supply the PCA and TA muscles, present in control animals and in animals allowed to survive for different times following laryngeal nerve crush injury
| Group | Area (μm) | Major axis (μm) | Minor axis (μm) | |
|---|---|---|---|---|
| PCA | Control | 466 ± 9 | 30 ± 0.4 | 29 ± 0.2 |
| 1 wpi | 426 ± 16 | 31 ± 0.8 | 18 ± 0.4 | |
| 2 wpi | 567 ± 17 | 35 ± 0.8 | 20 ± 0.4 | |
| 4 wpi | 558 ± 17 | 34 ± 0.6 | 21 ± 0.4 | |
| 8 wpi | 451 ± 14 | 32 ± 0.7 | 18 ± 0.4 | |
| 12 wpi | 468 ± 18 | 32 ± 0.8 | 19 ± 0.6 | |
| TA | Control | 681 ± 17 | 37 ± 0.6 | 23 ± 0.3 |
| 1 wpi | 790 ± 42 | 44 ± 2.6 | 24 ± 1.1 | |
| 2 wpi | 658 ± 20 | 38 ± 0.7 | 22 ± 0.5 | |
| 4 wpi | 539 ± 13 | 33 ± 0.5 | 20 ± 0.3 | |
| 8 wpi | 525 ± 17 | 32 ± 0.7 | 21 ± 0.3 | |
| 12 wpi | 460 ± 21 | 32 ± 0.9 | 18 ± 0.6 |
Data are expressed as mean ± SEM.
PCA, posterior cricoarytenoid; TA, thyroarytenoid; wpi, weeks post-injury.
Fig. 8.
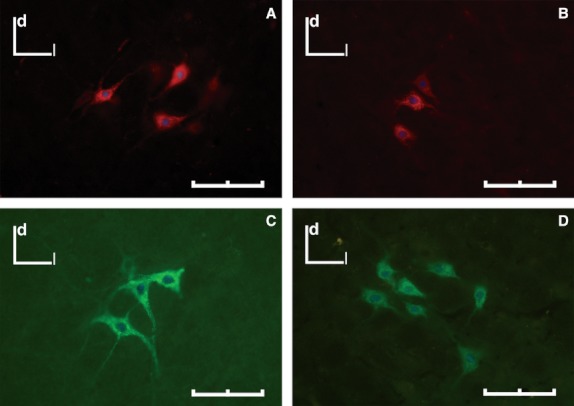
Labeled neurons within the Amb after an injection of CtB-AF594 (red) or CtB-AF488 (green) into cricoarytenoid muscle (A, B) and the TA muscle (C, D). (A, C) Control group. (B, D) At 4 wpi. d, dorsal; l, lateral. Scale bar: 100 μm.
Discussion
The somatotopic distribution of laryngeal motoneurons observed in this study is consistent with the majority of previous studies, but differs in detail from some (Gacek, 1975; Pásaro et al. 1983; Flint et al. 1991). In comparison to a previous study (Flint et al. 1991), but in agreement with others (Hinrichsen & Ryan, 1981; Portillo & Pásaro, 1988; Hirasugi et al. 2007; Hernández-Morato et al. 2013), our study shows that there is no transverse separation between abductor and adductor neurons, although there is a clear rostrocaudal arrangement of the different pools; the motoneurons supplying the TA muscle are located caudally to those supplying the PCA muscle, without any degree of overlap between them, as has previously been described (Portillo & Pásaro, 1988; Hirasugi et al. 2007; Van Daele & Cassell, 2009; Hernández-Morato et al. 2013). In the majority of cases, the motoneurons innervating the intrinsic laryngeal muscles are organized in continuous columns for each muscle (Yoshida et al. 1982; Davis & Nail, 1984; Bieger & Hopkins, 1987). The one exception being the motoneurons innervating the PCA muscle, which are arranged as two separated columns of motoneurons (Gacek, 1975; Pásaro et al. 1983). The differences between our results and those of previous studies may be explained as being partly due to the use of a different species and the employing of a more efficient retrograde tracer (horseradish peroxidase vs. CtB), as well as the use of a more effective injection technique (in our case, the spread of tracer was avoided by inserting a piece of parafilm and leaving the needle in place up to 2 min post-injection).
Our results demonstrate that following laryngeal nerve lesion there is a disorganization of the topography in the Amb. This is in agreement with earlier studies that have shown that the somatotopy of the Amb is not restored after a cryolesion or section and repair of the RLN (Nahm et al. 1990, 1993; Flint et al. 1991). Taken together, these results show that the regenerating axons of the injured RLN appear to grow back randomly in the larynx, resulting in a disorganization of the topographic organization of motoneurons of the Amb. This has been found to occur not only in the motoneurons within the Amb, but also in other motoneuron populations including the facial nucleus, following lesion of the facial nerve (Thomander, 1984; Aldskogius & Thomander, 1986), the nuclei that supply the extraocular muscles (Scherer, 1986; Sibony et al. 1986; Fernández et al. 1992), the hypoglossal nucleus after an injury in the hypoglossal nerve (Mizuno et al. 1980) and after lesion in the sciatic nerve (Fernández et al. 1992; English, 2005).
In the present study we found that the number of neurons that supplied two laryngeal muscles after a crush injury in the RLN was increased in the first weeks after injury and then gradually decreased, becoming similar to the control number. Previous studies that have determined the number of motoneurons innervating the PCA muscle at different survival times post-injury both in the guinea pig and the cat showed at 1- and 2-week survival times the number of neurons in the Amb drastically diminished, and that the numbers progressively increased returning to control values by the third month after the injury (Nahm et al. 1990, 1993), though there is considerable variability in the response. The number of motoneurons supplying the PCA and TA has been shown to return to normal values 15 weeks after a section of the RLN in the rat (Flint et al. 1991).
This study shows that at 1 week following injury the number of labeled PCA motoneurons in the Amb is much higher than the number of TA neurons. One explanation for this may be that there is collateral reinnervation of the PCA that originates from motoneuron axons that travel in the intact SLN following the RLN injury, and which then undergo intramuscular sprouting (Hydman & Mattsson, 2008). In support of this, it can also be observed that the branch that supplies the PCA is the first to exit from the RLN, which means that it is likely to be the first muscle to be reinnervated, resulting in a higher number of labeled neurons from this muscle than from the TA (Crumley & McCabe, 1982; Crumley, 2000). Results from one of the control groups support this interpretation. In these control experiments (control group 3), injection of the PCA muscle 1 week after RLN crush is followed immediately by section of the SLN. The results show that there is very little labeling of motoneurons in the brainstem. From this we conclude that the initial increased number of motoneurons labeled following PCA injection results from motoneurons whose sprouted axons each the muscle via the intact SLN.
This is further supported by the fact that at 1, 2 and 4 wpi there is a rostral extension of the PCA motoneuron pool in comparison to the control population, and this rostral region corresponds with the region occupied by the neurons whose axons travel along the SLN, as is demonstrated after application of tracer (dextran-amines) into the SLN or the RLN (Pascual-Font et al. 2011). Moreover, the results obtained in control group 3, in which the SLN was cut after injection of the tracer 1 week after the crush injury of the RLN, support this fact, as both the number and location of labeled motoneurons were similar to those of the normal animals, demonstrating that the rostral region found after the injection of PCA muscle corresponded to motoneurons whose axons travel in the SLN. This rostral location of the neurons supplying the PCA has not been described in previous studies on RLN injury, but these studies were performed in different animals other than the rat, such as cats or guinea pig (Nahm et al. 1990, 1993). Furthermore, the study made in the rat only described the position of these neurons at 15 wpi, when the connections from the SLN may have matured and disappeared, and therefore the rostral location may have been overlooked (Flint et al. 1991).
It is not clear, and it would be interesting to know, which muscle groups these motoneurons originally innervated as there can be no new production of motoneurons. In the human larynx there are extensive anastomoses between laryngeal nerves on the posterior surface of the larynx (Dilworth, 1921). While it cannot be assumed that there is exact similarity between rat and human larynx, it would seem likely that there are anastomoses between laryngeal nerves in the rat. Consequently, there are likely to be multiple routes available for the axons of motoneurons to reach their targets.
The variability in the number of neurons labeled following injection of the TA muscle, which at 1 wpi was lower than the normal control numbers and which from 2 weeks following injury was higher than those of the control, returning to normal values at 8 wpi, appears to be attributable solely to the inter-individual differences, as the differences between groups were not significant. Flint et al. (1991) have suggested that the lower number of one of the populations of laryngeal neurons in their experiments is due to selective cell death of neurons. However, Hydman et al. (2005) failed to observe death of neurons of the Amb within the first month following resection of the RLN.
The movement of the vocal folds was recovered after 8 wpi, as it has described in a previous study after RLN crush injury in which the evaluation of vocal fold mobility assessed by endoscopy yielded consistently normal findings at 6 wpi (Tessema et al. 2008, 2009). This recovery is delayed up to 16 wpi when the injury is a transection and anastomosis (Pitman et al. 2011).
After RLN injury, some contralateral motoneurons were found to supply the laryngeal muscles. This bilateral representation was not found in normal muscles. This has been described also in other models, including the oculomotor and trochlear nerves (Fernández et al. 1985, 1987, 1992), the sciatic nerve and the facial nerve (Fernández et al. 1992). Fernández et al. (1992) suggested that the muscles are reinnervated by the axons of uninjured motoneurons located in the contralateral motor nuclei, sprouting across the midline, to innervate the denervated muscles. In agreement with our results, these contralateral connections are lost at longer survival times following nerve injury (Fernández et al. 1992).
The changes of somatotopic organization are interpreted as being the result of the misdirected regrowth of axons in the post-lesion nerve stump and of collateral sprouting. The first stages of the laryngeal nerve regeneration would appear to include some collateral reinnervation and axonal sprouting, but these early connections are lost once original axons make contact and mature.
Although the somatotopic organization of the Amb is lost after a crush injury of the RLN and does not recover in the times studied here, the movement of the vocal folds as well as the number of neurons that supply the TA and PCA muscles recovered within 8 weeks, indicating that the central nervous system of the rat has a great capacity of plasticity.
Acknowledgments
The authors are grateful to Dr Marc Rodriguez-Niedenführ for his critical review of the manuscript. This work was supported by a grant from the Spanish Government (FIS07-0451 and FIS10-02721), and by funds obtained through postgraduate training courses by the UCM920547 group.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Representative diagrams illustrating the distribution of the labelled neurons in the medulla oblongata.
Fig. S2. Transversal sections of the medulla oblongata showing labelled neurons within the nucleus ambiguus after the injection of CtB-AF488 in the intrinsic laryngeal thyroarytenoid muscle 4 weeks post injury.
References
- Aldskogius H, Thomander L. Selective reinnervation of somatotopically appropriate muscles after facial nerve transaction and regeneration in the neonatal rat. Brain Res. 1986;375:126–134. doi: 10.1016/0006-8993(86)90965-0. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kumai Y, Yumoto E, et al. Effects of nerve-muscle pedicle on immobile rat vocal folds in the presence of partial innervation. Ann Otol Rhinol Laryngol. 2010;119:823–829. doi: 10.1177/000348941011901206. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Brushart T, Mesulam MM. Alternation in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980;208:603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- Crumley RL. Mechanisms of synkinesis. Laryngoscope. 1979;89:1847–1854. doi: 10.1288/00005537-197911000-00020. [DOI] [PubMed] [Google Scholar]
- Crumley R. Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol. 2000;109:365–371. doi: 10.1177/000348940010900405. [DOI] [PubMed] [Google Scholar]
- Crumley RL, McCabe BF. Regeneration of the recurrent laryngeal nerve. Otolaryngol Head Neck Surg. 1982;90:442–447. doi: 10.1177/019459988209000414. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Nail BS. On the location and size of laryngeal motoneurons in the cat and rabbit. J Comp Neurol. 1984;20:13–32. doi: 10.1002/cne.902300103. [DOI] [PubMed] [Google Scholar]
- Dilworth TF. The nerves of the human larynx. J Anat. 1921;56:48–52. [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Fernández E, Pallini R, Sbriccoli A. Changes in the central representation of the extraocular muscles in the rat oculomotor nucleus after section and repair of the third cranial nerve. Neurol Res. 1985;7:199–201. doi: 10.1080/01616412.1985.11739722. [DOI] [PubMed] [Google Scholar]
- Fernández E, Pallini R, Gangitano C, et al. Oculomotor nerve regeneration in rats. Functional, histological, and neuroanatomical studies. J Neurosurg. 1987;67:428–437. doi: 10.3171/jns.1987.67.3.0428. [DOI] [PubMed] [Google Scholar]
- Fernández E, Pallini R, Marchese E, et al. Reconstruction of peripheral nerves: the phenomenon of bilateral reinnervation of muscles originally innervated by unilateral motoneurons. J Neurosurg. 1992;30:364–369. doi: 10.1227/00006123-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Flint PW, Downs DH, Coltrera MD. Laryngeal synkinesis following reinervation in the rat. Neuroanatomic and physiologic study using retrograde fluorescent tracers and electromyography. Ann Otol Rhinol Laryngol. 1991;100:797–806. doi: 10.1177/000348949110001003. [DOI] [PubMed] [Google Scholar]
- Gacek RR. Localization of laryngeal motor neurons in the kitten. Laryngoscope. 1975;85:1841–1861. doi: 10.1288/00005537-197511000-00007. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Hernández-Morato I, Pascual-Font A, Ramírez C, et al. Somatotopy of the neurons innervating the cricothyroid, posterior criciarytenoid and thyroarytenoid muscles of the rat′s larynx. Anat Rec. 2013;296:470–479. doi: 10.1002/ar.22643. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CFL, Ryan T. Localization of laryngeal motoneurons in the rat: morphologic evidence for dual innervation? Exp Neurol. 1981;74:341–355. doi: 10.1016/0014-4886(81)90174-6. [DOI] [PubMed] [Google Scholar]
- Hirasugi K, Hisa Y, Setsu T, et al. Ambiguus motoneurons innervating laryngeal and esophageal muscles are malpositioned in the Reelin-deficent mutant rat, Shaking Rat Kawasaki. Acta Otolaryngol. 2007;127:213–220. doi: 10.1080/00016480600794479. [DOI] [PubMed] [Google Scholar]
- Hydman J, Mattsson P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve. 2008;38:1280–1289. doi: 10.1002/mus.21124. [DOI] [PubMed] [Google Scholar]
- Hydman J, Svensson M, Kuylenstierna R, et al. Neuronal survival and glial reactions alter recurrent laryngeal nerve resection in the rat. Laryngoscope. 2005;115:619–624. doi: 10.1097/01.mlg.0000161362.43320.b2. [DOI] [PubMed] [Google Scholar]
- Inagi K, Ford CN, Rodríguez AA, et al. Efficacy of repeated botulinum toxin injections as a function of timing. Ann Otol Rhinol Laryngol. 1997;106:1012–1019. doi: 10.1177/000348949710601204. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brainstem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980a;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brainstem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac and gastrointestinal branches. J Comp Neurol. 1980b;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Lawn AM. The localization, in the nucleus ambiguus of the rabbit, of the cells of origin of motor nerve fibers in the glossopharyngeal nerve and various branches of the vagus nerve by means of retrograde degeneration. J Comp Neurol. 1966a;127:293–306. doi: 10.1002/cne.901270210. [DOI] [PubMed] [Google Scholar]
- Lawn AM. The nucleus ambiguus of the rabbit. J Comp Neurol. 1966b;127:307–320. doi: 10.1002/cne.901270211. [DOI] [PubMed] [Google Scholar]
- Lobera R, Pásaro R, Gonzalez-Barón S, et al. A morphological study of ambiguus nucleus in the rat and cat. Neurosci Lett. 1981;23:125–130. doi: 10.1016/0304-3940(81)90028-8. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb of the mouse. Phil Trans R Soc Lond B. 1981a;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The sizes of motoneurons supplying the hindlimb of the mouse. Proc R Soc Lond B. 1981b;213:201–216. doi: 10.1098/rspb.1981.0062. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Uemura-Sumi M, Matsuda K, et al. Non-selective distribution of hypoglossal nerve fibres after section and resuture: a horseradish peroxidase study in the cat. Neurosci Lett. 1980;19:33–37. doi: 10.1016/0304-3940(80)90251-7. [DOI] [PubMed] [Google Scholar]
- Nahm I, Shin T, Chiba T. Regeneration of the recurrent laryngeal nerve in the guinea pig. Reorganization of motoneurons after freezing injury. Am J Otolaryngol. 1990;11:90–98. doi: 10.1016/0196-0709(90)90005-g. [DOI] [PubMed] [Google Scholar]
- Nahm I, Shin T, Watanabe H, et al. Misdirected regeneration of injured recurrent laryngeal nerve in the cat. Am J Otolaryngol. 1993;14:43–48. doi: 10.1016/0196-0709(93)90009-v. [DOI] [PubMed] [Google Scholar]
- Pásaro R, Lobera B, González-Barón S, et al. Localización de las motoneuronas de los músculos intrínsecos de la laringe de la rata. Rev Esp Fisiol. 1981;37:317–322. [PubMed] [Google Scholar]
- Pásaro R, Lobera B, González-Barón S, et al. Cytoarchitectonic organization of laryngeal motoneurons within the nucleus ambiguus of the cat. Exp Neurol. 1983;82:623–634. doi: 10.1016/0014-4886(83)90085-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Font A, Hernández-Morato I, McHanwell S, et al. The central projections of the laryngeal nerves in the rat. J Anat. 2011;219:217–228. doi: 10.1111/j.1469-7580.2011.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou JW. Motor neurons of the laryngeal nerves. Anat Rec. 1991;230:551–556. doi: 10.1002/ar.1092300415. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th edn. London: Elsevier Academic Press; 2005. [Google Scholar]
- Pitman MJ, Weissbrod P, Roark R, et al. Electromyographic and histologic evolution of the recurrent laryngeal nerve from transection and anastomosis to mature reinnervation. Laryngoscope. 2011;121:325–331. doi: 10.1002/lary.21290. [DOI] [PubMed] [Google Scholar]
- Portillo F, Pásaro R. Location of motoneurons supplying the intrinsic laryngeal muscles of rats. Horseradish peroxidase and fluorescence double-labeling study. Brain Behav Evol. 1988;32:220–225. doi: 10.1159/000116549. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du Syste‘me Nerveux de l′homme et des vertébrés. Paris: Malaine; 1909. [Google Scholar]
- Rice DH. Laryngeal reinnervation. Laryngoscope. 1982;92:1049–1059. [PubMed] [Google Scholar]
- Scherer SS. Reinnervation of the extraocular muscles in goldfish is nonselective. J Neurosci. 1986;6:764–773. doi: 10.1523/JNEUROSCI.06-03-00764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H, Ruebsamen R, Ruehle C. Localization of brain stem motoneurons innervating the laryngeal muscles in the rufous horseshoe bat. Brain Res. 1981;230:41–50. doi: 10.1016/0006-8993(81)90390-5. [DOI] [PubMed] [Google Scholar]
- Sibony PA, Evinger C, Lessell S. Retrograde horseradish peroxidase transport after oculomotor nerve injury. Invest Ophthalmol Vis Sci. 1986;27:975–980. [PubMed] [Google Scholar]
- Szentágothai J. Die lokalisation der kehlkopfmuskulatur in deu vaguskernen. Zeit Anat Entwicklun. 1943;112:704–710. [Google Scholar]
- Tessema B, Pitman MJ, Roark RM, et al. Evaluation of functional recovery of recurrent laryngeal nerve using transoral laryngeal bipolar electromyography: a rat model. Ann Otol Rhinol Laryngol. 2008;117:604–608. doi: 10.1177/000348940811700810. [DOI] [PubMed] [Google Scholar]
- Tessema B, Roark RM, Pitman MJ, et al. Observations of recurrent laryngeal nerve injury and recovery using a rat model. Laryngoscope. 2009;119:1644–1651. doi: 10.1002/lary.20293. [DOI] [PubMed] [Google Scholar]
- Thomander L. Reorganization of the facial motor nucleus after peripheral nerve regeneration. An HRP study in the rat. Acta Otolaryngol. 1984;97:619–626. doi: 10.3109/00016488409132939. [DOI] [PubMed] [Google Scholar]
- Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience. 2009;162:501–524. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, et al. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Kelley DB, Campbell BA. Central control of ultrasonic vocalizations in neonatal rats: I. Brain stem motor nuclei. J Comp Physiol Psychol. 1980;94:596–605. doi: 10.1037/h0077699. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Miyazaki T, Hirano M, et al. Arrangement of motoneurons innervating the intrinsic laryngeal muscles of cats as demonstrated by horseradish peroxidase. Acta Otolaryngol. 1982;94:329–334. doi: 10.3109/00016488209128920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


