Abstract
The peptides orexin A (OXA) and orexin B, deriving from the cleavage of the precursor molecule prepro-orexin, bind two G-coupled transmembrane receptors, named as receptor 1 (OX1R) and receptor 2 for orexin, showing different affinity-binding properties. First discovered in the rat hypothalamus, orexins and their receptors have been also found in many peripheral tissues where they exert neuroendocrine, autocrine and paracrine functions. Because inconclusive data on their localization in the mammalian prostate are reported, the aim of this study was to investigate the presence of prepro-orexin, OXA and OX1R in the human normal and hyperplastic gland. Immunohistochemistry revealed the localization of both OXA and OX1R in the cytoplasm of the follicular exocrine epithelium of all tested normal and hyperplastic prostates. Positive immunostaining was mainly observed in the basal cells of the stratified epithelium, and only rarely in the apical cells. The expression of mRNAs coding for prepro-orexin and OX1R and of proteins in the tissues was also ascertained by polymerase chain reaction and Western blotting analysis, respectively. In order to gain insights into the functional activity of OXA in the prostate, we administered different concentrations of OXA to cultured prostatic epithelial cells PNT1A. We first demonstrated that PNT1A cells express OX1R. The addition of OXA did not affect PNT1A cell proliferation, while it enhanced cAMP synthesis and Ca2+ release from intracellular storage. Overall, our results definitely demonstrate the expression of OXA and OX1R in the human prostate, and suggest an active role for them in the metabolism of the gland.
Keywords: human prostate, immunohistochemistry, in vitro cultured prostatic cells, orexin A, receptor 1 for orexins
Introduction
Two independent groups of researchers (de Lecea et al. 1998; Sakurai et al. 1998) simultaneously discovered in the rat hypothalamus two novel peptides, named orexin A (OXA) and B (OXB). These peptides, deriving from the proteolytic cleavage of a common precursor, prepro-orexin, link two G-coupled membrane receptors, receptor 1 (OX1R) and 2 (OX2R) for orexins: OX1R is highly selective for OXA, while OX2R binds both peptides with similar affinity.
Orexins spread out from the original hypothalamic nuclei towards many cerebral areas, thus regulating multiple body functions, such as food assumption, sleep/wake cycle, blood pressure and heart rate, sexual behaviour and arousal, plasma corticosterone levels and adrenal/gonadal functions (Taylor & Samson, 2003; Martyńska et al. 2006; Spinazzi et al. 2006). A critical role for orexins and their cognate receptors in the pathogenesis of narcolepsy in humans and mice has been also established (Cao & Guillerminault, 2011).
More recently, the presence of orexins and their receptors in the gastrointestinal and genital tract of mammals has been reported (Jöhren et al. 2001; Ehrström et al. 2005; Russo et al. 2008; Pavone et al. 2009; Tafuri et al. 2009, 2010). In the male genital tract, the principal cells of rat epididymis (Pavone et al. 2009) and the Sertoli cells of rat testes (Barreiro et al. 2005; Tafuri et al. 2010) have been shown to provide a local source of OXA. Expression of OX1R mRNA in the seminal vesicles, penis and epididymis of humans (Karteris et al. 2004), in the testes of sheep (Zhang et al. 2005), chicken (Ohkubo et al. 2003) and rat (Jöhren et al. 2001; Barreiro et al. 2004; Assisi et al. 2012), and in the rat epididymis (Tafuri et al. 2009) has been demonstrated. By contrast, contradictory results on the localization of the peptide and its receptor 1 in the prostate have been reported. Russo et al. (2008) described the presence of OXA in neuroendocrine (NE) cells of the urethra, and in exocrine cells of cattle prostate. The expression of genes coding for prepro-orexin and OX1R in the urethro-prostatic complex of cattle was also demonstrated (Russo et al. 2008). Conversely, Nakabayashi et al. (2003) failed to demonstrate the presence of OXA in the human prostate, whereas the presence of OX2R, but not of prepro-orexin and OX1R, was found in normal and hyperplastic prostates of men (Malendowicz et al. 2011).
Here, we investigated the localization of OXA and OX1R in human normal and hyperplastic prostates by an immunohistochemical technique, and the expression of prepro-orexin and OX1R by reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting analyses. Furthermore, in order to gain insights into the role of OXA in the prostate, we evaluated the activity of the peptide on cell proliferation, cAMP synthesis and Ca2+ release in cultured human prostatic epithelial cells, PNT1A. These cells have been proved to be a good model for the analysis of cellular processes and can be considered as non-tumorigenic cells showing molecular and biochemical properties close to the normal prostate epithelium (Mitchell et al. 2000).
Materials and methods
Antibodies and chemicals
Goat polyclonal anti-OXA (sc-8070) and anti-OX1R antibodies (sc-8073), their respective blocking peptides (sc-8070 P, sc-8073 P), and mouse monoclonal anti-chromogranin A (Chr A) antibody (sc-47714) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit polyclonal anti-prepro-orexin antibody (AB3096), its blocking peptide (AG774) and monoclonal anti-tubulin antibody (MAB1637) from Chemicon International (Temecula, CA, USA); biotinylated rabbit anti-goat secondary antibodies and avidin–biotin complex (PK-6105) from Vector Laboratories (Burlingame, CA, USA); horseradish peroxidase-conjugated anti-goat IgG (A-5420) and anti-rabbit IgG (A-0545) from Sigma Chemical (St Louis, MO, USA). Triazol (15596-026) was purchased from Invitrogen (Carlsbad, CA, USA); the enhanced chemiluminescence (ECL) kit (RPN2209), the GFX PCR DNA and Gel Purification Kit (27-9602-01) from Amersham (Little Chalfont, UK); the DC protein assay kit (500-0111) from Bio-Rad Laboratories (Hercules, CA, USA); the primers for human prepro-orexin, OX1R and β-actin from Primm (Milan, Italy); the kit for PCR and RT-PCR (A1280) from Promega (Madison, WI, USA); and ethylenediamine tetra-acetic-acid (EDTA; 60-00-4) from Sigma Aldrich. The peptide OXA (003-30) was provided by Phoenix Pharmaceuticals (Burlingame, CA, USA); fluorescent probe QF8 (21095) by ABD Bioquest (Sunnyvale, CA, USA); cAMP ELISA kit (900-066) by Enzo Life Sciences (Lausen, Switzerland); the 3-(4,5-dimethylthiazol-2-yl)-2,5-difeniltetrazolium bromide (MTT) assay kit (10009365) by Cayman Chemicals (Ann Arbor, MI, USA).
Prostatic tissue collection
Tissue samples of normal and hyperplastic prostates were obtained from patients who were hospitalized at the Urological Department of the Regional Hospital ‘A. Cardarelli’ (Naples), and underwent total cystectomy for bladder carcinoma (n = 5; 55–65 years old) or prostatectomy for hyperplasia of the gland (n = 7; 52–76 years old), respectively. The procedure was carried out according to the rules established by the Ethical Committee of University of Naples Federico II. Samples were fixed in Bouin's fluid for 24–48 h, washed in water, dehydrated in a series of ascending alcohols, and embedded in Paraplast. Microtome sections (5–7 μm) were stained by the avidin–biotin immunohistochemical method. Other tissue samples were frozen in liquid nitrogen soon after collecting, and stored at −80 °C until being used to perform RT-PCR and Western blotting analyses.
Immunohistochemistry
Paraplast sections were deparaffinized by xylene, hydrated in descending alcohols and incubated in a 3% hydrogen peroxidase solution for 30 min to inhibit endogenous peroxidase. After three washes in phosphate-buffered saline (PBS), pH 7.2, the sections were covered with normal rabbit serum for 30 min in order to avoid aspecific tissue binding of the secondary antibody. Then, they were incubated with anti-OXA or anti-OX1R antibody overnight at 4 °C. Sometimes, serially cut sections were alternately incubated with the antibodies directed against OXA or OX1R and an antibody directed against the protein Chr A, a marker protein of NE cells, in order to distinguish the exocrine cells of the prostate from the endocrine components of the gland. These latter have been termed NE cells or paraneurons, and Chr A was considered their own marker (Pearse, 1977; Fujita, 1980). The day after, the sections were washed three times in PBS and incubated with biotinylated rabbit anti-goat secondary antibody and with freshly prepared ABC reagent, in two consecutive steps of 30 min each at room temperature. The site of the specific immunological reaction was visualized by 3-3'diaminobenzidine as final staining. The preparations were observed by a Nikon Eclipse E 600 light microscope, and microphotographs were taken using a 100 × objective lens and a Nikon Coolpix 8400 digital camera.
Negative controls were performed pre-absorbing the primary antibody with an excess of the relative antigen (100 μg mL−1), and incubating the complex on sections in the specific step. They were always negative.
Cell culture
Human prostate epithelial cells PNT1A were purchased from ATCC 180 (Rockville, MD, USA). PNT1A cells were grown in Dulbecco's modified Eagle's medium (DMEM; ECB750IL, Euroclone, Milan, Italy) containing heat-inactivated 10% fetal bovine serum, 1% l-glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin.
Preparation of tissue homogenates and cell lysates
Normal and hyperplastic prostate samples were homogenized by an Ultraturrax L-407 at 4 °C with 5 mL 1.5 g−1 tissue of buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 10 mm NaF, 0.5 g 100 mL−1 deoxycholic acid, 0.1 g 100 mL−1 sodium dodecyl sulphate (SDS), 1% (v/v) Nonidet P-40, 1 mm phenylmethyl-sulphonyl fluoride (PMSF), 10 μg mL−1 aprotinin, 10 μg mL−1 leupeptin and 1 mm Na3VO4. Homogenates were centrifuged at 15 000 g for 10 min at 4 °C. Total protein amounts of samples were determined by the Bio-Rad DC protein assay.
Confluent PNT1A cells were washed in PBS, and lysed with 0.5 mL RIPA buffer containing 50 mm Tris–HCl (pH 7.4), 150 mm NaCl, 1% (v/v) Nonidet P40, 1 mm EDTA, 0.25 g 100 mL−1 sodium deoxycholate, 10 mm NaF, 10 μm Na3VO4, 1 mm PMSF and a protease inhibitor cocktail (10 μg mL−1 aprotinin, 10 μg mL−1 pepstatin, 10 μg mL−1 leupeptin). Cell lysates were incubated at 4 °C for 30 min, and centrifuged at 16 000 g for 15 min at 4 °C. The total amount of proteins in the samples was determined by the Bio-Rad DC protein assay.
RNA extraction and RT-PCR analysis
Total RNA was extracted from normal and hyperplastic prostate samples by using Triazol solution. The RNA was re-suspended in 50 μL diethyl pyrocarbonate-treated water, and stored at −80 °C until used. Synthesis of cDNAs for the detection of human prepro-orexin and OX1R was performed by using the Promega RT system. The following specific primers were used: forward 5′-CACAATTGACAGCCTCAAGG-3′ and reverse 5′-AATGGAGACTCGTCTTTATT-3′ for prepro-orexin; forward 5′-TATGTGGCTGTGTTCGTCGT-3′ and reverse 5′-CCCAGCGTTCATCACAGAC-3′ for OX1R. These primers were designed in such a way that the forward and the reverse primers span different exons, so that the amplification product obtained from the cDNA would be of a different length from that obtained from any contaminant genomic DNA comprising intronic sequences. Furthermore, to definitely rule out the possibility of amplifying genomic DNA, one PCR was carried out prior to RT of the RNA. As internal control for RT and reaction efficiency, amplification of beta actin mRNA was carried out in parallel in each sample, using the primer pair: forward 5′-TCACCCTGAAGTACCCCATC-3′ and reverse 5′-GGCTGGAAGAGTGCCTCA-3′. As a negative control for all reactions, distilled water was used in place of cDNA, whereas a positive control was performed using whole rat brain homogenates. The PCR products were separated on a 2% agarose gel, and visualized by ethidium bromide using a 1-kb DNA ladder to estimate the band sizes. The bands were cut off from the gel, purified using Amersham GFX PCR DNA and gel purification kits, and sequenced by Primm (GenBank accession no.: NP-001515 for human prepro-orexin, NP-001516.2 for human OX1R and HQ154074 for human beta actin).
Western blotting
Samples containing equal amount of proteins (100 μg) were boiled for 5 min in SDS buffer [50 mm Tris-HCl (pH 6.8), 2 g 100 mL−1 SDS, 10% (v/v) glycerol, 0.1 g 100 mL−1 bromophenol blue and 5% (v/v) beta mercaptoethanol], run on a 12.5% SDS/polyacrylamide gel, and transferred to nitrocellulose using a Mini trans-blot apparatus (Bio-Rad Laboratories). Membranes were blocked for 1 h at room temperature with TBS-T buffer [150 mm NaCl, 20 mm Tris HCl (pH 7.4), 0.1% Tween 20] containing 5 g 100 mL−1 milk. The blots were incubated overnight with polyclonal antibody directed against prepro-orexin or OX1R diluted 1 : 1000 in TBS-T containing 2.5 g 100 mL−1 milk. Then, the membranes were washed three times with TBS-T, and incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit and anti-goat IgG diluted 1 : 3000 in TBS-T containing 2.5 g 100 mL−1 milk. The proteins were visualized by ECL. To ensure specificity, pre-absorption of prepro-orexin or OX1R antibody with their relative control peptide was performed before blotting and, as positive control whole rat brain homogenates were used. To monitor loading of gel lanes, the blots were stripped and re-probed using anti-γ-tubulin monoclonal antibody.
Cell proliferation assay
Cells at 80–90% of confluence were harvested using a trypsin-EDTA solution, and seeded at low concentration into 96-well plates. After 24 h, the culture medium was removed and replaced with test medium. The cells were then incubated with OXA diluted in the same medium at different concentrations in the range 10−8–10−6 m. Control cells were treated with the medium alone without the addition of OXA. Cell proliferation was evaluated by measuring the activity of cellular enzymes that reduce the tetrazolium dye MTT to its insoluble form formazan, using a MTT assay kit, following the manufacturer's instructions. After 24 h of incubation time, the absorbance at 570 nm was measured by a Cary 50 spectrophotometer (Agilent Technologies, Milan, Italy).
cAMP quantification
Confluent PNT1A cells were seeded into 24-well plates at a density of 3 × 105 in DMEM medium, and intracellular release of cAMP was determined using a cAMP Elisa kit following the manufacturer's instructions. Briefly, PNT1A cells were incubated with 10−8, 10−7 and 10−6 m OXA for 30 min, 1 and 2 h time intervals. The incubation was stopped by adding 0.1 m HCl. Cell lysates were centrifuged at 600 g at room temperature, and surpernatant was utilized to evaluate cAMP release.
Intracellular Ca2+ measurement
Confluent PNT1A cells were seeded into transparent bottom 96-well plates at a concentration of 3 × 104 cells/well in phenol red and Ca2+-free medium for 1 h in the dark. The medium was previously added with the fluorescent probe QF8 (Kd = 232 nm) diluted in PBS containing 0.02% pluronic F-127 acid and 0.2 mm probenecid. Then, the cells were washed twice in PBS, and immersed in Ca2+-free medium containing OXA at concentrations of 10−8, 10−7 and 10−6 m. The plates were immediately read at 514 nm by a microplate reader Synergy 2 (Biotek, Bad Friedrichshall, Germany), and the relative data were acquired by Gen5 software (Biotek). Calibration of the fluorescence experiments was performed in vitro according to the method of Grynkiewicz et al. (1985) in which the intracellular calcium concentration is determined by the following equation:
where Kd is the apparent dissociation constant of the probe for Ca2+, F is the fluorescence of the sample with Ca2+ probe, Fmin is the fluorescence of the sample with no Ca2+ bound, and Fmax is the fluorescence of the sample saturated with Ca2+.
Statistical analysis
All experiments were independently performed at least three times, and the data were expressed as means ± SEM. The overall statistical significance was evaluated by the Kruskal–Wallis test. The two-tailed Mann–Whitney test was used to evaluate treated groups compared with the appropriate control group.
Results
OXA and OX1R immunohistochemical detection
OXA- and OX1R-immunoreactivity was found in the cytoplasm of the follicular exocrine epithelium of all normal (Fig. 1) and hyperplastic (Fig. 2) prostates. Immunoreactivity showed a focal distribution in the glands: it was clearly observed in cells scattered in small areas of the parenchyma, whereas large zones of the epithelium were unreactive. The extension of positive areas varied both in normal and hyperplastic prostates. The immunoreactive structures appeared as small granules (Fig. 1A) scattered in the cytoplasm. Such granules gradually clustered in the apical or basal portions of the cells, and sometimes filled these portions giving rise to pictures of intense positivity (Figs 1A, C and D, and 2A–D). In the areas of wide diffusion, deeply stained cells often were lined in a row along the basal membrane of the follicle (Fig. 2A, B and D). Both OXA and OX1R staining was mainly localized in the basal cells of the stratified epithelium, and only rarely in the apical cells (Figs 1B–D and 2D).
Fig. 1.
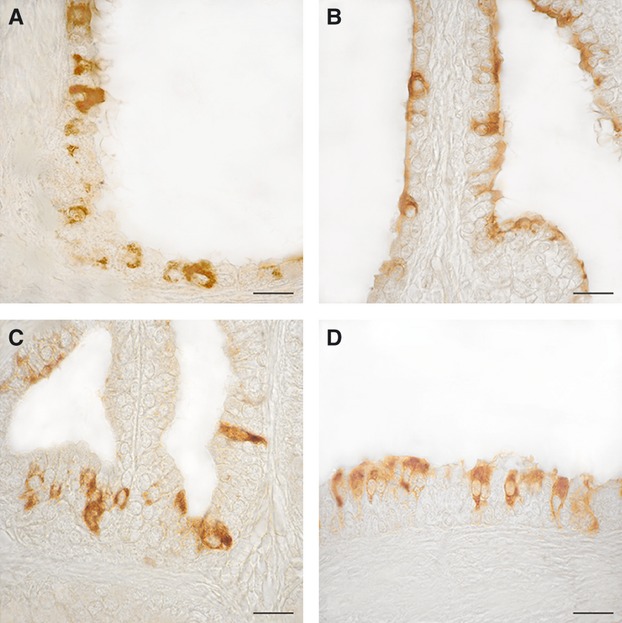
OXA- (A and B) and OX1R- (C and D) immunoreactivity in the human normal prostate. (A) Positive cells scattered along the monolayered epithelium of a prostatic follicle. (B) The majority of positive cells visible in this micrograph contain differently stained granular material in the apical portion of their cytoplasm. (C and D) Positive cells lined along the stratified epithelium of three follicles. Some of them are elongated in shape and completely filled with clusters of immunoreactive granules. Avidin–biotin immunohistochemical method. Scale bars: 20 μm.
Fig. 2.
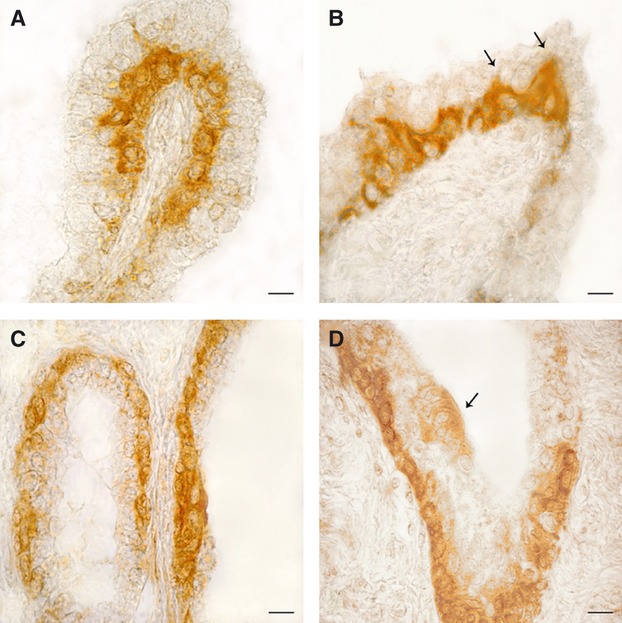
OXA- (A and B) and OX1R- (C and D) immunoreactivity in the human hyperplastic prostate. (A) The basal membrane of an intrafollicular, papillar-like structure is lined by a continuous row of intensely stained cells. (B) A peculiarity often shown by positive basal cells is the presence of a slender cytoplasmic extension (arrows) directed towards the follicular lumen and intermingled between the negative apical cells. (C) Almost all basal cells of the follicular epithelium contain OX1R-immunoreactive material, which completely fills their cytoplasm. (D) Invagination of a prostatic follicle completely lined by positive basal cells. The arrow points to a small cluster of low intensely stained apical cells close in contact with the follicular fluid. Avidin–biotin immunohistochemical method. Scale bars: 20 μm.
Although a quantitative analysis of immunoreactive material was not performed, the careful observation of a huge amount of histological sections always showed a more abundant localization of both peptides OXA and OX1R in the hyperplastic prostates rather than in the normal ones.
The staining of serially cut sections alternating anti-OXA or anti-OX1R antibody and anti-Chr A antibody demonstrated that OXA and its specific receptor 1 were never contained in NE cells (data not shown).
Expression of prepro-orexin and OX1R mRNA and proteins
The expression of mRNAs coding for prepro-orexin and OX1R in normal and hyperplastic prostates was analysed by RT-PCR. This analysis resulted in the amplification of specific DNA fragments of 576 bp for prepro-orexin and 463 bp for OX1R both in normal (Fig. 3A, lns. 3) and hyperplastic tissues (Fig. 3A, lns. 4). A 469-bp transcript was obtained from the amplification of β-actin cDNA in all tested samples (Fig. 3A, lns. 2–4, bottom). No amplification products were obtained when distilled water was used in place of cDNA (negative control; Fig. 3A, lns. 5), whereas expression of DNA fragments for prepro-orexin and OX1R was detected in whole rat brain homogenate that was used as positive control (Fig. 3A, lns. 2).
Fig. 3.

Expression of prepro-orexin and OX1R mRNAs and the proteins in human normal and hyperplastic prostates (A and B) and expression of OX1R in PNT1A cultured cells (C). (A) RT-PCR analysis. Lane 1, DNA ladder; lane 2, prepro-orexin and OX1R mRNA transcripts from whole rat brain (positive control); lanes 3 and 4, prepro-orexin and OX1R mRNA transcripts from normal and hyperplastic prostate samples, respectively; lane 5, negative control (no cDNA input). The bottom of (A) reports the beta-actin mRNA transcripts (internal control). (B) Western blotting analysis. Lane 1, homogenates from whole rat brain (positive control); lanes 2 and 3, homogenates from normal and hyperplastic prostate tissue, respectively; lane 4, prostate homogenates treated with the antisera directed against prepro-orexin or OX1R pre-absorbed with their respective control peptides (negative control). (C) Western blotting analysis. Lane 1, homogenates from whole rat brain (positive control); lane 2, homogenates from PNT1A cell lysate; lane 3, cell lysates treated with the antiserum directed against OX1R pre-absorbed with its control peptide (negative control). The upper blots of (B) and (C) were stripped and re-probed with an anti-tubulin monoclonal antibody to ensure equal loading of proteins in all lanes (lower blots). Molecular mass markers are indicated on the left of the Western blotting panels. Similar results were obtained from four separate experiments of identical design.
The presence of prepro-orexin and OX1R proteins in normal and hyperplastic prostates was confirmed by Western blotting, using rabbit and goat polyclonal antibodies raised against a 17-amino acid peptide mapping near the C-terminus of mouse prepro-orexin or against a peptide mapping near the C-terminus of OX1R of rat origin, respectively. The detected prepro-orexin and OX1R proteins showed a molecular mass of 16 kDa and 50 kDa, respectively, both in normal (Fig. 3B, lns. 2) and hyperplastic tissues (Fig. 3B, lns. 3). The specificity of the response was confirmed by pre-incubation of the prepro-orexin or OX1R antibody with their respective blocking peptide. There was no expression of prepro-orexin and OX1R in these preparations (Fig. 3B, lns. 4), whereas the presence of the proteins was detected in the whole rat brain homogenate (Fig. 3A, lns 1). The stripping of the upper blots and their re-probing with a monoclonal anti-tubulin antibody demonstrated equal loading of proteins in all lanes (Fig. 3B, bottom).
OX1R detection in PNT1A cultured cells
The expression of OX1R was also investigated in the human prostate cell line PNT1A. Samples collected from cell cultures at about 90% of confluence were subjected to SDS–polyacrylamide gel electrophoresis and Western blotting. The profile obtained showed a band corresponding to a protein with molecular mass of 50 kDa corresponding to the molecular mass of OX1R (Fig. 3C, ln. 2). The specificity of the response was confirmed by pre-incubation of the OX1R antibody with its respective blocking peptide. There was no expression of OX1R in this preparation (Fig. 3C, ln. 3), whereas the presence of the protein was detected in the whole rat brain homogenate (Fig. 3C, ln 1). The stripping of the upper blot and its re-probing with a monoclonal anti-γ-tubulin antibody demonstrated equal loading of proteins in all lanes (Fig. 3C, lns. 1–3, bottom).
OXA activity on cell proliferation, cAMP production and Ca2+ release
The results of MTT proliferation assay showed that OXA, at the doses of 10−8, 10−7 and 10−6 m, was unable to exert any effect on the proliferation of PNT1A cultured cells after 24 h of peptide administration (Fig. 4A).
Fig. 4.
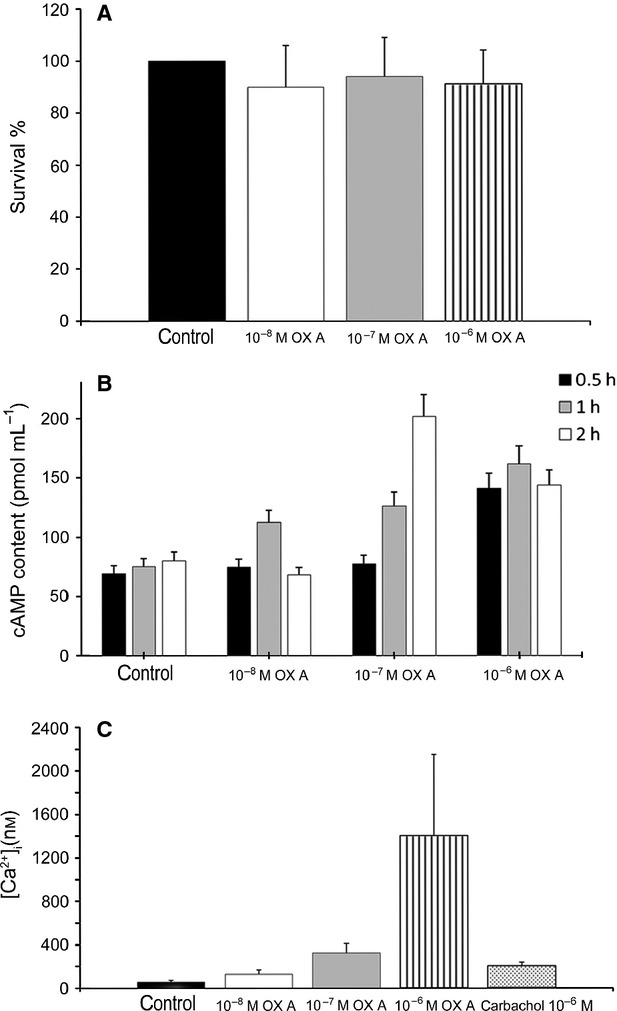
Effects of different OXA concentrations on PNT1A cells. (A) Results from MTT-based cell proliferation assay. The data, expressed as mean ± SEM of four independent experiments, are reported as the survival percentage as compared with control cells, after 24 h of OXA administration. (B) cAMP production induced by OXA at different time intervals. Data are expressed as mean ± SEM of three independent experiments: 0.5 h 10−6 m compared with control, P < 0.05; 1 h each dose compared with control, P < 0.05, 2 h 10−7, 10−6 m compared with control, P < 0.05. (C) Intracellular Ca2+ release induced by different OXA doses. Control was calcium-free medium exclusively, other samples were calcium-free. Carbachol 10−6 m was used as positive control. Data are expressed as mean ± SEM of three independent experiments. All experimental groups have a value of P < 0.05 compared with control.
In order to investigate OXA activity on metabolic pathways of cells, cAMP content of lysates from cells treated with different OXA concentrations at three exposure times was measured. Our results demonstrated that OXA promotes cAMP release (Fig. 4B). The earliest effect was achieved by 30 min of cell exposure to 10−6 m OXA, the lowest one was registered after 1 h of cell treatment with 10−8 m OXA, while the strongest effect was observed after 2 h of cell exposure to10−7 m OXA.
The effect of OXA on intracellular Ca2+ release was tested exposing PNT1A cells, previously loaded with the Ca2+ fluorescent probe QF8, to different concentrations of OXA. Our results demonstrated that the highest concentration of the peptide (10−6 m) causes an intracellular Ca2+ increase 26-fold higher than control, and sevenfold higher than that caused by the same concentration of carbachol, which is a well-known Ca2+ release inducer. The peptide showed a stimulatory effect on intracellular Ca2+ release also at the other tested concentrations (10−7 and10−8 m; Fig. 4C).
Discussion
We recently reported the expression of both OXA and OX1R in the exocrine epithelium of the prostate in cattle (Russo et al. 2008). In the present study, our immunohistochemical and biochemical analyses definitely demonstrated the localization of OXA and its highly selective receptor 1 in the human normal and hyperplastic prostate. The receptor OX1R is also expressed by the PNT1A cells, which resemble human normal epithelial prostatic cells.
Previous studies failed to detect OXA and OX1R in human prostate tissues (Nakabayashi et al. 2003; Malendowicz et al. 2011). The discrepancy between our results and those of the other research groups is not surprising, as conflicting results also exist on the localization of orexins and their receptors in some peripheral organs, the best studied of which is the human adrenal gland. While Karteris et al. (2001) detected only OX2R in the adrenal cortex, Mazzocchi et al. (2001) found OX1R and OX2R mRNAs expression in the zona fasciculata, zona reticularis and medulla, and OX1R mRNA alone in the zona glomerulosa. Later, Blanco et al. (2002) demonstrated the presence of OX1R and OX2R in the cortex and medulla, respectively. More recently, the presence of both receptors was shown in in vitro cultured cells from the two portions of the adrenal gland (Spinazzi et al. 2005a; Ziolkowska et al. 2005). The controversial results might be ascribed to the high turnover of cellular production and/or internalization of orexins and their receptors, as it occurs for other NE substances in many organs of the body. Moreover, the distribution of OXA and OX1R in the human and cattle (Russo et al. 2008) prostate is clustered in small areas of the gland separated by large zones of absence, so that a random sampling of tissue fragments can give rise to contrasting results.
The effect of orexins on proliferation of different types of in vitro cultured non-neoplastic cells is still debated. Both the orexins showed an inhibitory effect on the growth of calvarial osteoblast-like cells (Ziolkowska et al. 2008). Furthermore, while OXA stimulated the growth of preadipocytic 3T3 L1 cells, OXB showed an opposite effect in the same cell system (Zwirska-Korczala et al. 2007). The peptide OXB decreased the proliferation of hippocampal dentate gyrus neurons (Ito et al. 2008). Interestingly, OXA stimulated adrenocortical cell proliferation acting through OX1R, while it decreased cell growth when linked to OX2R (Spinazzi et al. 2005a). In our experimental conditions, OXA had no effect on epithelial prostatic PNT1A cell proliferation. Conversely, the peptide was able to stimulate cAMP synthesis in PNT1A cells. This finding well correlates with OXA-stimulating activity of adenyl cyclase (AC)/phosphokinase A pathway in human and rat adrenocortical cells via OX1R (Spinazzi et al. 2005b), and AC signalling in Chinese hamster ovary (CHO) cells (Holmqvist et al. 2005). Furthermore, we found that PNT1A cell exposure to OXA promotes a huge amount of Ca2+ release from cytoplasmic storage. These results are consistent with previously reported observations that OXA promotes stimulation of Gq/11 excitatory proteins bound to both orexin receptors, activation of phospholipase C and formation of inositoltriphosphate in CHO cells (Smart et al. 1999; Kane et al. 2000; Lund et al. 2000; Holmqvist et al. 2001; Ammoun et al. 2003; Larsson et al. 2005). Thus, OXA induces signals of metabolic activation in our in vitro cell system, although further studies are needed to elucidate the precise function(s) played by OXA in the prostatic exocrine epithelial cells.
The relationship between the orexinergic system and the human prostatic hyperplasia is far from being elucidated. An upregulation of OX2R synthesis, and a decrease of OXA blood levels in hyperplastic patients, led us to hypothesize a role of OX2R in the disease pathogenesis (Malendowicz et al. 2011). Our results also suggest an involvement of OX1R in the pathogenesis of human prostatic hyperplasia. Furthermore, our immunohistochemical data show a prevalent localization of OXA and OX1R in the basal portion of the follicular epithelium both in normal and hyperplastic glands. The basal cells of the prostate are involved in the renewal of the luminal epithelium (El-Alfy et al. 2000) and in the onset of carcinomas (Goldstein et al. 2010; Lawson et al. 2010). Thus, overall our findings strongly suggest that OXA plays a role both in normal physiology and proliferative diseases of the gland.
Conclusions
In conclusion, this research describes the presence of prepro-orexin, OXA and OX1R in normal and hyperplastic prostates of men, and demonstrates that OXA stimulates metabolic pathways of in vitro cultured prostatic cells. Such results provide a starting point for further studies aimed to clarify the role exerted by OXA and its receptor 1 in the exocrine epithelium of the gland, both in health and disease.
Acknowledgments
This study was supported by a grant from University of Naples Federico II (Naples, Italy).
Author contributions
All authors contributed equally to the production of this work.
References
- Ammoun S, Holmqvist T, Shariatmadari R, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- Assisi L, Tafuri S, Liguori G, et al. Expression and role of receptor 1 for orexins in seminiferous tubules of rat testis. Cell Tissue Res. 2012;348:601–607. doi: 10.1007/s00441-012-1394-8. [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Pineda R, Navarro VM, et al. Orexin 1 receptor messenger ribonucleic acid expression and stimulation of testosterone secretion by orexin-A in rat testis. Endocrinology. 2004;145:2297–2306. doi: 10.1210/en.2003-1405. [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Pineda R, Gaytan F, et al. Pattern of orexin expression and direct biological actions of orexin-A in rat testis. Endocrinology. 2005;146:5164–5175. doi: 10.1210/en.2005-0455. [DOI] [PubMed] [Google Scholar]
- Blanco M, García-Caballero T, Fraga M, et al. Cellular localization of orexin receptors in human adrenal gland, adrenocortical adenomas and pheochromocytomas. Regul Pept. 2002;104:161–165. doi: 10.1016/s0167-0115(01)00359-7. [DOI] [PubMed] [Google Scholar]
- Cao M, Guillerminault C. Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr Neurol Neurosci Rep. 2011;11:227–234. doi: 10.1007/s11910-010-0172-9. [DOI] [PubMed] [Google Scholar]
- Ehrström M, Gustafsson T, Finn A, et al. Inhibitory effect of exogenous orexin A on gastric emptying plasma leptin and the distribution of orexin and orexin receptors in the gut and pancreas in man. J Clin Endocrinol Metab. 2005;90:2370–2377. doi: 10.1210/jc.2004-1408. [DOI] [PubMed] [Google Scholar]
- El-Alfy M, Pelletier G, Hermo LS, et al. Unique features of the basal cells of human prostate epithelium. Microsc Res Tech. 2000;51:436–446. doi: 10.1002/1097-0029(20001201)51:5<436::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Fujita T. Paraneuron, its current implications. Biomed Res. 1980;1:3–9. [PubMed] [Google Scholar]
- Goldstein SA, Huang J, Guo C, et al. Identification of a cell origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Holmqvist T, Akerman KEO, Kukkonen JP. High specificity of human orexin receptors for orexins over neuropeptide Y and the other neuropeptides. Neurosci Lett. 2001;305:177–180. doi: 10.1016/s0304-3940(01)01839-0. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Ostman M, et al. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanism. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Gamo Y, et al. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–732. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, et al. Prepro-orexin and orexin receptors mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- Kane JK, Tanaka H, Parker SL, et al. Sensitivity of orexin-A binding to phospholipase C inhibitors, neuropeptide Y and secretin. Biochem Biophys Res Commun. 2000;272:959–965. doi: 10.1006/bbrc.2000.2880. [DOI] [PubMed] [Google Scholar]
- Karteris E, Randeva HS, Grammatopoulus DK, et al. Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab. 2001;86:4512–4519. doi: 10.1210/jcem.86.9.7849. [DOI] [PubMed] [Google Scholar]
- Karteris E, Chen J, Randeva HS. Expression of human prepro-orexin and signaling characteristics of orexin receptors in the male reproductive system. J Clin Endocrinol Metab. 2004;89:1957–1962. doi: 10.1210/jc.2003-031778. [DOI] [PubMed] [Google Scholar]
- Larsson KP, Peltonen HM, Bart G, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of TRPC channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Zong Y, Memarzadeh S, et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci USA. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;6:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PE, Shariatmandari R, Uustare A, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30 806–30 812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- Malendowicz W, Szyszka M, Ziolkowska A, et al. Elevated expression of orexin receptor 2 (HCRTR2) in benign prostatic hyperplasia is accompanied by lowered serum orexin A concentrations. Int J Mol Med. 2011;27:377–383. doi: 10.3892/ijmm.2010.590. [DOI] [PubMed] [Google Scholar]
- Martyńska L, Polkowska J, Wolińska-Witort E, et al. Orexin A and its role in the regulation of the hypothalamo-pituitary axes in the rat. Reprod Biol. 2006;6:29–35. [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Gottardo L, et al. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab. 2001;86:778–782. doi: 10.1210/jcem.86.2.7233. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Abel P, Ware M, et al. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000;85:932–944. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- Nakabayashi M, Suzuki T, Takahashi K, et al. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol. 2003;205:43–50. doi: 10.1016/s0303-7207(03)00206-5. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Tsukada A, Shamoto K. cDNA cloning of chicken orexin receptor and tissue distribution: sexually dimorphic expression in chicken gonads. J Mol Endocrinol. 2003;31:499–508. doi: 10.1677/jme.0.0310499. [DOI] [PubMed] [Google Scholar]
- Pavone LM, Tafuri S, Avallone L, et al. Expression of orexin A and its receptor 1 in the vestibular glands of the cattle genital tract. Anat Rec. 2009;292:202–206. doi: 10.1002/ar.20833. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. The diffuse neuroendocrine system and the APUD concept: related “endocrine” peptides in brain, intestine, pituitary, placenta and anuran cutaneous glands. Med Biol. 1977;55:115–125. [PubMed] [Google Scholar]
- Russo F, Pavone LM, Tafuri S, et al. Expression of orexin A and its receptor 1 in the bovine urethroprostatic complex. Anat Rec. 2008;291:169–174. doi: 10.1002/ar.20641. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behaviour. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIRP. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi R, Rucinski M, Neri G, et al. Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab. 2005a;90:3544–3549. doi: 10.1210/jc.2004-2385. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Ziolkowska A, Neri G, et al. Orexins modulate the growth of cultured rat adrenocortical cells, acting through type 1 and type 2 receptors coupled to the MAPK p42/p44- and p38-dependent cascades. Int J Mol Med. 2005b;15:847–852. [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Rossi GP, et al. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev. 2006;58:46–57. doi: 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- Tafuri S, Pavone LM, Lo Muto R, et al. Expression of orexin A and its receptor 1 in the rat epididymis. Regul Pept. 2009;5:1–5. doi: 10.1016/j.regpep.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Tafuri S, Lo Muto R, Pavone LM, et al. Novel localization of orexin A in the tubular cytotypes of the rat testis. Regul Pept. 2010;164:53–57. doi: 10.1016/j.regpep.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. The other side of the orexins: endocrine and metabolic actions. Am J Physiol Endocrinol Metab. 2003;284:13–17. doi: 10.1152/ajpendo.00359.2002. [DOI] [PubMed] [Google Scholar]
- Zhang S, Blache D, Vercoe PE, et al. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 2005;124:81–87. doi: 10.1016/j.regpep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Ziolkowska A, Spinazzi R, Albertin G, et al. Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the OX1 receptor. J Steroid Biochem Mol Biol. 2005;96:423–429. doi: 10.1016/j.jsbmb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Ziolkowska A, Rucinski M, Tyczewska M, et al. Orexin B inhibits proliferation and stimulates specialized function of cultured rat calvarial osteoblast-like cells. Int J Mol Med. 2008;22:749–755. [PubMed] [Google Scholar]
- Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol. 2007;58:53–64. [PubMed] [Google Scholar]


