Abstract
Background
The European spruce bark beetle, Ips typographus, and the North American mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae), are severe pests of coniferous forests. Both bark beetle species utilize aggregation pheromones to coordinate mass-attacks on host trees, while odorants from host and non-host trees modulate the pheromone response. Thus, the bark beetle olfactory sense is of utmost importance for fitness. However, information on the genes underlying olfactory detection has been lacking in bark beetles and is limited in Coleoptera. We assembled antennal transcriptomes from next-generation sequencing of I. typographus and D. ponderosae to identify members of the major chemosensory multi-gene families.
Results
Gene ontology (GO) annotation indicated that the relative abundance of transcripts associated with specific GO terms was highly similar in the two species. Transcripts with terms related to olfactory function were found in both species. Focusing on the chemosensory gene families, we identified 15 putative odorant binding proteins (OBP), 6 chemosensory proteins (CSP), 3 sensory neuron membrane proteins (SNMP), 43 odorant receptors (OR), 6 gustatory receptors (GR), and 7 ionotropic receptors (IR) in I. typographus; and 31 putative OBPs, 11 CSPs, 3 SNMPs, 49 ORs, 2 GRs, and 15 IRs in D. ponderosae. Predicted protein sequences were compared with counterparts in the flour beetle, Tribolium castaneum, the cerambycid beetle, Megacyllene caryae, and the fruit fly, Drosophila melanogaster. The most notable result was found among the ORs, for which large bark beetle-specific expansions were found. However, some clades contained receptors from all four beetle species, indicating a degree of conservation among some coleopteran OR lineages. Putative GRs for carbon dioxide and orthologues for the conserved antennal IRs were included in the identified receptor sets.
Conclusions
The protein families important for chemoreception have now been identified in three coleopteran species (four species for the ORs). Thus, this study allows for improved evolutionary analyses of coleopteran olfaction. Identification of these proteins in two of the most destructive forest pests, sharing many semiochemicals, is especially important as they might represent novel targets for population control.
Keywords: Ips typographus, Dendroctonus ponderosae, Gene ontology, Transcriptome, Odorant receptor, Ionotropic receptor, Gustatory receptor, Odorant binding protein, Chemosensory Protein, Sensory neuron membrane protein
Background
The European spruce bark beetle, Ips typographus L., and the North American mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae: Scolytinae) are serious pests of coniferous forests. I. typographus mainly attacks Norway spruce (Picea abies) in Eurasia, whereas D. ponderosae infests several species of pine in western North America. Currently, large-scale D. ponderosae outbreaks have resulted in unprecedented economic losses and turned North American forests into major sources of carbon release [1]. The olfactory sense drives bark beetle behaviors that are important for fitness, such as the localization of suitable hosts and mates [2]. In the search for suitable host material, bark beetles respond to volatiles that emanate from both host and non-host plants [3-5]. However, most individuals locate trees by means of an aggregation pheromone that is released by beetles that have already attacked the tree. This signal is responsible for coordinated mass-attacks, which often lead to the death of the host tree [2] and large-scale forest destruction. Due to their ecological and economic impact, an extensive knowledge base on bark beetle chemical ecology and olfactory physiology has been established [2,3,6,7]. However, information on the molecular aspects of odor detection has been lacking until now.
In insects, volatile molecules are detected by olfactory sensory neurons (OSNs) that are housed within special structures (sensilla) predominantly on the antennae, and to a lesser extent on the maxillary palps. The cell membrane of OSNs contains receptor proteins that bind odor ligands [8,9]. The binding of a ligand to a receptor protein is the key event in olfactory transduction, as it converts a chemical signal in the environment into an electrical signal that can be interpreted by the insect nervous system [10].
Receptors from three large and divergent multigene families are expressed in insect OSNs [9,11-13], namely the odorant receptors (OR), ionotropic receptors (IR), and gustatory receptors (GR), the latter group notably containing carbon dioxide-detecting receptors [14,15]. However, most GRs are expressed in gustatory receptor neurons in taste organs and are involved in contact chemoreception. These GRs typically detect different sugars, bitter compounds, and contact pheromones [12].
Insect ORs are seven-transmembrane domain proteins [16,17] with a reversed membrane topology (intracellular N-terminus) compared to vertebrate ORs, which are G-protein coupled receptors [18]. Insect ORs and GRs are distantly related members of the same superfamily [19]. In general, ORs (and GRs) show little sequence homology to each other and they are unrelated to vertebrate ORs. The conventional exchangeable OR that determines ligand specificity [20] forms heteromers of unknown stoichiometry with a conserved co-receptor, known as Orco [21]. Orco is ubiquitously expressed in OSNs that express ORs [22,23] and necessary for olfactory responses and for localization of the conventional OR in the cell membrane [18,24]. Putative insect ORs have been identified mainly in species with sequenced genomes [8,25]. Recently, however, studies on antennal transcriptomes have led to the identification of OR sets in several moth species [26-28] and one beetle [29]. The ORs respond to a variety of volatile chemicals [20,30], including pheromones [31] and plant or microbe-derived compounds [32]. Some ORs are highly defined in their response specificity [32], whereas others appear more broadly tuned, especially at high stimulus concentrations [20].
IRs were recently discovered as another class of receptors involved in chemoreception [9]. They are related to ionotropic glutamate receptors (iGluRs) that function in synapse communication, but have atypical binding domains. IRs have been identified throughout protostome lineages (including arthropods, mollusks, annelids and nematodes) and, thus, constitute a far more ancient group of receptors than the ORs [33]. IRs form complexes with up to three subunits, including odor-specific receptors and one or two broadly expressed co-receptors [34]. In insects, the IRs are divided into two major groups: the “antennal IRs” that have an olfactory function and are conserved across insect orders, and the species-specific “divergent IRs”, some of which have been assigned a tentative role in taste [33]. Antennal IRs in Drosophila have different odor specificity compared to the ORs and respond to nitrogen-containing compounds (e.g. ammonia and amines), acids, and aromatics (i.e. phenylacetaldehyde) [34].
In addition to the receptor genes, other multigene families encode proteins with critical roles in olfaction. Odorant binding proteins (OBP) are small soluble proteins (generally 135–220 amino acids long) with two or three disulfide bridges [35,36]. OBPs are highly abundant in the sensillar lymph of insects and are thought to solubilize hydrophobic molecules and deliver them to the receptors [35]. Studies have shown conflicting results whether or not OBPs affect the response specificity of OSNs [37]. At least in some studies, the specificity of pheromone receptors was improved by the presence of OBPs (i.e. a class of OBPs called pheromone binding proteins, PBPs) [38,39]. Some evidence suggests that OBPs might undergo odor-induced conformational changes, with a change in the OBP itself triggering the response of the OSN [40]. In insects with sequenced genomes, the number of OBP coding genes normally ranges from ca. 40–60 [35].
Chemosensory proteins (CSP) constitute another class of small binding proteins (ca. 130 amino acids long). They are more conserved than OBPs and are characterized by the presence of 4 cysteines that form two disulfide bridges [41]. CSPs may have shared a common ancestor with the OBPs near the origin of the arthropods [42]. Like OBPs, CSPs are present in high concentration in chemosensory sensilla (outer sensillum lymph [43,44]). However, the majority of them are also expressed in various non-sensory tissues [45] and they seem to play a role in development, moulting [45], and leg regeneration [46]. Some CSPs bind pheromone compounds [41,47], but their exact role in chemosensory systems remains uncertain [35,48]. Most Drosophila genomes contain only 4 CSP coding genes and T. castaneum has 20 [35]. The genome of Aedes aegypti mosquitoes contains 43 members of this family, the largest number found in insects so far [49].
Finally, the sensory neuron membrane proteins (SNMP) are proteins of the CD36 family that associate with pheromone-responding OSNs [50]. Their functional significance is still poorly understood, but SNMP is crucial for proper pheromone detection in D. melanogaster and also required for activation of a pheromone receptor in Heliothis virescens moths [51]. In contrast, SNMP was dispensable for responses of a fly receptor (DmelOR22a) to fruit-related esters [51]. Insects generally have two representatives of SNMPs (SNMP1 and SNMP2), although copy numbers of each orthologue seem to vary somewhat across species [52].
In the largest insect order, Coleoptera, ORs have been identified from only two species: from the genome of the red flour beetle, Tribolium castaneum[53], and recently from the antennal transcriptome of the cerambycid beetle, Megacyllene caryae[29]. Members of the other olfactory gene families have been identified only in T. castaneum[33,35,52-54]. Consequently, additional beetle species need to be investigated to reach a better understanding on the molecular biology of coleopteran and insect olfaction.
In this study, we assembled and analyzed bark beetle antennal transcriptomes from next-generation sequencing. We report the results from gene ontology (GO) annotation as well as sets of putative OBPs, CSPs, SNMPs, ORs, GRs, and IRs in I. typographus and D. ponderosae. Identification of the chemosensory genes in these devastating insect pests is especially relevant because of their potential as novel targets for pest control.
Methods
Mountain pine beetle
The source of D. ponderosae (obtained from lodgepole pine, Pinus contorta) antennal tissue and the method of sequencing have been reported previously in a larger transcriptome study [55]. From this transcriptome dataset originating from several tissue types, sequences originating only from a non-normalized antenna-specific cDNA library were re-assembled for the analyses presented in the present study. This included 12,142 paired-end Sanger reads, 1,147 single-end Sanger reads and 1,048,708 Roche 454 reads. Newbler (version 2.6, Roche) was used for assembly using the "-cdna" switch for transcriptome assemblies and a 45 bp cutoff to eliminate short reads. For identification of OBPs, CSPs, and SNMPs in D. ponderosae we also used a combination of Sanger-specific (CAP3 assembly) data and transcriptome assemblies from other tissues and life stages, since these proteins may have sensory or non-sensory functions in non-antennal tissues. We did not have such assemblies for I. typographus.
European spruce bark beetle
Insects, RNA extraction and cDNA synthesis
I. typographus was reared on Norway spruce (Picea abies) logs in an environmental chamber (25°C, 70% RH, 20:4 L:D photoperiod) [56], starting from individuals collected from their natural habitat near Asa and Älmhult, southern Sweden. Emerged adults were kept in a state of low activity in a refrigerator (1–5°C) before being used for RNA extraction.
Two hundred adult I. typographus were collected in a 50 ml plastic tube, approximately two weeks after their emergence. The tube was submerged in liquid nitrogen, after which it was vigorously shaken using a vortex shaker to separate extremities from the body. Body parts were suspended in -20°C acetone (>99%, Fisher Scientific AB, Sweden) and passed through meshes that filtered out the antennae. After removal of the acetone, 0.6 ml TRI reagent (Ambion) was added to the antennae and the sample was homogenized using a Tissue-tearor (model 98370–365, Bartlesville, OK, USA). Total RNA was extracted following the TRIZOL protocol, but using 1-bromo-3-chloropropane instead of chloroform. 1.7 μg total RNA was sent to Evrogen (Moscow, Russia) for synthesis of duplex-specific-nuclease normalized cDNA [57,58].
Sequencing and assembly
The I. typographus cDNA was sequenced at LGC Genomics (Berlin, Germany), using 454 GS/FLX sequencing (Roche Applied Science, Mannheim, Germany) with titanium chemistry, to produce 350,000 reads for a total of 114 megabases. Furthermore, Illumina sequencing was performed at the Max Planck Institute for Molecular Genetics in Berlin to generate a further 3.6 million reads for a total of 122 megabases.
Short or low-quality reads, as well as linker and adapter sequences were removed by the Crossmatch program (454 reads, Incogen Inc., Williamsburg, VA, USA) or by the built-in sequence cleanup of Seqman Ngen (Illumina reads, DNAStar, Madison, WI, USA). The 454 reads were assembled using Seqman Ngen to generate a backbone; subsequently, the Illumina reads were mapped onto this backbone using Seqman Ngen to correct for technology-inherent read errors. The resultant contigs were annotated using a Codequest Workstation (Timelogic/Active Motif, Carlsbad, CA) [27].
Annotation
For an initial assessment of the two assembled beetle antennal transcriptomes, gene ontology (GO) annotation was performed using Blast2GO [59,60]. Blast2GO annotation associates genes or transcripts with GO terms using hierarchical vocabularies. Genes are described in terms related to molecular function, biological process, or cellular component, allowing for meta-analyses of gene populations [27,61].
The BLAST step was performed with a lenient E-value cutoff at 0.1 to account for the high sequence variability among the olfactory gene families. The mapping step was done using default settings, whereas a lenient E-value (0.1) and lower annotation cut-off (55) and GO-weight (5) were used in the first annotation step to increase the proportion of annotated transcripts. Annotation was further enhanced by merging annotation with results of InterProScan database search at the EBI [62], ANNEX procedure, and the Blast2GO validation step. A subsequent GO-slim step was not used, as this procedure removed the low frequency odorant protein families from the annotation.
For annotation of ORs, IRs, GRs, OBPs, CSPs, and SNMPs in I. typographus and D. ponderosae, contigs were analyzed with tBLASTx searches against custom-made databases and the non-redundant nucleotide collection at NCBI. Additionally, HMM-based searches of the PANTHER database of domain family profiles were done. We identified non-redundant translated proteins with reciprocal BLAST using the comprehensive datasets available for OBPs and CSPs [42], as well as SNMPs [52].
For contigs/isotigs with hits against genes of interest, open reading frames were identified and the annotation verified by additional BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searches. Contigs containing suspected sequencing errors (mainly insertions/deletions in homopolymer regions) were edited manually after identifying miss-assemblies through manual inspection of the assembly files, ESTs, or genomic data (D. ponderosae) [63]. The suffix “FIX” was added to the gene name of such edited sequences, and also to those extended by RACE-PCR (below).
TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) was used to predict transmembrane domains of candidate ORs, IRs, and GRs. For all proteins studied, amino acid sequences were aligned using MAFFT [64], and maximum-likelihood analysis and dendrogram construction were subsequently performed with FastTree [65]. Dendrograms were colored and arranged in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). To ensure that sequences corresponded to unigenes (and not to fragments of the same gene), only those that showed sufficient overlap in multiple sequence alignments were included in the analysis. In addition, for contigs that shared >98.5% amino acid identity only one “copy” (the contig with the longest ORF) was included. I. typographus 454- and Illumina sequences have been submitted to EBI (project accession number ERP001792). The D. ponderosae antennal Sanger and 454 sequence data have previously been submitted to NCBI (accession numbers GT344964-GT358252 and SRX132062, respectively). All bark beetle contigs/isotigs have been submitted to the Transcriptome Shotgun Assembly (TSA) sequence database at NCBI (accession numbers GACR00000000 and GABX00000000 for I. typographus and D. ponderosae, respectively) or to GenBank (D. ponderosae genes with representative full-length cDNA clones) (see Additional file 1 for accession numbers for the individual olfactory genes).
RACE-PCR
The assembled contigs from the 454- and Illumina sequencing of the Ips transcriptome did not always constitute full-length transcripts. Therefore, for better resolution of phylogenetic analyses, some sequences encoding putative ORs were elongated using RACE-PCR (Rapid Amplification of cDNA Ends; SMARTer cDNA amplification kit, Clontech) with a nested protocol following the manufacturer’s instructions. Total RNA from 300 adult beetle antennae (extracted using RNeasy MiniKit, Qiagen) was used as template to generate RACE-ready cDNA. Primer design was performed manually, but aided with Tm-calculations and self-complementarity checks using Oligo Calc (http://www.basic.northwestern.edu/biotools/OligoCalc.html). Amplified and extended DNA was cloned (TOPO TA cloning kit dual promoter, PCRII®-TOPO® vector, Invitrogen) before being sequenced (Eurofins MWG Operon, Ebersberg, Germany).
Results
Assembly
The D. ponderosae antenna-specific assembly resulted in 19,523 isotigs from 15,736 isogroups and 19,343 singletons, of which 48 were Sanger reads. The isotigs assembled by Newbler were comparable with the contigs generated by other assemblers, with the exception that Newbler also considers alternative splice variants when producing the isotigs, and these are grouped into different isogroups. The N50 was 1,864 bp and the largest isotig was 8,483 bp. The I. typographus assembly resulted in 20,298 contigs with an N50 of 717 bp. The largest contig was 3,389 bp.
Gene ontology annotation
GO annotation indicated that the analyzed antennal transcriptomes of the two bark beetle species were highly similar with respect to GO terms (Figure 1, Additional file 2). In I. typographus, 8,713 contigs (43%) were associated with GO terms. In D. ponderosae, this number was 10,713 (55%). Thus, a substantial proportion of contigs in both species was not associated with any GO term, and possibly these contigs represent orphan genes. Among the annotated contigs, GO terms related to basic cell functions were the most abundant; however, contigs with GO terms related to olfaction were also present, such as “odorant binding”, “signal transducer activity” (Figure 1A), and “response to stimulus” (Figure 1B). Contigs with GO terms associated with enzymatic activity were well represented, such as “hydrolase activity” and “transferase activity” (Figure 1A).
Figure 1.
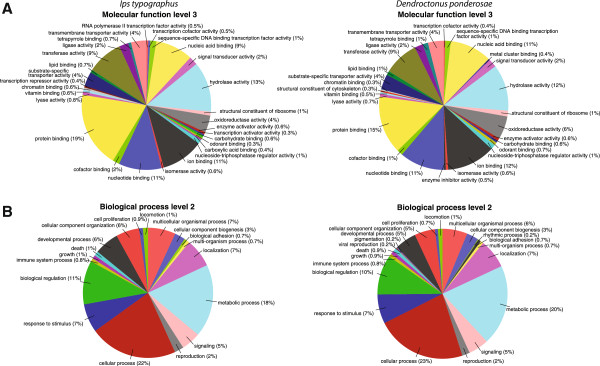
Gene ontology (GO) results. GO analysis corresponding to 8,713 contig sequences in Ips typographus and 10,713 isotigs in Dendroctonus ponderosae, as predicted for their involvement in A) molecular function (level 3 GO categorization) and B) biological process (level 2). For results presented as detailed bar diagrams, see Additional file 2.
Nonreceptor olfactory gene families
We identified 15 transcripts encoding putative OBPs in I. typographus, and 31 transcripts in D. ponderosae. All but five transcripts (ItypOBP1, 8, 9, 11, and 13) corresponded to full-length genes. One third of the transcripts identified in D. ponderosae were not found in the antennal cDNA library, but rather in the cDNA libraries from other body parts (Additional file 3).
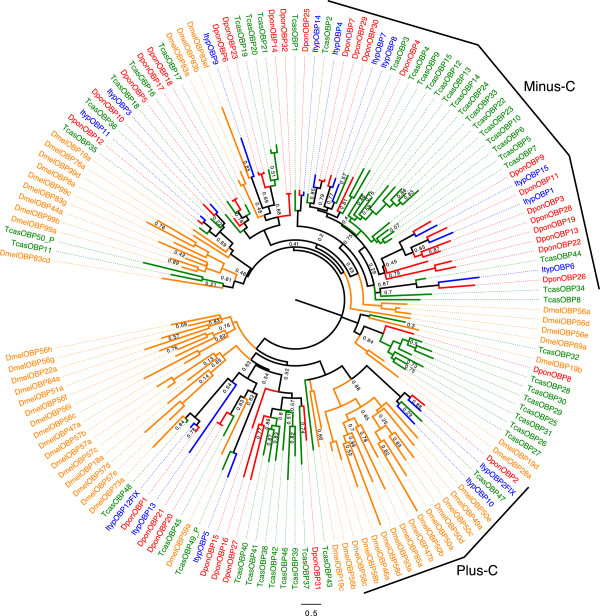
In general, OBPs can be classified into different phylogenetic groups. Classic OBPs are characterized by 6 cysteine residues at conserved positions. The Plus-C class has 4–6 additional cysteines and one characteristic proline, whereas the Minus-C class has lost cysteine residues, generally C2 and C5 [35,66]. In our sequence similarity dendrogram, the classic bark beetle OBPs were spread out on various branches (Figure 2) where they generally formed small subgroups together with OBPs mostly from T. castaneum.
Figure 2.
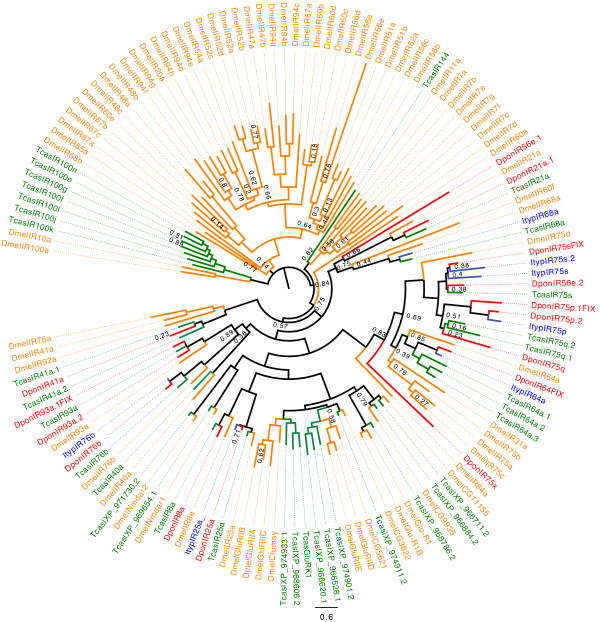
Maximum-likelihood dendrogram based on protein sequences of candidate odorant binding proteins (OBPs). Included are OBPs from Ips typographus (Ityp), Dendroctonus ponderosae (Dpon), Tribolium castaneum (Tcas) and Drosophila melanogaster (Dmel). One major beetle-specific expansion of Minus-C OBPs is evident. Bark beetle proteins in this expansion have lost cysteine residue C2 and C5. Two OBPs in I. typographus and one in D. ponderosae belong to the Plus-C group characterized by additional cysteine residues. Numbers refer to support values, which are only displayed when <; 0.9.
Two OBPs in I. typographus (ItypOBP2 and ItypOBP10) and one OBP in D. ponderosae (DponOBP2) were of the Plus-C type and were grouped together with the Plus-C OBP (TcasOBP47) from T. castaneum (Figure 2). ItypOBP2 and DponOBP2 shared 45% amino acid identity. Members of the Minus-C class, i.e. 12 DponOBPs, 6 ItypOBPs, and 18 TcasOBPs, formed a large clade (Figure 2). Within this clade, we found a bark beetle-specific expansion, containing ItypOBP1, ItypOBP15, DponOBP3, DponOBP9, DponOBP11, DponOBP13, DponOBP19, DponOBP22, and DponOBP28. All bark beetle full-length Minus-C OBPs had lost C2 and C5.
Six bark beetle OBP orthologous pairs shared >50% amino acid identity between species (ItypOBP14/DponOBP25: 78%; ItypOBP11/DponOBP12: 76%; ItypOBP12FIX/DponOBP1: 74%; ItypOBP7/DponOBP30: 60%; ItypOBP4/DponOBP7: 57%; ItypOBP3/DponOBP10: 51%). There were several OBP pairs with high amino acid identity in D. ponderosae (DponOBP14/DponOBP32: 98% identity; DponOBP20/DponOBP21: 97%; DponOBP17/DponOBP18: 96%; DponOBP7/DponOBP29: 91%).
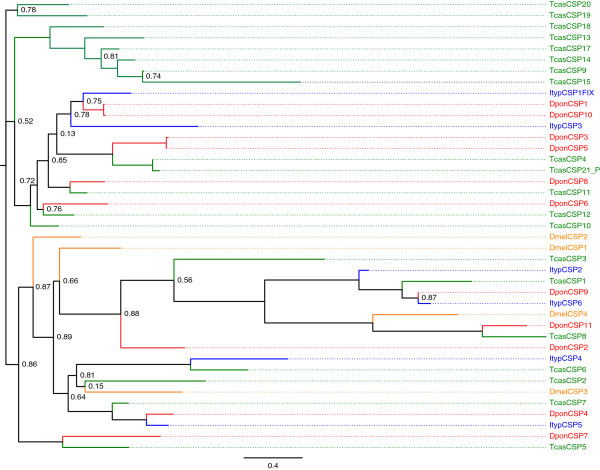
We identified 6 transcripts encoding putative CSPs in I. typographus, and 11 transcripts in D. ponderosae (Figure 3). Five of the transcripts encoded partial proteins (ItypCSP1-4 and 6, of which 1 and 4 lacked only a few C-terminal amino acids), whereas all the others represented full-length genes. Four of the transcripts identified in D. ponderosae were not found in the antennal cDNA library, but rather in the cDNA libraries from other body parts (Additional file 4). The bark beetle CSPs were present on different branches throughout the dendrogram (Figure 3), and no major bark beetle-specific expansion of CSP lineages was evident. Amino acid identity among candidate simple orthologues in the two bark beetles was high (e.g. ItypCSP6/DponCSP9: 91%; ItypCSP5/DponCSP4: 75%; ItypCSP1FIX/DponCSP1: 64%). Two CSP pairs in D. ponderosae (DponCSP3/DponCSP5 and DponCSP1/DponCSP10) had the highest (98%) amino acid identity.
Figure 3.
Maximum-likelihood dendrogram based on protein sequences of candidate chemosensory proteins (CSPs). Included are CSPs from Ips typographus (Ityp), Dendroctonus ponderosae (Dpon), Tribolium castaneum (Tcas) and Drosophila melanogaster (Dmel). Numbers refer to support values, which are only displayed when <; 0.9.
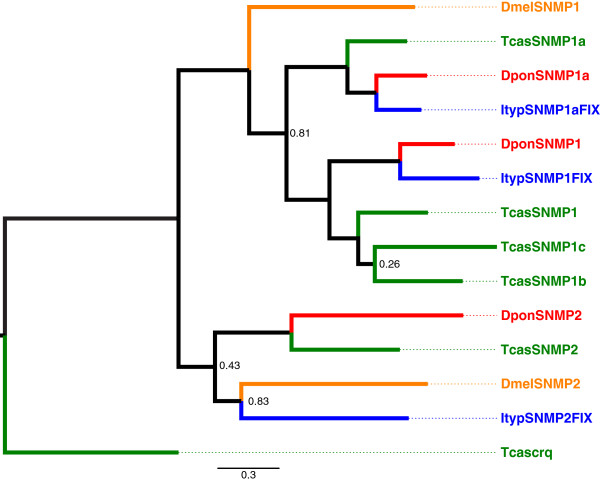
In each bark beetle species, we found two orthologues of SNMP1 (SNMP1 and SNMP1a), and one orthologue of SNMP2 (Figure 4). ItypSNMP1a was present only as a fragment, whereas transcripts for the others likely represented full-length or very close to full-length genes. The bark beetle SNMPs grouped together with orthologues in T. castaneum, with the exception of ItypSNMP2 that paired up with SNMP2 in D. melanogaster. SNMP1 and SNMP1a appeared more conserved across the two bark beetles with 58% and 66% amino acid identity, respectively, compared to the SNMP2 orthologues that shared 28% identity.
Figure 4.
Maximum-likelihood dendrogram based on protein sequences of candidate sensory neuron membrane proteins (SNMPs). Included are SNMPs from Ips typographus (Ityp), Dendroctonus ponderosae (Dpon), Tribolium castaneum (Tcas) and Drosophila melanogaster (Dmel). The T. castaneum orthologue of Croqemort, a non-SNMP member of the CD36 family, was used as outgroup to root the tree. Numbers refer to support values, which are only displayed when <; 0.9.
Receptor encoding genes
Odorant receptors
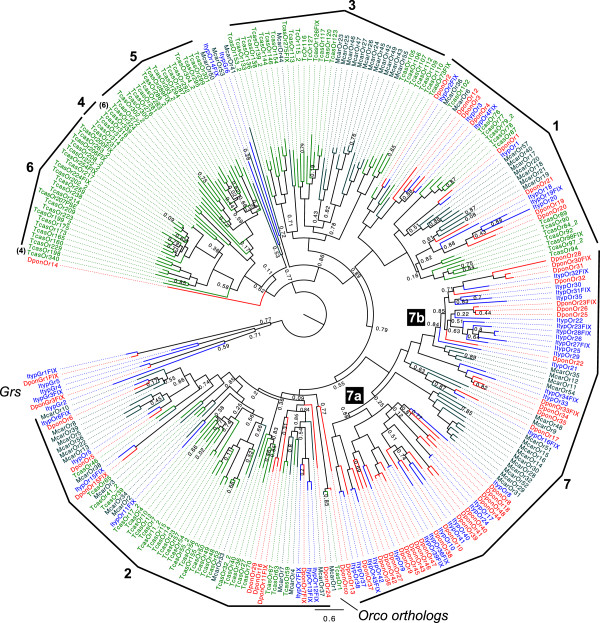
Similar numbers of putative OR encoding transcripts were annotated in the two bark beetle species. We identified 43 OR candidates in I. typographus. Eleven of these were likely representing full-length genes, encoding proteins with more than 374 amino acids. Partial transcripts encoding ItypOR6, 7, 12, 13, 19, 31, 36, and 43 were extended by 3’-RACE-PCR. In D. ponderosae, the number of candidate OR transcripts was 49, and the number of full-length candidates was 27. In addition, 4 short partial transcripts in I. typographus and 6 in D. ponderosae were left unlabeled and excluded from analysis, since unigene identity could not be conclusively confirmed. The shortest partial OR candidate included was ItypOR38 (62 amino acids). Two pairs of receptors, i.e. ItypOR17 and ItypOR24, as well as ItypOR36 and ItypOR39, showed the highest amino acid identity (98% for both pairs).
Sequences of the bark beetle ORs were compared with those of M. caryae and T. castaneum. For the latter species we included only those ORs with confirmed expression in the adult head [53]. Several OR subgroups of various size and content could be distinguished (Figure 5). In order to standardize the numbering of coleopteran OR subfamilies, we numbered these subgroups from 1 to 7 according to previous studies [29,53]. The majority (59 ORs, 64%) of bark beetle ORs were present within group 7, which also contained 16 ORs from M. caryae, but no ORs from T. castaneum. Fifty-one of these bark beetle ORs formed two subgroups (group 7a and 7b, with 31 and 20 ORs, respectively) that were completely devoid of receptors from the other two beetle species. However, considering only the bark beetle ORs, only minor species-specific subgroups (containing 3–5 ORs) could be seen and they were found within group 7a and 7b (Figure 5). In addition to the bark beetle specific subgroups, group 7 also contained two M. caryae-specific subgroups (with 9 and 4 McarORs, respectively) that each formed a sister group to either of the two bark beetle specific subgroups. Finally, a fifth subgroup within group 7 contained ORs from all three species, indicating conservation of some OR sequences among the three xylophagous species. The complete lack of T. castaneum receptors within group 7, and the presence of specific subgroups in the other species, indicate broad expansions of OR lineages in bark beetles and cerambycids and/or losses of corresponding OR lineages in T. castaneum.
Figure 5.
Maximum-likelihood dendrogram based on protein sequences of candidate odorant receptors (ORs) and gustatory receptors (GRs). Included are ORs and GRs from Ips typographus (Ityp), Dendroctonus ponderosae (Dpon), Tribolium castaneum (Tcas) and Megacyllene caryae (Mcar). The branch containing bark beetle GRs was used as outgroup to root the tree. The different subgroups (numbered 1–7 according to [29,53], and 7a-7b) are discussed in the main text. Originally, TcasOr339 and TcasOr340 were found within group 4 and 6 [53], as indicated here by the numbers in brackets. Numbers at nodes refer to support values, which are only displayed when <; 0.9.
Expansions of OR lineages were also seen in T. castaneum. Forty-five (41%) TcasORs formed a large group that was exclusive to the flour beetle. Within this group, the previously defined coleopteran OR subgroups 4–6 could be found [53]. These subgroups were collectively rooted by a smaller clade containing receptors from I. typographus and M. caryae.
Receptor group 3 contained ORs only from T. castaneum and M. caryae. The lack of bark beetle ORs in this group suggested that these OR lineages have been lost in bark beetles (or not represented in our transcriptome assemblies), while retained in cerambycids. Within group 3, subgroups that were specific for T. castaneum or specific for M. caryae could be found.
The dendrogram also contained two groups (1 and 2) with OR representatives from all four species (although group 2 was dominated by expanded T. castaneum lineages). We found most of the candidate 1:1 orthologous relationships among the bark beetle ORs within groups 1 and 2 (and a few in subgroup 7a) (e.g. Ityp/DponOR1-10, 15). For these candidate orthologous pairs, amino acid identity was 54–69% (excluding fragments <;100 amino acids, i.e. OR4 and OR8). The Orco orthologues rooted group 2.
The co-receptor Orco was identified in the antenna-specific assembly of D. ponderosae, but surprisingly not in the antennal transcriptome assembly of I. typographus. However, by using PCR with primers designed from a conserved region close to the C-terminus of the DponOrco, we amplified a 62 amino acid fragment of Orco from I. typographus antennal-specific cDNA. This ItypOrco fragment shared 97% amino acid identity with DponOrco. As expected, Orco in D. ponderosae shared high amino acid identity with Orco orthologues in M. caryae (McarOR1, 85% identity) and T. castaneum (TcasOR1, 82 % identity).
Gustatory receptors
Six candidate GR-encoding transcripts were identified (Figure 5) in I. typographus, including putative conserved carbon dioxide receptors (GR1-3) [15]. Two GR candidates (GR1 and GR3) were identified in D. ponderosae. Interestingly, GR2 was not found in our D. ponderosae antenna-specific assembly, but was recovered from the draft genome [63] and from larval RNAseq data.
GR6 in I. typographus could tentatively be assigned to the trehalose receptor 1 in T. castaneum (67% amino acid identity). Since GRs and ORs are members of the same superfamily, both were included in the same dendrogram analysis, in which GRs formed a distinct clade (Figure 5). All GRs except for ItypGR6 grouped within this clade.
Ionotropic receptors
We identified 7 transcripts for putative ionotropic receptors in I. typographus, and 15 transcripts in D. ponderosae (Figure 6). We found bark beetle orthologues for all 10 conserved antennal IRs with representatives in T. castaneum[33]. However, we did not find all of them in both species. In D. ponderosae, we identified candidates for IR21a, IR41a, IR64a, IR76b, IR93a (a1 and a2), five members of the IR75 group, as well as the co-receptors IR25a and IR8a. Transcripts for DponIR25a, DponIR8a, DponIR75p.1FIX, DponIR75p.2, DponIR75q, and DponIR76b likely corresponded to full-length genes (or very close to), whereas all the other identified IRs were represented as partial genes. Candidate IR fragments located on 8 isotigs in D. ponderosae were discarded from our dendrogram analysis, as they were too short to confidently assign them unigene status. However, among these, two fragments (both 99 amino acids long) shared 72%, and 69% amino acid identity with TcasIR40a and TcasIR68a, respectively. Thus, in D. ponderosae it seems like orthologues for all conserved antennal IRs found in T. castaneum[33] were present. In contrast, we identified candidates only for IR25a, IR64a, IR68a, IR76b, and three IR75 members in I. typographus. Thus, several orthologues found in D. ponderosae and T. castaneum were lacking in the I. typographus assembly. IR8a, which is a broadly expressed co-receptor, necessary for odor responses [34] and present in all insects studied to date [33], was one of the receptors lacking in I. typographus.
Figure 6.
Maximum-likelihood dendrogram based on protein sequences of candidate ionotropic receptors (IRs). Included are IRs from Ips typographus (Ityp), Dendroctonus ponderosae (Dpon), Tribolium castaneum (Tcas) and Drosophila melanogaster (Dmel). Numbers refer to support values, which are only displayed when <; 0.9.
Discussion
The gene sets reported here represent significant additions to the pool of identified olfactory genes in Coleoptera. Prior to this study, members of the major chemosensory gene families in Coleoptera had been identified only from the genome of T. castaneum (except for the ORs also identified in the antennal transcriptome of the cerambycid, M. caryae). Additionally, as the genes identified here underlie the aggregation behavior that results in tree killing by mass-attack, they represent novel targets for management programs of two of the world’s most destructive forest pests.
In general, we identified somewhat larger numbers of transcripts encoding putative olfactory proteins (i.e. ORs, IRs, OBPs, and CSPs) in D. ponderosae than in I. typographus. The greater depth of the 454-sequencing and the access to Sanger data for D. ponderosae likely account for this difference. In addition, duplex-specific nuclease cDNA normalization (performed only for I. typographus) seems to result in overrepresentation of shorter full-length transcripts, which might explain the lower number of OR and IR transcripts identified in I. typographus, and also the absence of Orco transcripts in the transcriptome assembly. However, despite the slight difference in methodology, the GO annotation demonstrated a remarkable overall similarity in the types of genes (with respect to associated GO terms) that are expressed in the antennae of the two species. GO annotation was previously conducted for the antennal transcriptome of Manduca sexta moths by Grosse-Wilde et al. [27], and comparison with their data reveals a striking similarity to the bark beetles analyzed here. Indeed, several GO terms with putative relation to olfactory function showed identical (or near identical) relative abundance, suggesting a kind of across-order conservation of gene expression patterns in antennae, although the data say nothing about expression levels of the individual genes themselves.
Odorants are thought to interact with OBPs or CSPs in the sensillum lymph prior to the ligand-receptor interaction. The numbers of OBPs identified in the bark beetles (15 in I. typographus and 31 in D. ponderosae) are clearly lower than the 49 OBP-encoding genes reported in the genome of T. castaneum[54]. The same is true for the CSPs, for which we identified 6 transcripts in I. typographus and 11 in D. ponderosae compared with 20 putative CSP encoding genes in the T. castaneum genome [35]. However, it might be misleading to compare the number of genes identified in a genome with the number of transcripts in a specific tissue at a specific life stage. Some of the genes might, for instance, be expressed only in the larva [53,67]. Indeed, many of the identified OBPs and CSPs (ca. one third of the transcripts) in D. ponderosae were not identified from the antennal library, but seem to be expressed only in non-olfactory tissue. Similar patterns have been found also in other insects, suggesting that these proteins may have physiological functions independent of olfaction [41,45,68].
SNMPs are associated with pheromone-responsive OSNs in Lepidoptera and Diptera [50,51]. In D. melanogaster, SNMP1 was shown to be necessary for proper OSN responses to the pheromone compound cis-vaccenyl acetate, but not for OSN responses to food-related fruit esters [51]. Benton et al. [51] also demonstrated that SNMP was required for activation of Heliothis virescens (Lepidoptera) pheromone receptor HR13 by its corresponding ligand when heterologously expressed in Drosophila neurons. It was suggested that the hydrophobic tail of the fatty-acid derived dipteran and lepidopteran pheromone molecules necessitates the presence of SNMP. If so, that raises the question why bark beetles that do not use pheromone compounds with long hydrophobic tails [2] express SNMPs in their antennae.
The numbers of putative OR-encoding transcripts identified in the two bark beetles (43 in I. typographus and 49 in D. ponderosae) are close to the number reported in the antennal transcriptome of M. caryae (57 ORs) [29], but lower than the number expressed in the head of adult T. castaneum (111 ORs), and much lower than the number in the T. castaneum genome (341 OR-encoding genes, including 79 pseudogenes) [53]. In other insects, the number of seemingly intact OR-encoding genes identified from genomes is highly variable [25], ranging from only 10 in the human body louse, Pediculus humanus[69], to ca. 300 in the fire ant, Solenopsis invicta[70]. It is not fully understood how the number of ORs relates to the ecology of an insect. In our case, one could expect that the flour beetle might have a less complex sense of smell than the forest dwelling beetles, since it has presumably adapted to an environment with a lower “semiochemical diversity” [71]. This would suggest a lower number of receptors, contrary to our results. Therefore, the chemical ecology of T. castaneum may be more complex than currently understood as also suggested by [53]. However, it is unknown how many of the 111 ORs that are expressed in the adult head are actually expressed in the olfactory organs of T. castaneum. In addition, it is likely that some bark beetle ORs have been missed in our transcriptome analysis (especially in Ips due to the lower sequencing depth), underestimating the true number of antennal-expressed bark beetle ORs.
Species (or taxon)-specific expansions of OR lineages are seen in most insects studied e.g. [72,73], and some of the largest expansions have been found in Hymenoptera, particularly in the jewel wasp, Nasonia vitripennis[74]. The pattern of OR lineage expansion and conservation observed in the present study likely reflects the evolutionary and ecological relatedness among the four beetle species. The beetle taxa analysed here all belong to the more derived part of Coleoptera (Cucujiformia) [75]. However, the Curculionidea (with Ips and Dendroctonus) and Tenebrionidea (with Tribolium) superfamilies are the two furthest separated clades within Cucujiformia, sharing a common ancestor ca. 230–240 Mya. Thus, it may come as no surprise that the ORs of these two taxa largely fall into different subgroupings in the tree. On the other hand, the Curculionidea is a sister group to the Chrysomeloidea (including the longhorns) [75] and, likewise, the closer relatedness of these taxa seems to be reflected in the OR subgroupings. Within Scolytinae, the Ips and Dendroctonus genera are separated by ca. 80 Mya [76]. However, despite the fact that Culex and Aedes mosquitoes are separated by only ca. 40 Mya [77], they show more distinct species-specific OR lineage expansions than the bark beetles [78], indicating that ecological adaptation and life cycle also play important roles in shaping the OR repertoire of a species [25]. On this note, the bark beetles and the cerambycid utilize similar types of host material, i.e. conifer trees and hardwood, respectively [2,79], whereas T. castaneum has been associated with human populations and stored products, for at least a few thousand years [80].
However, not all ORs were grouped in taxon-specific expansions; some subfamilies contained ORs from all four species. This might indicate preservation of ancestral functional patterns within Coleoptera, but since non-coleopteran ORs were left out from the analysis we are careful to draw any conclusions based on this finding (i.e. the clades might contain receptors also from insects outside Coleoptera).
The close clustering of OR sequences from the two bark beetles raises the question about how similar the semiochemical environment is for I. typographus and D. ponderosae. They both live in conifers and would thus be expected to share several biologically relevant compounds. Due to their status as very serious forest pests, the plant- and beetle-produced compounds that they respond to are well studied in these two species. Mainly based on a set of review papers [2,3,7,81-83], we compiled a table of all compounds that have been shown to be physiologically and/or behaviorally active in I. typographus and D. ponderosae (Additional file 5). For 29 (54%) of the 54 listed compounds, there is evidence of shared bio-activity. Not surprisingly, the host compounds show a large overlap (61%), but there is also a large overlap (56%) among pheromone compounds of beetle origin. For the non-host volatiles, the overlap is lower (40%). One might speculate that the extent of this shared “chemosphere” of semiochemicals could account for the low degree of species-specific diversifications among the bark beetle ORs and the other proteins studied here. However, functional data is required to test this hypothesis.
We identified only a small number of putative GR-encoding transcripts (6 in I. typographus; 2 in D. ponderosae) from the antennal transcriptomes. The identified bark beetle GRs included transcripts for carbon dioxide receptors, suggesting that the antennae of bark beetles detect carbon dioxide. In addition, the presence of GR1-3 in I. typographus indicates that carbon dioxide is detected by a heterotrimer receptor, like in mosquitoes, Bombyx mori, and T. castaneum[15,84]. However, GR2 was not found in the analyzed transcriptome of D. ponderosae. Hence, it is possible that D. ponderosae uses a heterodimer receptor for carbon dioxide detection (like D. melanogaster) [85], but it seems unlikely that expression of GR2 would have been lost in only one of the bark beetle species analyzed here.
All the conserved antennal IRs that previously were found in T. castaneum were also identified in D. ponderosae. However, some of them were missing in the I. typographus data. As IRs are associated with coeloconic sensilla that are relatively rare on the Ips antenna [86], it is possible that the missing IR transcripts are expressed only in a few neurons. A lower expression level results in a higher probability that these transcripts were missed during the random sequencing of the Ips cDNA, which had a lesser depth than for D. ponderosae. Generally in insects, the antennal IR subfamily constitutes only a portion of the total number of IRs. The others belong to the divergent IRs, a subfamily that shows species-specific expansions that are particularly large in Diptera [33]. In D. melanogaster, expression of divergent IRs was detected only in gustatory organs [9,33]. This is consistent with the scarcity of divergent IRs in the bark beetle antennal transcriptomes.
Conclusions
We have carried out comprehensive analyses of the antennal transcriptomes of two major tree-killing bark beetle species. At an abstract level, the prevalence of transcript types with respect to associated GO terms was highly similar in the two beetles. In addition, we annotated members of six major gene families that encode proteins with crucial roles in chemoreception. Thus, these proteins have now been identified in three coleopteran species (four species considering the ORs). In combination with the previously published data, the gene sets identified here now allow for improved evolutionary analysis of coleopteran olfaction. We found clear expanded bark beetle-specific lineages mainly among the ORs, suggesting that in comparison to the other analyzed protein families ORs are more tightly linked to sensory specialization and adaptation to specific ecological niches and a shared space of semiochemicals.
The results from the present study will also be fundamental for future functional studies. Functional characterization is needed in order to connect the available physiological and ecological knowledge with the molecular information presented here. Identification and de-orphanization of receptor proteins in bark beetles is especially relevant, since they may represent new targets for integrated pest management strategies.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MNA and EGW contributed equally to this work, by carrying out lab work (MNA) and assemblies (EGW) for I. typographus, as well as bioinformatic analysis, sequence alignment, sequence editing, and data analysis for ORs, GRs, and IRs of both species. MNA drafted the manuscript. EGW constructed trees for all gene families. CIK and MMSY performed antenna-specific assembly for D. ponderosae, as well as bioinformatic analysis, sequence alignment, sequence editing, and data analysis for OBPs, CSPs, and SNMPs from both species. JMB performed lab work, bioinformatic analysis (I. typographus ORs, GRs), and data analysis. ML sequenced representative cDNA clones of D. ponderosae genes that appeared to be full-length for submission to NCBI. FS conducted the GO annotations and compilation of shared semiochemicals. CIK, YH, JB, BSH, and FS conceived of the study, coordinated, and designed the study. All authors contributed to study design and manuscript preparation. All authors read and approved the final manuscript
Supplementary Material
Accession numbers for genes encoding olfactory proteins that were submitted to GenBank and the Transcriptome Shotgun Assembly sequence database (TSA) at NCBI.
Gene ontology results. Gene ontology analyses as in Figure 1, but here represented as bar diagrams that have a higher resolution. A) Molecular function level 3 in Ips typographus, B) molecular function level 3 in Dendroctonus ponderosae, C) biological process level 2 in I. typographus, and D) biological process level 2 in D. ponderosae.
Presence of odorant binding proteins in various tissues of Dendroctonus ponderosae. Analyses of Sanger-specific data and normalized as well as non-normalized transcriptome assemblies from various body parts of D. ponderosae indicate that large sets of odorant binding proteins were found exclusively in non-antennal tissues. The numbers in the tables represent reads found. The clones were sequenced from both 5' and 3' directions. For further information see Keeling et al. [55].
Presence of chemosensory proteins in various tissues of Dendroctonus ponderosae. Analyses of Sanger-specific data and normalized as well as non-normalized transcriptome assemblies from various body parts of D. ponderosae indicate that 4 of the 11 identified chemosensory proteins were found exclusively in non-antennal tissues. The numbers in the tables represent reads found. The clones were sequenced from both 5' and 3' directions. For further information see Keeling et al. [55].
Shared chemosphere of I. typographusandD. ponderosae. List of 54 semiochemicals that are produced by the two bark beetle species, or present in their host or non-host plants and whether the compounds are active at a physiological and/or behavioral level in each species.
Contributor Information
Martin N Andersson, Email: martin_n.andersson@biol.lu.se.
Ewald Grosse-Wilde, Email: grosse-wilde@ice.mpg.de.
Christopher I Keeling, Email: ckeeling@alumni.sfu.ca.
Jonas M Bengtsson, Email: jonas.bengtsson@fmach.it.
Macaire MS Yuen, Email: myuen@mail.ubc.ca.
Maria Li, Email: mlif@msl.ubc.ca.
Ylva Hillbur, Email: ylva.hillbur@slu.se.
Jörg Bohlmann, Email: bohlmann@mail.ubc.ca.
Bill S Hansson, Email: hansson@ice.mpg.de.
Fredrik Schlyter, Email: fredrik.schlyter@slu.se.
Acknowledgements
We are thankful to Sascha Bucks (MPI, Jena) for invaluable support in early laboratory work, and to Bernd Timmermann (MPI, Berlin) for next generation sequencing. We also acknowledge William Walker III (SLU, Alnarp) for identifying Orco in Ips, and Liwen Song for help with the bioinformatic analysis. We acknowledge two anonymous reviewers for useful comments on the submitted manuscript. MNA, YH, and FS were funded by the Department of Plant Protection Biology, SLU, Alnarp, and by the Linnaeus program, “Insect chemical ecology, ethology, and evolution, ICE3”. JMB was supported by the Swedish International Development Agency (Sida/SAREC). Work in Jena (EGW, BSH) was funded by the Max Planck Society. CIK, MMSY, JB, and ML were supported with funding from Genome Canada, Genome British Columbia, and Genome Alberta (Tria 1 and Tria 2 projects, http://www.thetriaproject.ca) and the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery Grant, Strategic Project Grants, and an E.W.R. Steacie Memorial Fellowship to JB). During manuscript preparation, MNA was funded by the Swedish Research Council (VR).
References
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452(7190):987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- Schlyter F, Birgersson GA. In: Pheromones of non-Lepidopteran insects associated with agricultural plants. Hardie J, Minks AK, editor. CAB International, Oxford; 1999. Forest beetles; pp. 113–148. [Google Scholar]
- Zhang Q-H, Schlyter F. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric For Entomol. 2004;6(1):1–19. doi: 10.1111/j.1461-9555.2004.00202.x. [DOI] [Google Scholar]
- Andersson MN, Larsson MC, Blaženec M, Jakuš R, Zhang Q-H, Schlyter F. Peripheral modulation of pheromone response by inhibitory host compound in a beetle. J Exp Biol. 2010;213:3332–3339. doi: 10.1242/jeb.044396. [DOI] [PubMed] [Google Scholar]
- Erbilgin N, Krokene P, Kvamme T, Christiansen E. A host monoterpene influences Ips typographus (Coleoptera: Curculionidae, Scolytinae) responses to its aggregation pheromone. Agric For Entomol. 2007;9:135–140. doi: 10.1111/j.1461-9563.2007.00329.x. [DOI] [Google Scholar]
- Byers JA. In: Bark and wood boring insects in living trees in Europe, a synthesis. Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF, editor. Kluwer Academic Publishers, Dordrecht; 2004. Chemical ecology of bark beetles in a complex olfactory landscape; pp. 89–134. [Google Scholar]
- Andersson MN. Mechanisms of odor coding in coniferous bark beetles: From neuron to behavior and application. Psyche. 2012;2012:Article ID 149572. [Google Scholar]
- de Bruyne M, Baker TC. Odor detection in insects: volatile codes. J Chem Ecol. 2008;34(7):882–897. doi: 10.1007/s10886-008-9485-4. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Touhara K. Insect olfaction: receptors, signal transduction, and behavior. Results Probl Cell Differ. 2009;47:121–138. doi: 10.1007/400_2008_10. [DOI] [PubMed] [Google Scholar]
- Touhara K, Vosshall L. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11(3):188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. PNAS. 2007;104(9):3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9(19):1–14. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne P, Warr C, Freeman M, Lessing D, Kim JC JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/S0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Vosshall L, Amrein H, Morozov P, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/S0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4(2):240–257. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. PNAS. 2003;100:14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Hansson BS. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011;36(6):497–498. doi: 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452(7190):1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452(7190):1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9(12):951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- Bengtsson JM, Trona F, Montagné N, Anfora G, Ignell R, Witzgall P, Jacquin-Joly E. Putative chemosensory receptors of the codling moth, Cydia pomonella, identified by antennal transcriptome analysis. PLoS One. 2012;7(2):e31620. doi: 10.1371/journal.pone.0031620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde E, Kuebler LS, Bucks S, Vogel H, Wicher D, Hansson BS. Antennal transcriptome of Manduca sexta. PNAS. 2011;108(18):7449–7454. doi: 10.1073/pnas.1017963108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legeai F, Malpel S, Montagné N, Monsempes C, Cousserans F, Merlin C, François MC, Maïbèche-Coisné M, Gavory F, Poulain J. An Expressed Sequence Tag collection from the male antennae of the Noctuid moth Spodoptera littoralis: a resource for olfactory and pheromone detection research. BMC Genomics. 2011;12(1):86. doi: 10.1186/1471-2164-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RF, Hughes DT, Luetje CW, Millar JG, Soriano-Agatón F, Hanks LM, Robertson HM. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem Mol Biol. 2012;42:499–505. doi: 10.1016/j.ibmb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su C-Y, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhara K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. PNAS. 2004;101(47):16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HKM, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, Wicher D, Sachse S, Knaden M, Becher PG, Seki Y, Hansson BS. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151(6):1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6(8):e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity. 2009;103(3):208–216. doi: 10.1038/hdy.2009.55. [DOI] [PubMed] [Google Scholar]
- Vogt RG. In: Insect pheromone biochemistry and molecular biology. Blomquist G, Vogt RG, editor. Academic Press, San Diego; 2003. Biochemical diversity of odor detection: OBPs, ODEs and SNMPs; pp. 391–445. [Google Scholar]
- Leal WS. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2012;58(1):373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Große-Wilde E, Svatoš A, Krieger J. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem Senses. 2006;31(6):547–555. doi: 10.1093/chemse/bjj059. [DOI] [PubMed] [Google Scholar]
- Große-Wilde E, Gohl T, Bouché E, Breer H, Krieger J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur J Neurosci. 2007;25(8):2364–2373. doi: 10.1111/j.1460-9568.2007.05512.x. [DOI] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133(7):1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63(14):1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira FG, Rozas J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: Origin and evolutionary history of the chemosensory system. Genome Biol Evol. 2011;3:476–490. doi: 10.1093/gbe/evr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli S, Ceron F, Scaloni A, Monti M, Monteforti G, Minnocci A, Petacchi R, Pelosi P. Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur J Biochem. 1999;262(3):745–754. doi: 10.1046/j.1432-1327.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- Jin X, Zhang S-G, Zhang L. Expression of odorant-binding and chemosensory proteins and spatial map of chemosensilla on labial palps of Locusta migratoria (Orthoptera: Acrididae) Arthropod Struct Dev. 2006;35(1):47–56. doi: 10.1016/j.asd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Wanner KW, Isman MB, Feng Q, Plettner E, Theilmann DA. Developmental expression patterns of four chemosensory protein genes from the Eastern spruce budworm, Chroistoneura fumiferana. Insect Mol Biol. 2005;14(3):289–300. doi: 10.1111/j.1365-2583.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- Kitabayashi AN, Arai T, Kubo T, Natori S. Molecular cloning of cDNA for p10, a novel protein that increases in the regenerating legs of Periplaneta americana (American cockroach) Insect Biochem Mol Biol. 1998;28(10):785–790. doi: 10.1016/S0965-1748(98)00058-7. [DOI] [PubMed] [Google Scholar]
- Bohbot J, Sobrio F, Lucas P, Nagnan-Le Meillour P. Functional characterization of a new class of odorant-binding proteins in the moth Mamestra brassicae. Biochem Biophys Res Commun. 1998;253(2):489–494. doi: 10.1006/bbrc.1998.9806. [DOI] [PubMed] [Google Scholar]
- Liu R, He X, Lehane S, Lehane M, Hertz-Fowler C, Berriman M, Field LM, Zhou JJ. Expression of chemosensory proteins in the tsetse fly Glossina morsitans morsitans is related to female host-seeking behaviour. Insect Mol Biol. 2012;21(1):41–48. doi: 10.1111/j.1365-2583.2011.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Leal WS. Characterization of olfactory genes in the antennae of the Southern house mosquito, Culex quinquefasciatus. J Insect Physiol. 2011;57(7):915–929. doi: 10.1016/j.jinsphys.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Vogt RG, Miller NE, Litvack R, Fandino RA, Sparks J, Staples J, Friedman R, Dickens JC. The insect SNMP gene family. Insect Biochem Mol Biol. 2009;39(7):448–456. doi: 10.1016/j.ibmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450(7167):289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- Nichols Z, Vogt RG. The SNMP/CD36 gene family in Diptera, Hymenoptera and Coleoptera: Drosophila melanogaster, D. pseudoobscura, Anopheles gambiae, Aedes aegypti, Apis mellifera, and Tribolium castaneum. Insect Biochem Mol Biol. 2008;38(4):398–415. doi: 10.1016/j.ibmb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Engsontia P, Sanderson AP, Cobb M, Walden KKO, Robertson HM, Brown S. The red flour beetle's large nose: An expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem Mol Biol. 2008;38(4):387–397. doi: 10.1016/j.ibmb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Tribolium genome sequencing consortium. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452(7190):949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Keeling CI, Henderson H, Li M, Yuen M, Clark EL, Fraser JD, Huber DPW, Liao NY, Docking TR, Birol I, Chan SK, Taylor GA, Palmquist D, Jones SJM, Bohlmann J. Transcriptome and full-length cDNA resources for the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major insect pest of pine forests. Insect Biochem Mol Biol. 2012;42:525–536. doi: 10.1016/j.ibmb.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Faccoli M, Schlyter F. Conifer phenolic resistance markers are bark beetle antifeedant semiochemicals. Agric For Entomol. 2007;9(3):237–245. doi: 10.1111/j.1461-9563.2007.00339.x. [DOI] [Google Scholar]
- Bogdanova EA, Shagin DA, Lukyanov SA. Normalization of full-length enriched cDNA. Mol Biosyst. 2008;4(3):205–212. doi: 10.1039/b715110c. [DOI] [PubMed] [Google Scholar]
- Zhulidov PA, Bogdanova EA, Shcheglov AS, Vagner LL, Khaspekov GL, Kozhemyako VB, Matz MV, Meleshkevitch E, Moroz LL, Lukyanov SA. Simple cDNA normalization using kamchatka crab duplex-specific nuclease. Nucleic Acids Res. 2004;32(3):e37. doi: 10.1093/nar/gnh031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. The Gene Ontology Consortium. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarga A, Valentin F, Anderson M, Lopez R. Web services at the European bioinformatics institute. Nucleic Acids Res. 2007;35(suppl 2):W6–W11. doi: 10.1093/nar/gkm291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling CI, Yuen MMS, Liao NY, Docking TR, Chan SK, Taylor GA, Palmquist DL, Jackman SD, Nguyen A, Li M, Henderson H, Janes JK, Zhao Y, Pandoh P, Moore R, Sperling FAH, Huber DPW, Birol I, Jones SJM, Bohlmann J. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biology. In press. [DOI] [PMC free article] [PubMed]
- Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9(4):286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree2 - Approximately maximum-likelyhood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 2002;12(9):1357–1369. doi: 10.1101/gr.239402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. PNAS. 2008;105(17):6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo K, Smith DP. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 2001;159(3):1059–1072. doi: 10.1093/genetics/159.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, Lee SH, Robertson HM, Kennedy RC, Elhaik E, Gerlach D, Kriventseva EV, Elsik CG, Graur D, Hill CA, Veenstra JA, Walenz B, Tubio JMC, Ribeiro JMC, Rozas J, Johnston JS, Reese JT, Popadic A, Tojo M, Raoult D, Reed DL, Tomoyasu Y, Kraus E, Mittapalli O, Margam VM. et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. PNAS. 2010;107(27):12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm Y, Wang J, Riba-Grognuz O, Corona M, Nygaard S, Hunt BG, Ingram KK, Falquet L, Nipitwattanaphon M, Gotzek D, Dijkstra MB, Oettler J, Comtesse F, Shih C-J, Wu W-J, Yang C-C, Thomas J, Beaudoing E, Pradervand S, Flegel V, Cook ED, Fabbretti R, Stockinger H, Long L, Farmerie WG, Oakey J, Boomsma JJ, Pamilo P, Yi SV, Heinze J. et al. The genome of the fire ant Solenopsis invicta. PNAS. 2011;108(14):5679–5684. doi: 10.1073/pnas.1009690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q-H, Schlyter F. Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos. 2003;101(2):299–310. doi: 10.1034/j.1600-0706.2003.111595.x. [DOI] [Google Scholar]
- Bohbot J, Pitts RJ, Kwon HW, Rützler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007;16(5):525–537. doi: 10.1111/j.1365-2583.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja C, Shi P, Butlin RK, Robertson HM. Large gene family expansions and adaptive evolution for odorant and gustatory receptors in the pea aphid, Acyrthosiphon pisum. Mol Biol Evol. 2009;26(9):2073–2086. doi: 10.1093/molbev/msp116. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Gadau J, Wanner KW. The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol Biol. 2010;19:121–136. doi: 10.1111/j.1365-2583.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- Hunt T, Bergsten J, Levkanicova Z, Papadopoulou A, Wild R, Hammond PM, Ahrens D, Balke M, Caterino MS, Gómez-Zurita J, Ribera I, Barraclough TG, Bocakoca M, Bocak L, Vogler AP, StJohn O. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–1916. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- McKenna DD, Sequeira AS, Marvaldi AE, Farrell BD. Temporal lags and overlap in the diversification of weevils and flowering plants. PNAS. 2009;106(17):7083–7088. doi: 10.1073/pnas.0810618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besansky NJ, Fahey GT. Utility of the white gene in estimating phylogenetic relationships among mosquitoes (Diptera: Culicidae) Mol Biol Evol. 1997;14(4):442–454. doi: 10.1093/oxfordjournals.molbev.a025780. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC. et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. doi: 10.1126/science.1191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzel MD, Hanks LM. Role of host plant volatiles in mate location for three species of longhorned beetles. J Chem Ecol. 2005;31(1):213–217. doi: 10.1007/s10886-005-6735-6. [DOI] [PubMed] [Google Scholar]
- Dawson PS. Life history strategy and evolutionary history of Tribolium flour beetles. Evolution. 1977;31(1):226–229. doi: 10.2307/2407562. [DOI] [PubMed] [Google Scholar]
- Borden JH, Pureswaran DS, Lafontaine JP. Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae) J Econ Entomol. 2008;101(4):1266–1275. doi: 10.1603/0022-0493(2008)101[1266:SBOMFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pureswaran DS, Borden JH. New repellent semiochemicals for three species of Dendroctonus (Coleoptera: Scolytidae) Chemoecology. 2004;14(2):67–75. doi: 10.1007/s00049-003-0260-2. [DOI] [Google Scholar]
- Pureswaran DS, Gries R, Borden JH. Antennal responses of four species of tree-killing bark beetles (Coleoptera: Scolytidae) to volatiles collected from beetles, and their host and nonhost conifers. Chemoecology. 2004;14(2):59–66. doi: 10.1007/s00049-003-0261-1. [DOI] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, Van Loon JJA, Takken W, Carlson JR, Zweibel LJ. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17(18):1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2006;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Hallberg E. Sensory organs in Ips typographus (Insecta: Coleoptera) - Fine structure of antennal sensilla. Protoplasma. 1982;111:206–214. doi: 10.1007/BF01281968. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Accession numbers for genes encoding olfactory proteins that were submitted to GenBank and the Transcriptome Shotgun Assembly sequence database (TSA) at NCBI.
Gene ontology results. Gene ontology analyses as in Figure 1, but here represented as bar diagrams that have a higher resolution. A) Molecular function level 3 in Ips typographus, B) molecular function level 3 in Dendroctonus ponderosae, C) biological process level 2 in I. typographus, and D) biological process level 2 in D. ponderosae.
Presence of odorant binding proteins in various tissues of Dendroctonus ponderosae. Analyses of Sanger-specific data and normalized as well as non-normalized transcriptome assemblies from various body parts of D. ponderosae indicate that large sets of odorant binding proteins were found exclusively in non-antennal tissues. The numbers in the tables represent reads found. The clones were sequenced from both 5' and 3' directions. For further information see Keeling et al. [55].
Presence of chemosensory proteins in various tissues of Dendroctonus ponderosae. Analyses of Sanger-specific data and normalized as well as non-normalized transcriptome assemblies from various body parts of D. ponderosae indicate that 4 of the 11 identified chemosensory proteins were found exclusively in non-antennal tissues. The numbers in the tables represent reads found. The clones were sequenced from both 5' and 3' directions. For further information see Keeling et al. [55].
Shared chemosphere of I. typographusandD. ponderosae. List of 54 semiochemicals that are produced by the two bark beetle species, or present in their host or non-host plants and whether the compounds are active at a physiological and/or behavioral level in each species.