Abstract
Background
Although vitamin D deficiency has been studied in various adult populations, there are few data on the prevalence of this nutritional deficiency among healthy adolescents in the United Arab Emirates (UAE). This study was conducted to determine the prevalence of vitamin D deficiency and to examine its correlates in adolescents aged 15 to 18 years.
Methods
This was a cross-sectional study in urban schools. Healthy adolescents (N=315) from a sample of 8 schools were randomly selected from the 142 schools in Al Ain, Abu Dhabi Emirate. Outcomes measured included serum concentrations of 25-hydroxy vitamin D (25OHD), plasma lipids, blood sugar, blood pressure and anthropometric data, nutrition and lifestyle variables.
Results
Fourty-one participants (19.7%) were vitamin D deficient (serum 25OHD level ≤15 ng/mL [≤37.5 nmol/L]. Using a cutoff level of 25(OH) D of ≤20 ng/ml [≤50 nmol/l] 143 participants (45.4%) were vitamin D insufficient. Overall 65.1% of study participants were either vitamin D deficient or insufficient. The prevalence of vitamin D deficiency varied between boys (10%) and girls (28%). In a final multivariate model, serum 25(OH) D concentrations were inversely correlated with female gender, consumption of fast food per week, and body mass index and positively correlated with physical activity scores after adjustment for age.
Conclusions
Vitamin D deficiency and insufficiency were highly prevalent in adolescents, and more common in girls.
Keywords: Vitamin D deficiency, Adolescents, United Arab Emirates
Background
Inadequate exposure to sunlight and low nutritional intake of vitamin D result in low serum concentrations of circulating 25(OH) D, a condition known as hypovitaminosis D [1,2]. Severe vitamin D deficiency in children leads to nutritional rickets and it is associated with developmental delays and impaired growth [3]. Therefore, adequate stores of vitamin D are crucial for musculoskeletal health, especially since peak bone mass achieved early in life is a predictor of osteoporosis risk in adulthood [4]. In addition, there is a growing body of evidence that vitamin D deficiency is associated with type 1 diabetes [5] and cardiovascular disease (CVD) risk factors such as hypertension, hyperglycemia and metabolic syndrome [6-8]. The extreme consequences of vitamin D deficiency such as rickets in children and osteomalacia in adults have been almost eliminated in some developed countries through adequate diet, food fortification, and the encouragement of moderate sunlight exposure [9].
Vitamin D deficiency remains a problem globally [10,11]. Vitamin D deficiency in women in Arab countries has been attributed to inadequate exposure of skin to sunlight due to a very conservative style of dress (e.g. niqab, hijab) that covers most of the body when they are outside [12,13]. In a recent study, Arab-American women practicing a conservative style of dress showed a significantly higher prevalence of vitamin D deficiency compared to their counterparts practicing a less conservative style of dress [14]. In the United Arab Emirates (UAE), female youth similarly maintain a very conservative style of dress that limits sunlight exposure. Population-based data are lacking about the status of vitamin D in UAE adolescents. Therefore, the objectives of this study were to determine the prevalence of 25-hydroxyvitamin D (25[OH] D) deficiency and its correlation with gender, diet and CVD risk factors.
Methods
A cross-sectional study of healthy adolescents aged 12 to 18 years was conducted in Al Ain city in the United Arab Emirates during March and April of 2010. Abu Dhabi is the largest of the seven emirates that make up the United Arab Emirates and the city of Al Ain (“the spring” in Arabic) is a fertile oasis city located approximately 160 kilometers east of the Abu Dhabi capital.
Study participants were recruited as part of a metabolic syndrome project where 8 schools out of a total of 142 schools were randomly selected and adolescents in grades between 6 and 12 enrolled. The targeted sample size (n=1630) was selected with probability proportional to the school size. At the second stage, classes in each school were randomly selected by using the even and odd numbers of each grade. The final sample (n=1018) included participants who had fasted for at least 8 hours, agreed to draw blood, and had not used any regular medications or had any chronic medical conditions that might affect growth, body composition, dietary intake or physical activity and cigarette smoking. Serum 25(OH) D concentrations were measured in a sub-sample of participants, aged 15 to 18 years (n=315). The latitude of schools did not change in Al Ain city. We did not collect information on the use of sunscreen.
The study protocol was approved by the Human Research Ethics Committee of the Al Ain, Medical District. All the participants and their parents provided informed consent.
Data collection
Personal identification numbers were assigned to each participant to maintain anonymity. The identification number was used to confirm consent status and to link students to their respective schools, clinical measurements and blood samples. A questionnaire was developed to obtain relevant information related to age, gender, ethnicity, mother’s education level, dietary habits, and physical activity. The questionnaire was translated in Arabic and validated by a pilot test using 30 volunteers. In the dietary habit section of the questionnaire, milk and milk product consumption data were categorized as less than once per day, once per day and more than once per day. The short version of the International Physical Activity Questionnaire [14] was used to assess the physical activity status in the study participants. Part of the physical activity questionnaire asked information on hours spent watching television, playing video games, and computer use. Combined television, video, and computer hours were categorized as none, ≤2 hours, 3 to 4 hours and >4 hours per day.
Measurements and laboratory analysis
A training workshop on standardizing the method of anthropometric and blood pressure measurement was conducted by qualified trainers prior to data collection, and involved nurses of all study schools. All measurements were performed at the same time of the day (i.e. early morning between 8 and 11 am) for all participants. Height was measured in centimeters (cm) using a stadiometer (SECA) with the participant standing in an upright position without wearing shoes. Waist circumference was measured in centimeters (cm) using an un-stretched measuring tape placed around the midpoint between the bottom of the rib cage and above the tip of the iliac crest. Body weight was measured to the nearest 0.2 kilograms (kg) using a digital scale (SECA) with the participant standing in an upright position without shoes and in light clothing. Triplicate readings of height, weight and waist circumference were taken and the average was considered to be the participant’s measurement.
Following at least five minutes of rest in a seated position, blood pressure (BP) was measured on the right arm using a standard mercury sphygmomanometer with an appropriate cuff size. All participants were required to refrain from smoking, consuming caffeine and participating in any moderate- or vigorous-intensity physical activity at least 60 minutes before their blood pressure measurement. Three consecutive measures were obtained at one-minute intervals and the average of the last two readings was considered to be the participant's blood pressure.
A five milliliter venous blood sample was obtained from the subjects by qualified nurses using standardized tubes. Prior to blood sampling, all participants had been instructed to fast for at least eight hours and abstain from smoking. Blood samples were sent to Tawam hospital laboratory within two hours of blood draw where they underwent standardized (quality controlled) analyses. Serum 25 (OH) D concentrations were measured by radioimmunoassay (DiaSorin, Stillwater, MN). The intra-assay and inter-assay for coefficients of variation were 8.3% and 3.2% respectively. The fasting plasma glucose (FBS), high density lipoproteins (HDL) and triglycerides (TG) were analyzed by the DXC 800 Analyzer (Beckman Coulter, Fullerton, CA, USA) using the appropriate conventional laboratory reagents, enzymatic and calorimetric techniques.
Definitions
Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. BMI was used to classify participants as either healthy/normal weight (BMI 5th to 75th percentile), overweight (BMI between the 85th and 95th percentiles) or obese (BMI ≥95th percentile) according to the 2000 Centers for Disease Control and Prevention growth charts using Anthro program in Epi-Info software [15].
Metabolic syndrome (MetS) was defined using the diagnostic criteria proposed by the International Diabetes Federation [16]; namely, waist circumference ≥90th percentile or ≥94th percentile (for youth aged 16 years or older), triglycerides concentrations ≥150 mg/dL(1.7 mmol/L), HD-cholesterol <40 mg/dL(1.03 mmol/L) or <50 mg/dL (1.29 mmol/L) for female adolescents aged ≥16 years and older, fasting plasma glucose (FPG) concentrations of >100mg/dL (5.6 mmol/L) and blood pressure (BP) ≥130/80 mmHg.
Elevated blood pressure was defined by using percentiles for systolic and diastolic values on the basis of height percentile, age and gender. Values >95th percentiles were considered elevated [17].
The 25 (OH) levels are the most commonly measured indicator of vitamin D status. We defined vitamin D deficient as having serum 25OHD level ≤15 ng/mL [≤37.5 nmol/L] and vitamin D insufficient as 25OHD level ≤20 ng/mL [≤50 nmol/L], respectively [18,19].
Statistical analysis
All data were normally distributed. Descriptive statistics (frequencies, means, standard deviations) were used to estimate serum 25(OH) D concentrations by age group, gender, nationality, mother’s education level, food intake, physical activity, nutritional status and by presence of cardiovascular risk factors such as high blood pressure, high blood glucose, low HDL-cholesterol, high triglycerides and metabolic syndrome. The analyses were conducted using Epi-info, and SPSS (v.19). A one way ANOVA and student t-test was used to analyze the association between serum 25(OH) D concentrations and potentially associated variables. Statistical significance was defined as p-values <0.05. Serum 25 (OH) D concentrations were considered as a continuous outcome variable for multivariate analyses to avoid power loss associated with categorization [20] and stepwise linear regression analysis was used to identify the significant covariates of serum 25 (OH) D concentrations.
Results
The age of study participants (n=315) ranged from 15 to 18 years and 52% were female (n=165). The mean age (SD) was 16 years for females (0.6) as well as for males (0.9). The mean distribution of 25(OH) D concentrations across characteristics of 315 study participants is listed in Table 1. There were no significant differences in 25(OH) D concentrations between Emirati youth and youth from other Gulf and Arab countries. The majority of the study participants (63%) were Emirati nationals and the rest (37%) were from other Arab and Gulf countries.
Table 1.
Mean 25 (OH) D concentrations by socio-demographic characteristics, physical activity, and dietary habits of UAE adolescents aged 12 to 18 Years, 2010
|
Characteristic |
All |
25 (OH) D Level, ng/mL |
|
|---|---|---|---|
| n | Mean (95%CI) | p-value | |
| Overall |
315 |
23.8 (22.1-24.7) |
NA |
|
Age, Y |
|
|
|
| 15-16 |
142 |
24.2 (22.4-25.9) |
|
| 17-18 |
173 |
23.1 (21.8-24.6) |
0.373 |
|
Gender, % |
|
|
|
| Male |
150 |
26.2 (24.6-27.8) |
|
| Female |
165 |
21.2 (19.8-22.7)) |
0.000 |
|
Nationality |
|
|
|
| Emirati |
198 |
23.2 (21.8-24.3) |
|
| Other Gulf or Arab Countries (References) |
117 |
24.6 (21.3-26.5) |
0.364 |
|
Mother education |
|
|
|
| No formal education |
47 |
21.5 (19.2-23.8) |
|
| Up to secondary |
162 |
23.5 (22.0-25.0) |
|
| College/University |
49 |
24.8 (21.3-28.2) |
0.127 |
|
Physical Activity Score |
|
|
|
| Low |
115 |
22.1 (20.2-23.9) |
|
| Moderate |
102 |
23.0 (21.2-24.8) |
|
| High |
95 |
26.3 (24.3-28.3) |
0.005 |
|
Television and Computer Use, h/day |
|
|
|
| Non |
27 |
27.1 (22.5-31.7) |
|
| Up to 2 hours |
99 |
24.6 (22.7-26.5) |
|
| More than 2 hours |
186 |
22.5 (21.3-23.9) |
0.030 |
|
Milk and milk product intake |
|
|
|
| Less than once per day |
141 |
22.3 (21.0-23.7) |
|
| Once per day |
85 |
24.8 (22.3-27.3) |
|
| More than once per day |
89 |
24.4 (22.3-26.5) |
0.127 |
|
Meals at Fast Food in past 7 days |
|
|
|
| Never |
64 |
26.2 (23.3-29.1) |
|
| Once per week |
148 |
23.4 (21.8-24.9) |
|
| More than once per week |
103 |
22.3 (20.6-24.1) |
0.042 |
|
Carbonated soft drink intake in a day |
|
|
|
| Less than once a day |
171 |
23.8 (22.3-25.3) |
|
| Once or two times a day |
70 |
21.4 (19.6-23.1) |
|
| More than two times a day |
74 |
25.2 (22.6-27.8) |
0.058 |
|
Vegtables intake in a day |
|
|
|
| Less thance once a day |
170 |
23.1 (21.6-24.4) |
|
| Once daily |
71 |
23.8 (21.1-26.4) |
|
| More than once a day | 74 | 24.8 (22.5-27.0) | 0.417 |
Out of 315 study participants, 41 (19.7%) were vitamin D deficient (serum 25OHD concentrations ≤15 ng/mL [≤37.5 nmol/L]. Using a cutoff of (25OHD concentrations ≤20 ng/mL [≤50 nmol/L], 143 participants (45.4%) were vitamin D insufficient. We removed 6 participants who were on medication for diabetes or hypertension. Only boys reported (15%) current cigarette smoking and none of the girls had history of current smoking.
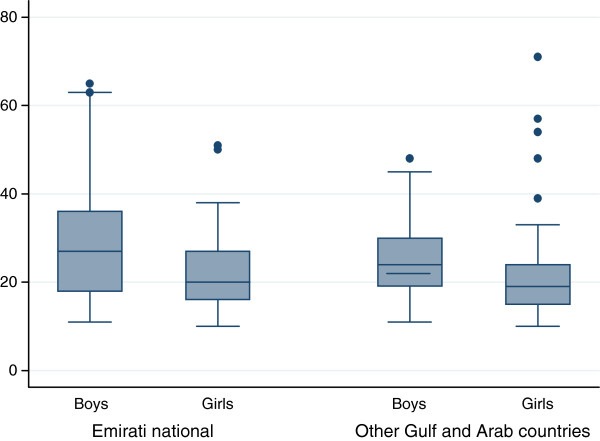
Among Emirati adolescents, a higher proportion of females (32.0%) were vitamin D deficient compared to their male counterparts (8.0%) and this difference was significant (p<0.05). Similarly female adolescents from other Gulf and Arab countries had higher prevalence of vitamin D deficiency (23.5%) compared to their male counterparts (14.3%) and this difference was significant (p<0.05). Mean serum 25 (OH) D concentrations was 20.8 ng/mL (95% CI 18.8-22.8) in Emirati females as compared to Emirati males (mean 25.2 ng/mL; 95% CI 23.7-26.8). Mean serum 25 (OH) D concentrations was 21.9 ng/mL (95% CI; 19.8-23.9) in female adolescents from other Gulf and Arab countries compared to their males counterparts (mean 28.3; 95% CI 24.6-31.9) as shown in Figure 1.
Figure 1.
Box plot of participants’ serum 25-hydroxyvitamin D (25OHD) concentration (ng/mL) by gender and nationality, (n=315), Al Ain, United Arab Emirates, 2010.
Mean 25 (OH) D concentrations were highest in those who had the highest physical activity score, intermediate among adolescents with moderate physical activity and the lowest in those with low physical activity score (p<0.005 for each pair-wise comparison). Study participants who spent more time watching television, playing video games, or using computers were more likely to have lower 25 (OH) D concentrations (Table 1). Furthermore, participants who ate at a fast food restaurant at least once in the past week were also more likely to have low 25 (OH) D levels.
Table 2 shows mean 25 (OH) D concentrations across BMI categories and other cardiovascular risk factors. Mean 25 (OH) D concentrations were lower among adolescents classified as obese. Although not statistically significant, vitamin D concentrations were lower among those with central obesity. Higher vitamin D concentrations were linked to lower HDL-C. Vitamin D concentrations were also lower among those who had elevated blood pressure and metabolic syndrome but these associations were not significant statistically.
Table 2.
Mean 25 (OH) D levels by cardiovascular disease risk factors of UAE adolescents aged 12 to 18 years (n=315), AL Ain, 2010
|
Variable |
All |
Mean 25 (OH) D Level, ng/mL |
|
|---|---|---|---|
| n | (95% CI) | p-value | |
| BMI |
|
|
|
| <85 percentile |
212 |
23.8 (21.9-25.9) |
|
| ≥85th percentile |
50 |
25.8 (22.5-29.2) |
|
| ≥95th percentile |
52 |
20.6 (18.2-22.4) |
0.023 |
| Waist circumference ≥90th percentile |
|
|
|
| Yes |
25 |
20.5 (17.8-23.2) |
|
| No |
290 |
23.9 (22.7-25.0) |
0.101 |
| High blood pressure |
|
|
|
| Yes |
35 |
23.2 (20.5-25.9) |
|
| No |
279 |
23.7 (22.5-24.9) |
0.342 |
| Fasting Glucose ≥100 mg.dL |
|
|
|
| Yes |
17 |
21.3 (18.5-24.1) |
|
| No |
298 |
23.7 (22.6-24.9) |
0.098 |
| HDL-cholesterol ≤40 mg mg/dL |
|
|
|
| Yes |
174 |
24.9 (23.4-26.4) |
|
| No |
141 |
22.1 (20.5-23.6) |
0.011 |
| Triglycerides ≥ 110 mg/dL |
|
|
|
| Yes |
16 |
23.2 (17.8-28.5) |
|
| No |
299 |
23.6 (22.5-24.8) |
0.860 |
| Metabolic syndrome |
|
|
|
| Yes |
26 |
22.0 (19.7-24.2) |
|
| No | 288 | 23.8 (22.6-24.9) | 0.151 |
Table 3 shows stepwise multivariate linear regression analyses that were conducted to identify independent determinants of Vitamin D concentrations. In this model, all significant (p<0.05) variables from earlier analyses were included to simultaneously adjust for other variables in the model. After adjustment for age, female gender, consumption of fast food per week, and BMI were independently correlated with decreased vitamin D level. Physical activity scores were positively correlated with vitamin D level.
Table 3.
Multivariate linear regression model for plasma 25 (OH) D concentrations
| Variable | β, Estimate ± SE | p-value |
|---|---|---|
| Intercept |
46.943 ± 12.056 |
0.000 |
| Age |
NS |
|
| Gender |
−3.878 ± 1.258 |
0.002 |
| Fast food per week |
−2.103 ± 0.801 |
0.009 |
| Body mass index |
−0.230 ± 0.095 |
0.017 |
| Physical activity score |
1.378 ± 0.698 |
0.050 |
| HDL cholesterol |
NS |
|
| Carbonated soft drink intake | NS |
NS indicates not significant.
Discussion
We found a high prevalence of vitamin D deficiency among otherwise healthy adolescents in our school-based study population despite living in a sunny subtropical area (latitude 24º N, longitude 55º E). More importantly a substantially higher proportion of female adolescents had vitamin D deficiency compared to their male counterparts. As part of our exclusion criteria we did not include six subjects who were on treatment for diabetes or hypertension and they might had even lower concentrations of vitamin D. To the best of our knowledge, no population-based data were available from previous studies investigating vitamin D levels in the adolescent population in the UAE.
The prevalence of vitamin D deficiency in adolescents in the UAE is very high, particularly in females, compared to their counterparts in other developed countries where vitamin D fortified foods are available, and people use vitamin D supplements [21,22]. Although the UAE and other Gulf countries have a sunny environment, skin sun exposure is low, and therefore vitamin D deficiency remains one of the major public health problems [23,24].
Both adult and adolescent females observe conservative dress codes that either require covering all body parts including face and hands (Niqab) or all body parts except the face and hands (Hijab). High prevalence of vitamin D deficiency has been noted in adolescent girls who observed conservative dress in Israel and Turkey [25,26]. Studies conducted in a Jordanian population revealed that women who observed Niqab and Hijab had significantly lower concentrations of vitamin D [27].
Inadequate nutritional intake of vitamin D may further contribute to low vitamin D concentrations. Laleye et al. [28] investigated the problem of vitamin D insufficiency in Emirati female students aged 19 to 23 years through a dietary intake assessment and found that over 70% of female students did not consume enough milk and other vitamin-D-rich foods. Data is lacking if UAE milk contains enough vitamin D and studies conducted elsewhere show that fortified milk did not contain the amount of vitamin D claimed on the label [29]. In the study from Al Ain in theUAE, 40% of participants reported consuming fortified milk with a calculated daily average intake of 88 international units of vitamin D [30].
Contrary to existing evidence, our findings did not show a significant association of blood pressure, plasma lipids and metabolic syndrome with 25 (OH) D concentrations. However, our analysis showed an inverse association between 25 (OH) D concentrations and BMI. Even after controlling for age and gender, body mass index remained an independent correlate of 25 (OH) D concentrations, consistent with findings from other studies [29,30]. The sequestration of vitamin D into adipose tissue has been proposed to explain this association [31]. This has important implications for the UAE population as recent figures estimate that 28% of male adolescents and 40% of female adolescents are either overweight or obese [32]. In our study, vitamin D concentrations were positively and significantly (p<0.05) correlated with physical activity and negatively with fast food intake. Other studies have shown that substantially large amounts of carbonated soft drinks were consumed with negligible amounts of milk at fast food places [33].
These findings must be interpreted in light of the acknowledged limitations. We were not able to measure the parathyroid hormone, a measure of bone health loosely associated with vitamin D status [34]. The study was cross-sectional, and therefore, causality cannot be inferred. Information on dietary and physical activity information was obtained by self-reports, with its inherent limitation. We did not evaluate the effect of seasonality as the study was conducted during months of March and April. Given the availability of sun shine almost throughout the year the seasonality may not be an important factor. Our results may not be generalizable to the entire UAE.
Conclusions
In conclusion, vitamin D deficiency and insufficiency were highly prevalent in females in the United Arab Emirates. Given the potential for serious morbidity, there is a need for urgent monitoring of vitamin D deficiency and timely correction of vitamin D status in the general UAE population by instituting public health measures such as encouragement of moderate sunlight exposure that has been shown to be effective in other Arab population [35].
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived, designed and implemented the experiments: MAS AEM AK MMN SJM FAM. Conceived, designed, analyzed the data, wrote the paper MAS NN. Wrote the paper AEM AK MMN SJM FAM NN MAS, JAK, ASD. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Shamma J Muhairi, Email: S_Almuhairi@UAEU.AC.AE.
Aaesha E Mehairi, Email: dr.almehairi@yahoo.com.
Aysha A Khouri, Email: Aysha_khouri@UAEU.AC.AE.
Muna M Naqbi, Email: muna_alnaqbi@UAEU.AC.AE.
Fatima A Maskari, Email: fatma.am@uaeu.ac.ae.
Juma Al Kaabi, Email: j.kaabi@uaeu.ac.ae.
Ayesha S Al Dhaheri, Email: ayesha_aldhaheri@uaeu.ac.ae.
Nico Nagelkerke, Email: niconagelkerke@gmail.com.
Syed M Shah, Email: syeds@uaeu.ac.ae.
Acknowledgements
This study was supported by a grant from the UAE University Individual Faculty Grant (# 01-14-8-11/09), Shah et al. Parents Assisting Children and Teachers (PACT) for Obesity Prevention), Seed grant by Faculty of Medicine and Health Sciences, UAE University (Al Maskari et al.). The laboratory investigations of this study were funded by John Hopkins Tawam Hospital – Al Ain, United Arab Emirates and help of laboratory staff at Tawam hospital is greatly appreciate. We greatly appreciate the editorial efforts of Dr. Tom Loney to improve the quality of English and other useful comments.
Role of the Sponsors: The funding organizations had no role in the design and conduct of the study; data collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clinc Nutr. 2008;87(4):1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Lessons for nutritional science from vitamin D. Am J Clin Nutr. 1999;69:825–826. doi: 10.1093/ajcn/69.5.825. [DOI] [PubMed] [Google Scholar]
- Gartner LM, Greer FR. American Academy of Pediatrics, Section on Breastfeeding and Committee on Nutrition. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111(4):908–910. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. Consensus Development Panel on Osteoporosis Prevention Diagnosis, and Therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9202):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- Krause R, Bühring M, Hopfenmüller W, Holick FM, Sharma AM. Untraviolet B and blood pressure. Lancet. 1998;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- Reis JP, Muhlen DV, Miller ER, Michos E, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med. 2011;155:820–826. doi: 10.7326/0003-4819-155-12-201112200-00004. [DOI] [PubMed] [Google Scholar]
- Welch TR, Bergstrom WH, Tsang RC. Vitamin D-deficient rickets; the reemergence of a once-conquered disease. J Pediatr. 2000;137(2):143–145. doi: 10.1067/mpd.2000.109008. [DOI] [PubMed] [Google Scholar]
- Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization Program (VDSP) Scand J Clin Lab Invest. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Rozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Yared MN, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone and Mineral Research. 2000;15(9):1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- Sedrani KM, Elidrissy AWTH, ElArabi KM. Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr. 1983;38:129–132. doi: 10.1093/ajcn/38.1.129. [DOI] [PubMed] [Google Scholar]
- Hobbs RD, Habib Z, Alromaihi D, Ldi L, Parikh N, Blocki F, Rao DS. Severe vitamin D deficiency in Arab-American women living in Dearborn, Michigan. Endocr Pract. 2009;15(1):35–40. doi: 10.4158/EP.15.1.35. [DOI] [PubMed] [Google Scholar]
- International Physical Activity Questionnaire (IPAQ) Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005. http://www.ipaq.ki.se/scoring.pdf. Accessed at 2010.
- Kuczmarski R, Ogden C, Guo S. CDC growth charts for the United States: methods and development. Vital and Health Statistics. 2002;246:1–190. Series 11. Data from the national health survey. [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. Metabolic Syndrome, a new world-wide definition, a Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on. High blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576. [PubMed] [Google Scholar]
- Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(suppl):1706S–1709S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- Gordon CM, DePeter KC, Feldman HA. Prevalence of vitamin D defiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose–response and trend analysis. Epidemiology. 1995;6(4):450–454. doi: 10.1097/00001648-199507000-00025. [DOI] [PubMed] [Google Scholar]
- Kumar J, Muntner P, Kaskel FJ, Hailpern SM. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the,Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clinic Nutr 2011. 2011;94(1):128–135. doi: 10.3945/ajcn.111.013268. [DOI] [PubMed] [Google Scholar]
- Bener A, Ali M, Hoffmann GF. High prevalence of vitamin D deficiency in young children in a highly sunny humid country: a global health problem. Minerva Pediatrica. 2009;61(1):15–22. [PubMed] [Google Scholar]
- Tsur A. Effect of different dress style on Vitamin D levels in healthy young Orthodox and Ultra-Orthodox students in Israel. Osteoporos. 2010. [DOI] [PubMed]
- Hatun S, Islam M, Cizmecioglu F, Kara B, Babaoglu K, Berk F, Sevim A. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218–222. doi: 10.1093/jn/135.2.218. [DOI] [PubMed] [Google Scholar]
- Mallah EM, Hamad MF, El Manaseer MA, Qinna NA NA, Idkaidek NM, Arafat TA, Matalka KZ. Plasma concentration of 25-hydroxyvitamin D among Jordanians: effect of biological and habitual factors on vitamin D status. BMC Clin Pathol. 2011;11:8. doi: 10.1186/1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laleye LC, Kerkadi AH, Wasesa AA, Rao MV, Aboubacar A. Assessment of vitamin D and vitamin A intake by female students at the United Arab Emirates University based on self-reported dietary and selected fortified food consumption. Ann Trop Paediatr. 2011;2(1):39–44. doi: 10.3109/09637486.2010.533159. [DOI] [PubMed] [Google Scholar]
- Chen TC, Heath H, Holic MF. An update on the vitamin D content of fortified milk from the United States and Canada. N Engl J Med. 1993;329:1507. doi: 10.1056/NEJM199311113292021. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Agarwal M, Hossain M, Kochyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in Summer: Adjustification for vitamin D supplementation of breat-feeding infants. J Pediatr. 2003;142:169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Sw NG, Zaghloul S, Ali H, Harrison G, Yeatts K, Sadig ME, Popkin BM. Nutrition in the United Arab Emirates. Clinical Nutrition: European J; 2011. pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SA, Vinyard BT. Fast food consumption of US adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr. 2004;23(2):163–168. doi: 10.1080/07315724.2004.10719357. [DOI] [PubMed] [Google Scholar]
- Abrams SA, Hawthrone KM, Chen Z. Supplementation with 1000 IU vitamin D leads to parathyroid hormone suppression, but not increased fractional calcium absorption in 4 to 8 years old children: a double blind randomized controlled trial. Am J Clin Nutri. 2012. [DOI] [PMC free article] [PubMed]
- Sedrani SH, Elidrissy ATH, El-Arabi KM. Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr. 1993;38:129–132. doi: 10.1093/ajcn/38.1.129. [DOI] [PubMed] [Google Scholar]