Abstract
The lungfish, the closest fish to tetrapods, has two types of sensory epithelia in the olfactory organ: the lamellar olfactory epithelium and the recess epithelium. The former resembles the olfactory epithelium of ordinary teleosts and the latter resembles the vomeronasal organ of tetrapods with respect to the G-protein expressions and the morphological properties of olfactory receptor cells. In contrast to the lamellar olfactory epithelium covering the surface of olfactory lamella, the recess epithelium, together with the glandular epithelium, lines the recesses at the base of olfactory lamellae and is separated from the surrounding tissues by nonsensory epithelium. In the present study, we examined the distribution of these recesses and the relationship between the recess epithelium and the associated gland in the nasal sac of lungfish. We found that the posterior part of the nasal sac contained more recesses than the anterior one, and the medial one contained more recesses than the lateral one. In addition, virtually all recesses consisted of both the recess epithelium and the glandular epithelium. Furthermore, the glandular epithelium was invariably situated proximal to the midline raphe of the nasal sac, and the recess epithelium distal to it. Possible roles of the recess epithelium and the glandular epithelium are discussed.
Keywords: associated gland, lungfish, olfaction, vomeronasal organ
Introduction
Most vertebrates have two distinct olfactory systems: the main olfactory system and the vomeronasal system. In the main olfactory system, the receptor cells in the olfactory epithelium (OE) project their axons to the main olfactory bulb. In the vomeronasal system, on the other hand, the receptor cells in the vomeronasal organ (VNO) project their axons to the accessory olfactory bulb. The OE and the VNO express different types of olfactory receptors, members of the G-protein-coupled receptors responsible for the detection of odoriferous substances. The OE expresses odorant receptors, and the VNO expresses vomeronasal receptors (Buck & Axel, 1991; Dulac & Axel, 1995; Herrada & Dulac, 1997). Unlike the OE, which is present in all vertebrates, the VNO is present in tetrapods but not in fish (Døving & Trotier, 1998). However, several lines of evidence suggest that the vomeronasal system is partly developed in some fish (Hansen et al. 2003, 2004).
Phylogenetically and genetically, the lungfish is known to be the closest fish to tetrapods (Takezaki et al. 2004; Diogo & Abdala, 2007). We have previously reported, by analyzing the expression of G-proteins and the ultrastructural properties of receptor cells, that the olfactory organ of African lungfish Protopterus annectens possesses two types of sensory epithelia (Nakamuta et al. 2012). These epithelia, designated as the lamellar OE and the recess epithelium, correspond to the OE of general teleosts and the VNO of tetrapods, and they cover the surface of olfactory lamellae and line the recesses situated at the base of lamellae, respectively. In general, the receptor cells in the VNO are equipped with microvilli, in contrast to the olfactory receptor cells, which show different types of ultrastructure between animal species (Døving & Trotier, 1998). In the olfactory organ of lungfish, the lamellar OE contains both ciliated receptor cells and microvillous receptor cells, whereas the recess epithelium contains only microvillous receptor cells (Nakamuta et al. 2012). Furthermore, the lectin histochemistry has suggested that axons from the recess epithelium project to the ventrolateral part of the olfactory bulb (Nakamuta et al. 2012). This correlates with two published studies on the olfactory system of lungfish. Franceschini et al. (2000) suggested, based on lectin histochemical analysis, that the ventrolateral part of the olfactory bulb in African lungfish P. annectens corresponds to the accessory olfactory bulb of tetrapods. González et al. (2010) revealed that the African lungfish Protopterus dolloi possesses a vomeronasal system similar to that of tetrapods, based on immunohistochemical analysis for a VNO specific marker, calbindin-D28k, and tract-tracing experiments using DiI.
Most terrestrial vertebrates have associated glands in their olfactory organs. For example, rodents possess Bowman's gland in the OE, Jacobson's gland in the VNO, and an unnamed gland in the septal organ of Masera (Taniguchi et al. 1993). On the other hand, aquatic vertebrates, including fish, usually possess no associated glands in their olfactory organs. However, lungfish have been suggested to possess an associated gland in their olfactory organ (Derivot, 1984). In fact, the recess at the base of olfactory lamella in the nasal sac of lungfish is lined with two types of epithelia, the recess epithelium and the glandular epithelium. The former consists of several layers of cells, whereas the latter consists of cylindrical cells with eosinophilic cytoplasm and round nuclei (Nakamuta et al. 2012). However, little is known about the nature of the associated gland in the olfactory organ of lungfish. In this study, we addressed the distribution of recesses and the relationship between the recess epithelium and the glandular epithelium.
Materials and methods
Animals
Five juvenile or adult African lungfish, P. annectens (three males and two females), ranging in length from 28 to 40 cm, were purchased from commercial suppliers. All of the experiments were performed in accordance with the Principles for Animal Experiments of Iwate University. In all cases, the fish were anesthetized with ice and euthanized by decapitation.
Morphological examination
The nasal sac was dissected out from four of the fish and fixed in Bouin's solution at 4 °C for 24 h. Specimens were routinely embedded in paraffin and cut sagittally at 6–8 m in thickness. Some sections were stained with periodic acid-Schiff (PAS), some with alcian blue (pH 2.5), and the others with hematoxylin-eosin (HE). Since three nasal sacs were partially damaged during processing, they were excluded from statistical analyses. The localization and structure of all recesses were determined by examining serial sections of whole nasal sacs (n = 5: right and left nasal sacs of a 28-cm female, right nasal sac of a 29-cm male, left nasal sac of a 30-cm male, and right nasal sac of a 30-cm male, see Supporting Information Tables S1–S5).
Immunohistochemistry
The nasal sac was dissected out from the fish of 40 cm in length, and fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) overnight at 4 °C. Specimens were cryoprotected and embedded in O.C.T. Compound (Sakura Finetek) and cut sagittally at 10–15 μm in thickness with a cryostat. After washing in phosphate-buffered saline (PBS) and again in 0.1% Triton X-100 in PBS, the sections were treated with 2% normal donkey serum in PBS for 30 min at room temperature to block non-specific binding, then incubated with a primary antibody, rabbit anti-Gαo antibody (1 : 500, MBL, 551), at 4 °C overnight. After washing in PBS and 0.1% Triton X-100 in PBS, the sections were incubated for 2 h at room temperature with a fluorescent-labeled secondary antibody, Tetramethyl Rhodamine Isothiocyanate-donkey anti-rabbit IgG (1 : 500, Jackson ImmunoResearch, 711-026-152). The antibodies were diluted in PBS containing 1% bovine serum albumin.
Statistical analyses
Recesses were counted in the distinct parts of the nasal sac: anterior half vs. posterior half, and lateral side vs. medial side. Anterior and posterior halves consisted of lamellae 1–8 and lamellae 9–16 in the case of nasal sacs with 16 lamellae (Tables S1–S4), and lamellae 1–9 and lamellae 10–18 in the case of a nasal sac with 18 lamellae (Table S5). Data are shown as mean ± standard error (SE). Statistical analyses were performed with a paired t-test.
Results
Structure of the lungfish olfactory organ
The gross anatomy of the lungfish olfactory organ is shown in Fig. 1. A pair of nasal sacs was situated anterior to the eyes. The nasal sacs were connected to the external environment with the anterior nostrils and to the oral cavity with the posterior nostrils (Fig. 1A,B). The olfactory lamellae were suspended from the dorsal, medial and lateral walls of the nasal sac and aligned on each side of the dorsal midline raphe (Fig. 1C). The numbers of lamellae were not different between the medial and lateral sides. On both sides, approximately 15–20 rows of lamellae were aligned perpendicular to the dorsal midline raphe (Fig. 1D).
Fig. 1.
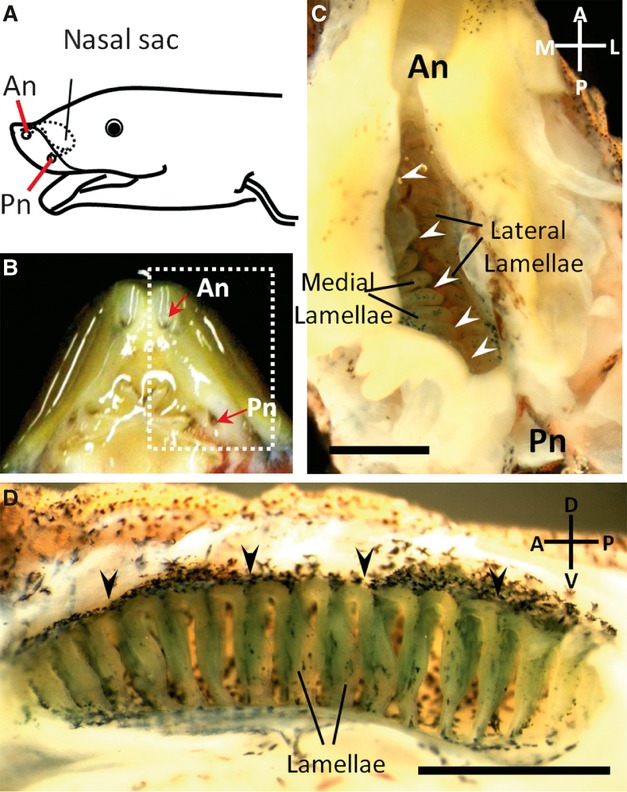
The olfactory organ of the lungfish. (A) Schematic drawing of the head viewed obliquely from the left. The anterior nostril (An) is situated ventrally near the rostral end of the upper jaw and connects the nasal sac to the external environment. The posterior nostril (Pn) opens into the oral cavity. (B) The ventral view of the upper jaw after removal of the lower jaw. (C) Close-up view of the area enclosed by the dotted line in (B). The ventral wall of the nasal sac has been dissected between the nostrils to expose the inside of the nasal sac. Arrowheads indicate the dorsal midline raphe. (D) Medial half of the nasal sac. Approximately 15–20 rows of lamella are aligned perpendicular to the dorsal midline raphe (arrowheads). Anterior is on the left, dorsal is on top. Scale bars: 2 mm (C,D).
Distribution and structure of the recesses
Serial sections stained with HE were examined to clarify the distribution and histological property of the recesses in the nasal sac of lungfish. The number of recesses was counted in relation to the individual lamellae and recesses were classified according to their epithelial types (Tables S1–S5). The roof in the posterior part of the nasal sac was higher than in the anterior part (Fig. 2). The glandular epithelium and the recess epithelium were observed only in the recesses (Fig. 3A–K). The recess epithelium consisted of several layers of epithelial cells (Fig. 3H–K) and was positive for Gαo, a vomeronasal receptor cell marker used in our previous study (Nakamuta et al. 2012) (Fig. 3L). The glandular epithelium consisted of cylindrical cells with basally situated round nuclei (Fig. 3A–G) and was negative for Gαo (Fig. 3L). The glandular epithelial cells showed punctate staining for PAS in the cytoplasm (Fig. 3M), and were not stained with alcian blue (pH 2.5) (Fig. 3N). The nasal sacs contained a total of 71, 68, 84, 96 and 89 recesses, respectively (Table 1). Of the 408 recesses examined, 371 (90.9%) were of mixed type, where both the glandular epithelium and the recess epithelium could be distinguished, and 37 (9.1%) were of unidentified types where cellular organization was not well preserved (Table 1). No recesses containing only the glandular epithelium or the recess epithelium were found. In all recesses, the glandular epithelium was situated proximally and the recess epithelium was situated distal to the midline raphe of the nasal sac (Fig. 3A–K). Significantly more recesses were contained in the posterior half (68.8 ± 6.5) of the nasal sac than in the anterior half (12.8 ± 1.7) (Fig. 4A). In addition, significantly more recesses were contained on the medial side (59 ± 3.7) than on the lateral side (22.6 ± 4.3) (Fig. 4B).
Fig. 2.
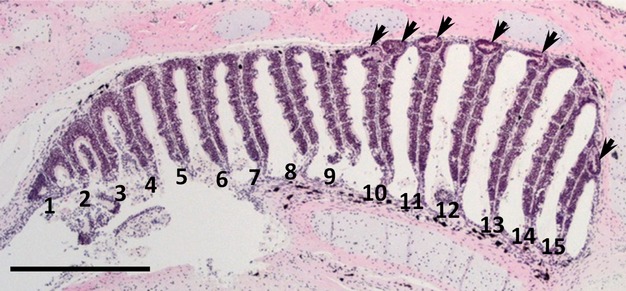
A representative section of the nasal sac stained with HE. Dorsal is on top and anterior is on the left. Each lamella is numbered from rostral to caudal. Arrows indicate recesses at the base of lamellae. More recesses are contained in the posterior part of the nasal sac than in the anterior one. The roof is higher in the posterior part of the nasal sac than in the anterior part. Scale bar: 1 mm.
Fig. 3.
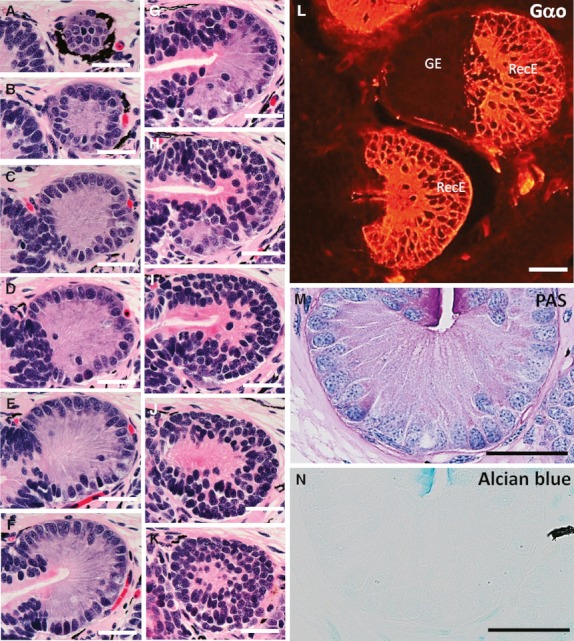
The recess epithelium and the glandular epithelium. (A–K) HE-stained representative sections (at 18-μm intervals) of a single recess serially sectioned: the most proximal side (A) and the most distal side (K) to the midline raphe of the nasal sac. The recess epithelium consists of several layers of epithelial cells, whereas the glandular epithelium consists of cylindrical cells with basally situated, round to oval nuclei. These sections demonstrate that the single recess contains both the glandular epithelium (A–G) and the recess epithelium (H–K). (L) Immunohistochemistry for Gαo in the recesses. In the upper recess, the recess epithelium (RecE) is positive for Gαo, whereas the glandular epithelium (GE) is negative. In the lower recess, only the RecE (Gαo-positive) is observed. (M,N) PAS or alcian blue-stained glandular epithelium. Punctate staining for PAS is observed in the cytoplasm of the glandular epithelial cells (M). The glandular epithelium is negative for alcian blue (N). Scale bars: 50 μm.
Table 1.
Total number and the cellular organization of recesses in the nasal sacs of lungfish
| Epithelial type | |||
|---|---|---|---|
| Nasal sac no.* | Total no. of recesses | Mixed type | Unidentified type |
| 1 | 71 | 65 | 6 |
| 2 | 68 | 68 | 0 |
| 3 | 84 | 77 | 7 |
| 4 | 96 | 83 | 13 |
| 5 | 89 | 78 | 11 |
| Total | 408 | 371 (90.9%) | 37 (9.1%) |
| Mean ± SE | 81.6 ± 5.3 | 74.2 ± 3.3 | 7.4 ± 2.2 |
1: Female, 28 cm in body length, right nasal sac; 2: Female, 28 cm in body length, left nasal sac; 3: Male, 29 cm in body length, right nasal sac; 4: Male, 30 cm in body length, left nasal sac; 5: Male, 30 cm in body length, right nasal sac.
Fig. 4.
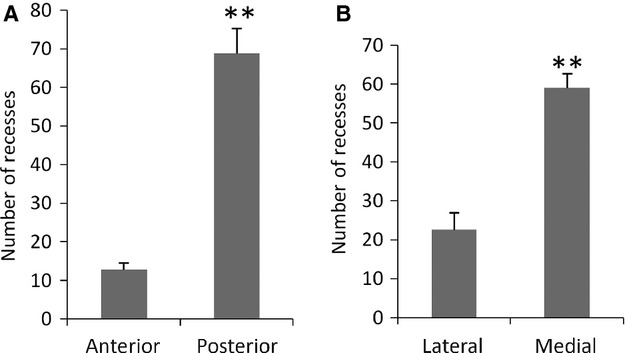
Quantitative assessment of the number of recesses in the distinct parts of nasal sac. Data are shown as mean ± SE (n = 5). (A) The number of recesses distributed in the anterior and posterior halves of the nasal sac. The anterior and posterior halves contain 12.8 ± 1.7 and 68.8 ± 6.5 recesses, respectively. **Significant difference between anterior and posterior half (P < 0.01). (B) The number of recesses distributed on the lateral and medial sides of the nasal sac. The lateral and medial sides contain 22.6 ± 4.3 and 59 ± 3.7 recesses, respectively. **Significant difference between lateral and medial sides (P < 0.01).
Discussion
Generally, fish lack the associated glands in their olfactory organ, whereas most terrestrial vertebrates are equipped with them (Getchell & Getchell, 1992). However, since lungfish depend almost entirely on the lung for respiration, sticking their heads out of water to breathe, they might have exceptionally developed an associated gland in their olfactory organ. Histological examination of the recesses in serial sections demonstrated that the glandular epithelium was situated proximally and the recess epithelium distally in relation to the midline raphe in each recess. Since this topographical relationship between the two epithelia was true for all recesses, it seems to have a significant importance for their interaction. In addition, this glandular epithelium is considered to be the associated gland of the recess epithelium and not that of the lamellar OE, because virtually all recesses were composed of both the recess epithelium and the glandular epithelium, and together these two epithelia were observed only in the recesses.
The glandular epithelial cells were positive for PAS and negative for alcian blue. They are suggested to contain neutral mucopolysaccharide as secretory granules because of punctate staining for PAS in their cytoplasm. Secretory products from the associated gland in the olfactory organ are known to contain some proteins which can interact with chemical substances and regulate their binding to the olfactory receptors. For example, vomeromodulin is produced by the associated gland of the VNO in rodents (Khew-Goodall et al. 1991) and odorant binding proteins are produced in the associated gland of the OE in newts (Iwasa et al. 2008). Apparently, further investigations are required to confirm whether the secretory products from the glandular epithelium in lungfish can bind some chemical substances and regulate chemoreception in the recess epithelium.
The nasal sacs of lungfish contained significantly more recesses on their medial side than on the lateral side, although there were the same number of lamellae on both sides. However, there were more sections from the medial half of the nasal sac than from the lateral side, indicating that the medial half of the nasal sac was larger than the lateral side. This might be why the medial side of the nasal sac contained more recesses than the lateral side. In addition, significantly more recesses were distributed in the posterior half of the nasal sac than in the anterior half. This uneven distribution of recesses might have a functional significance.
The properties of chemicals perceived by the sensory epithelium are closely related to the localization of sensory epithelium in the nasal cavity. For example, the olfactory epithelium and the middle chamber epithelium of semi-aquatic African clawed frog are suggested to detect airborne odorants and water-soluble odorants, respectively. The former lines the dorsally located principal chamber of the nasal cavity, and the latter the laterally located middle chamber (Hofmann & Meyer, 1991; Freitag et al. 1995; Oikawa et al. 1998). The recesses were distributed dorsally and caudally in the nasal sac of lungfish. It is not known what kinds of odorants are perceived by the recess epithelium at present. However, considering the localization of the recess epithelium in the nasal sac, it can be speculated that the lungfish detects airborne odorants by the recess epithelium. If air enters the nasal sac when they breathe, it is reasonable to conclude that the dorsal wall of the nasal sac is exposed to air. Furthermore, the posterior part of the nasal sac seems to be most suitable to detect airborne odorants because its roof is higher than the anterior part. It remains to be electrophysiologically verified whether the olfactory organ of lungfish responds to airborne odorants.
Acknowledgments
This work was supported by Grant-in-Aid for Graduate Students from The United Graduate School of Veterinary Sciences, Gifu University (S.N.).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1 Distribution and cellular organization of the recesses in the nasal sac (female, body length 28 cm, right nasal sac). The medial and lateral sides of the nasal sac were examined to determine whether the recesses in each lamella consist of the glandular epithelium, the recess epithelium, or both.
Table S2 Distribution and cellular organization of the recesses in the nasal sac (female, body length 28 cm, left nasal sac).
Table S3 Distribution and cellular organization of the recesses in the nasal sac (male, body length 29 cm, right nasal sac).
Table S4 Distribution and cellular organization of the recesses in the nasal sac (male, body length 30 cm, left nasal sac).
Table S5 Distribution and cellular organization of the recesses in the nasal sac (male, body length 30 cm, right nasal sac).
References
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Derivot JH. Functional anatomy of the peripheral olfactory system of the African lungfish Protopterus annectens Owen: macroscopic, microscopic, and morphometric aspects. Am J Anat. 1984;169:177–192. doi: 10.1002/aja.1001690206. [DOI] [PubMed] [Google Scholar]
- Diogo R, Abdala V. Comparative anatomy, homologies and evolution of the pectoral muscles of bony fish and tetrapods: a new insight. J Morphol. 2007;268:504–517. doi: 10.1002/jmor.10531. [DOI] [PubMed] [Google Scholar]
- Døving KB, Trotier D. Structure and function of the vomeronasal organ. J Exp Biol. 1998;201:2913–2925. doi: 10.1242/jeb.201.21.2913. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Franceschini V, Lazzari M, Ciani F. Cell surface glycoconjugates in the olfactory system of lungfish Protopterus annectens Owen. Acta Zool (Stockholm) 2000;81:131–137. [Google Scholar]
- Freitag J, Krieger J, Strotmann J, et al. Two classes of olfactory receptors in Xenopus laevis. Neuron. 1995;15:1383–1392. doi: 10.1016/0896-6273(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Getchell ML, Getchell TV. Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc Res Tech. 1992;23:111–127. doi: 10.1002/jemt.1070230203. [DOI] [PubMed] [Google Scholar]
- González A, Morona R, López JM, et al. Lungfishes, like tetrapods, possess a vomeronasal system. Front Neuroanat. 2010;4:130. doi: 10.3389/fnana.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Rolen SH, Anderson K, et al. Correlation between olfactory receptor cell type and function in the channel catfish. J Neurosci. 2003;23:9328–9339. doi: 10.1523/JNEUROSCI.23-28-09328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Anderson KT, Finger TE. Differential distribution of olfactory receptor neurons in goldfish: structural and molecular correlates. J Comp Neurol. 2004;477:347–359. doi: 10.1002/cne.20202. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Hofmann MH, Meyer DL. Functional subdivisions of the olfactory system correlate with lectin-binding properties in Xenopus. Brain Res. 1991;564:344–347. doi: 10.1016/0006-8993(91)91475-g. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Mangula G, Urano K, et al. Lipocalin family proteins expressed in the Bowman's gland of the newt olfactory organ. Jpn J Taste Smell Res. 2008;15:211–220. [Google Scholar]
- Khew-Goodall Y, Grillo M, Getchell ML, et al. Vomeromodulin, a putative pheromone transporter: cloning, characterization, and cellular localization of a novel glycoprotein of lateral nasal gland. FASEB J. 1991;5:2976–2982. doi: 10.1096/fasebj.5.14.1752363. [DOI] [PubMed] [Google Scholar]
- Nakamuta S, Nakamuta N, Taniguchi K, et al. Histological and ultrastructural characteristics of the primordial vomeronasal organ in lungfish. Anat Rec (Hoboken) 2012;295:481–491. doi: 10.1002/ar.22415. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Suzuki K, Saito TR, et al. Fine structure of three types of olfactory organs in Xenopus laevis. Anat Rec. 1998;252:301–310. doi: 10.1002/(SICI)1097-0185(199810)252:2<301::AID-AR16>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Takezaki N, Figueroa F, Zaleska-Rutczynska Z, et al. The phylogenetic relationship of tetrapod, coelacanth, and lungfish revealed by the sequences of forty-four nuclear genes. Mol Biol Evol. 2004;21:1512–1524. doi: 10.1093/molbev/msh150. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Arai T, Ogawa K. Fine structure of the septal olfactory organ of Masera and its associated gland in the golden hamster. J Vet Med Sci. 1993;55:107–116. doi: 10.1292/jvms.55.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


