Abstract
Objective
To determine how genetic factors might influence the progression of nonalcoholic fatty liver disease (NAFLD).
Design/Intervention
Beginning in adolescence, male C57BL6 (BL6) and 129/SVJ mice were fed control (n = 15/group) or high-fat (HF) diets (n = 30/group) for 6 months.
Main Outcome Measures
Assessed were body weight, insulin resistance, hepatic production of free radicals, expression of cytokines and fibrosis-related genes and severity of hepatic steatosis, injury and fibrosis.
Results
High-fat diets induced comparable obesity, hepatic steatosis and insulin resistance in the two strains. Compared with BL6 mice, 129/SVJ mice had impaired induction of antioxidant genes, generated three- to four-fold more free radicals and exhibited two-fold greater induction of profibrogenic cytokines (interleukin-4 and transforming growth factor-β1) and fibrosis-related genes (fibronectin and tissue inhibitor of metalloproteinase-1) (all P<0.05 for 129 vs BL6). Surprisingly, however, induction of collagen I α1 mRNA and accumulation of Sirius red-stained fibrils and hepatic hydroxyproline were similar in BL6 and 129/SVJ mice, and although patchy sinusoidal fibrosis emerged in both strains, neither developed bridging fibrosis.
Conclusions
Although BL6 and 129/SVJ mice with diet-induced obesity, insulin resistance and steatosis differed with respect to several factors that are thought to influence human NAFLD progression, they developed comparable liver fibrosis. Moreover, none of the risk factors for NAFLD-related cirrhosis in humans, including obesity, insulin resistance, chronic inflammatory and oxidant stress, steatohepatitis or activation of fibrogenic genes, proved to be sufficient to cause cirrhosis in these mice, even when exposure to one or more of these insults was very prolonged.
Keywords: fatty liver, fibrosis, genetic, high-fat diet, strain differences
Nonalcoholic fatty liver disease (NAFLD) is now the leading cause of chronic liver disease in the developed world and a major indication for liver transplantation (1, 2). However, the outcome of NAFLD is highly variable; only a quarter of individuals will develop progressive disease that is characterized by hepatic necroinflammation, fibrosis and cirrhosis. Elucidating the mechanisms involved in disease progression is, therefore, essential to our understanding of NAFLD and ability to identify those at risk of developing end-stage liver disease.
Nonalcoholic fatty liver disease is strongly associated with obesity. The vastmajority of obese individuals with NAFLD will have ‘simple’ hepatic steatosis, which is a stable and benign condition for decades (2). In others, however, hepatic necroinflammation evolves, cumulative liver damage ensues and cirrhosis ultimately results (3, 4). Inter-individual differences in susceptibility to hepatic steatosis, as well as subsequent progression to steatohepatitis and cirrhosis, are thought to reflect variability in exposures and responses to sequential insults (dubbed ‘hits’) (5). Diverse factors that challenge lipid homeostatic mechanisms are the initial ‘hits’ that provoke the onset of hepatic triglyceride accumulation (steatosis) and enhance hepatocyte vulnerability to injury. Progression to steatohepatitis is believed to result from superimposition of secondary ‘hits’, such as metabolic, oxidant and/or inflammatory stresses, that overw-helm cell survival mechanisms and provoke hepatocyte injury and death (6). More recently, our group has proposed that the death of mature hepatocytes provides a ‘third’ hit that enhances the risk of cirrhosis and hepatocellular carcinoma by inducing liver repair mechanisms (7).
Genetic factors are likely to modulate fatty liver disease pathogenesis by influencing both exposure and responses to the insults that drive liver disease initiation and progression. This concept is supported by studies of leptin-resistant fa/fa rats and leptin-deficient ob/ob mice. Fa/fa rats are much more susceptible to hepatic steatosis than wild-type rats when fed calorically dense diets enriched with fructose or sucrose (8). Leptindeficient, ob/ob mice are relatively protected from liver fibrosis, despite developing severe steatosis (9).
The ability to understand mechanisms that control NAFLD progression in humans would be greatly facilitated by the acquisition of small animal models that develop hepatic steatosis, and subsequent steatohepatitis and cirrhosis when exposed to typical risk factors for human NAFLD (e.g. high-calorie diets). However, reproducible models of progressive NAFLD have been difficult to establish in rodents with diet-induced obesity (10, 11). The reasons for failure remain uncertain. One possibility is that the duration of risk factor exposure was simply insufficient. Typically, rodents have been overfed for 8 weeks or less, a period corresponding to <10% of their life span. Most humans with NAFLD-related cirrhosis, on the other hand, are middle aged or older (4, 12) and have been overeating since at least adolescence, exposing themselves to NAFLD risk factors for a quarter of their lifespan or more. Another possibility is that commonly studied strains of rodents, such as C57BL6 mice, lack genetic factors that convey susceptibility to progressive liver damage.
This reasoning suggests that it may be feasible to develop a mouse model of progressive NAFLD by selecting a strain of mice that is naturally predisposed to one or more of the secondary ‘hits’ that trigger progression from steatosis to steatohepatitis (e.g. oxidant/inflammatory stress) and overfeeding them for a quarter of their life span to approximate the duration of risk factor in humans who develop progressive NAFLD. To evaluate this hypothesis, we fed adolescent C57BL6 mice and 129/SVJ mice high-fat (HF) diets for 6 months and compared NAFLD stage at the end of the treatment period in the two groups. C57BL6 mice generally develop steatosis and insulin resistance but little, if any, liver fibrosis when subjected to short periods of diet-induced obesity (13), and hence, were predicted to be relatively resistant to advanced NAFLD. 129/SVJ mice are frequently used as a source of embryonic stem cells (14). The 129/SVJ, specifically, has a mixed genetic background and thus contributes genetic factors that modulate the phenotypes of many transgenes (15). The 129/SVJ strain has been shown to be significantly more sensitive to heavy-metal-induced testicular toxicity and immune-mediated nephritis than the C57BL6 strain (16). These observations suggest that 129/SVJ mice may be predisposed to tissue damage elicited by oxidant and/or inflammatory stress, factors that are believed to promote progressive NAFLD (17, 18). Our results demonstrate that although steatosis, enhanced hepatic oxidant stress, steatohepatitis, T-helper type 2 (Th2) polarization of hepatic cytokine production and overactivation of fibrogenic pathways occurred in 129/SVJ mice during prolonged exposure to HF diets, these events were not sufficient to induce bridging fibrosis/cirrhosis. Similarities in the ultimate outcomes of HF-diet-induced liver damage in C57BL6 mice and 129/SVJ mice despite striking strain-related differences in oxidant stress and relative production of pro-(Th1) and anti-(Th2) inflammatory cytokines suggest the importance of other injury/repair mechanisms for progression of this disease.
Materials and methods
Animal model of liver injury
Adolescent, male C57BL6 and 129/SVJ mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). C57BL6 (n = 30) and 129/SVJ (n = 30) mice were fed with HF high-sucrose diet [fat 35%, sucrose 40%, total carbohydrate 9% (other than sucrose) and protein 16%] for 6 months as described (19). At the same time, two other groups of C57BL6 (n = 15) and 129/SVJ (n = 15) mice were fed with control diet [fat 12%, sucrose N/A, total carbohydrate 59% (other than sucrose) and protein 29%]. After 6 months, four mice from each group were randomly selected for liver free radical analysis as described below. All other mice were sacrificed at the same time to obtain serum and liver samples.
Animal care and surgical procedures were approved by the Duke University Medical Center Institutional Animal Care and Use Committee as set forth in the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health.
Collection of bile and electron spin resonance detection of free radical adducts
Bile collection as well as electron spin resonance (ESR) detection is a standard procedure to measure endogenous free radicals over time from liver tissue (20). Mice were anaesthetized with pentobarbital (50 mg/kg), the abdomen was opened and the bile duct was cannulated with PE-10 tubing. After the spin trapping reagent α-(4-pyridyl 1-oxide)-N-tert-butyl nitrone (0.5 kg) (Alexis Biochemicals, San Diego, CA, USA) was administrated intraperitoneally, the bile samples were collected for 3 h into 30 mM each of dipyridyl (25 µl/100 g) (Sigma Chemical Co., St Louis, MO, USA) and 30 mM bathcuproi-nedisulphonic acid (25 µl/100 g) (Sigma Chemical Co.) to prevent ex vivo radical formation. Samples were stored at −80 °C immediately until analysis of radical adducts by ESR spectroscopy (21).
Electron spin resonance spectra were obtained with a Bruker EMX ESR spectrometer equipped with a super high Q cavity operating at 9.77 GHz and room temperature. Other ESR spectrometer settings were as follows: magnetic field centre, 3494.4 G; magnetic field scan, 70 G; modulation frequency, 100 kHz; microwave power, 20 mW; modulation amplitude, 1.0 G; receiver gain, 5.0×104; time constant, 0.655 s; and conversion time, 0.164 s.
Serum biochemical measurements
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose and triglycerol levels were measured by the Bioreliance (Rockville, MD, USA). Serum insulin levels were assayed using 125I-Insulin, rat-specific, overnight radioimmunoassay (Linco Research, St Charles, MO, USA).
A measure of insulin resistance was obtained by calculating the insulin resistance index (insulin×glucose) (mg/dl)2.
Analysis of liver architecture and morphometry
Serial sections were stained with haematoxylin and eosin or Oil Red O using standard techniques, as described (22). To quantify liver fibrosis, 5 µm sections were stained with picrosirus red (Sigma) and counterstained with fast green (Sigma). The proportion of tissue stained with picrosirus red content was assessed by morphometric analysis with METAVIEW software (Universal Imaging Corp, Downtownington, PA, USA) as described (23). Collagen stained with sirius red was quantified in the sections that were randomly chosen (under ×20 magnification, 10 fields each from sample).
Histopathologic analysis
This was assessed using a criteria modified from Brunt et al. (24). Briefly, hepatic steatosis was graded as follows: grade 0, ≤ 5% of hepatocytes affected; grade 1, 5–33% of hepatocytes affected; grade 2, 34–66% of hepatocytes affected; or grade 3, >66% of hepatocytes affected. Hepatocyte ballooning and inflammation were also graded separately and a score of 0–3 assigned.
Hydroxyproline assay
Hydroxyproline content in whole liver specimens was quantified colorimetrically. Liver specimens were weighed, and 30 mg of freeze-dried sample was hydrolysed in 6N HCl at 110 °C for 16 h. The hydrolysate was evaporated under vacuum and the sediment was redissolved in 1 ml distilled water. Samples were filtered and then incubated with 0.5 ml of chloramine-T solution, containing 1.41 g of chloramine-T dissolved in 80 ml of acetate–citrate buffer and 20 ml of 50% isopropanol, for 20 min at room temperature. To this were added, 0.5 ml of Ehrlich’s solution, including 7.5 g of dimethylamino-benzaldehyde dissolved in 13 ml of 60% perchloric acid, and 30 ml isopropanol, and the mixture was incubated at 65 °C for 15 min. After cooling, the absorbance was read at 561 nm. Hydroxyproline concentration was calculated from a standard curve prepared with high-purity hydroxyproline (Sigma) and expressed as mg hydroxyproline/g liver.
Tissue triglyceride assay
Extraction of liver triglycerides with chloroform/methanol was modified from Burant et al. (25). The triglyceride content was determined using the Triglyceride Detection Kit Sigma.
Tissue caspase 3/7 activity
Caspase activity was assessed by amino-4-trifluoromethyl courmarin (AFC) release assays using the Fluor-Ace kit (Bio-Rad, CA, USA) according to the manufacturer’s instructions. Briefly, tissue lysates were incubated with 25 mmol/L z-DEVD-AFC (Enzyme and Systems Products, Livermore, CA, USA) at room temperature. The change in fluorescence (excitation at 370 nm and emission at 490 nm) was monitored after 60 and 120 min of incubation time and expressed as pico-moles of AFC release per microgram of protein.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assay
Five-micrometre liver sections were treated with Proteinase K for 20 min at 37 °C. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay was performed using the In Situ Cell Death Detection Kit, POD (Roche Applied Science, Mannheimm Germany) following the manufacturer’s instructions. Diaminobenzidine substrate was used to visualize TUNEL-positive cells (stained brown). TUNEL-positive hepatocytes were quantified by counting the number of positive cells per high-powered field. Ten high-powered fields were analysed for each sample.
mRNA quantification by real-time reverse transcriptase polymerase chain reaction
mRNAs were quantified by real-time reverse transcriptase polymerase chain reaction (RT-PCR) as per the manufacturer’s specifications (Eppendorf, Mastercycler Real-Time PCR, Hamburg, Germany). The sequences of primers are listed in Table 1. Total RNA was extracted from cells or whole livers using TRIzol (Invitrogen, Carlsbad, CA, USA). One microgram of RNA was reverse-transcribed using random primer and Superscript RNAse H-reverse transcriptase (Invitrogen). Samples were incubated at 20 °C for 10 min, 42 °C for 30 min; reverse transcriptase was inactivated by heating at 99 °C for 5 min and cooling at 5 °C for 5 min. Amplification reactions were performed using a SYBR Green PCR Master Mix (Applied Biosystems, Cheshire, UK). Five microlitres of diluted cDNA samples (1–5 dilution) were used for quantitative two-step PCR (a 10-min step at 95 °C, followed by 50 cycles of 15 s at 95 °C and 1 min at 65 °C) in the presence of 400 nM specific forward and reverse primers, 5 mM MgCl250 mM KCl, 10 mM Tris buffer (pH 8.3), 200 µM dATP, dCTP, dGTP and 400 µM dUTP and 1.25U of AmpliTaq Gold DNA polymerase (Perkin Elmer, Foster City, CA, USA). Each sample was analysed in triplicate. Target gene levels in treated cells or tissues are presented as a ratio to levels detected in corresponding control cells or tissues, according to the ΔCt method.
Table 1.
Primers for quantitative reverse transcriptase polymerase chain reaction analyses
| Gene | Sequence | Product size (base pairs) |
|---|---|---|
| SOD1 | ||
| Sense | GAGACCTGGGCAATGTGACT | 220 |
| Antisense | GTTTACTGCGCAATCCCAAT | |
| SOD2 | ||
| Sense | CCGAGGAGAAGTACCACGAG | 210 |
| Antisense | GCTTGATAGCCTCCAGCAAC | |
| SOD3 | ||
| Sense | TCTGCAGGGTACAACCATCA | 199 |
| Antisense | ACCTCCATCGGGTTGTAGTG | |
| GSS | ||
| Sense | GCCTCCTACATCCTCATGGA | 160 |
| Antisense | CCACATGCTTGTTCATCACC | |
| TNF-α | ||
| Sense | TCGTAGCAAACCACCAAGTG | 207 |
| Antisense | AGATAGCAAATCGGCTGACG | |
| IFN-γ | ||
| Sense | CATCAGCAACAACATAAGCGTCA | 202 |
| Antisense | CTCCTTTTCCGCTTCCTGA | |
| IL-4 | ||
| Sense | TCCTGCTCTTCTTTCTCG | 102 |
| Antisense | CTTCTCCTGTGACCTCGTT | |
| IL-10 | ||
| Sense | TGTGAAAATAAGAGCAAGGCAGTG | 85 |
| Antisense | CATTCATGGCCTTGTAGACACC | |
| IL-12 | ||
| Sense | GGAAGCACGGCAGCAGAATA | 180 |
| Antisense | AACTTGAGGGAGAAGTAGGAATGG | |
| TGF-β | ||
| Sense | TTGCCCTCTACAACCAACACAA | 103 |
| Antisense | GGCTTGCGACCCACGTAGTA | |
| α-SMA | ||
| Sense | AAACAGGAATACGACGAAG | 135 |
| cAntisense | CAGGAATGATTTGGAAAGGA | |
| Fibronectin | ||
| Sense | GTGGCTGCCTTCAACTTCTC | 132 |
| Antisense | GTGGGTTGCAAACCTTCAAT | |
| TIMP-1 | ||
| Sense | CCTTGCAAACTGGAGAGTGACA | 88 |
| Antisense | AAGCAAAGTGACGGCTCTGGT | |
| Collagen Iα | ||
| Sense | GAGCGGAGAGTACTGGATCG | 158 |
| Antisense | GCTTCTTTTCCTTGGGGTTC |
GSS, glutathione synthetase; IFN, interferon; IL, interleukin; SMA, smooth muscle actin; SOD, superoxide dismutase; TGF, transforming growth factor; TNF, tumour necrosis factor; TIMP, tissue inhibitor of metalloproteinase.
Statistical analysis
Results are expressed as mean±SEM. Significance was established using the Student t-test and analysis of variance when appropriate. Differences were considered significant when P <0.05.
Results
C57BL6 and 129/SVJ mice show comparable levels of obesity and hepatic steatosis after a 6-month high-fat diet
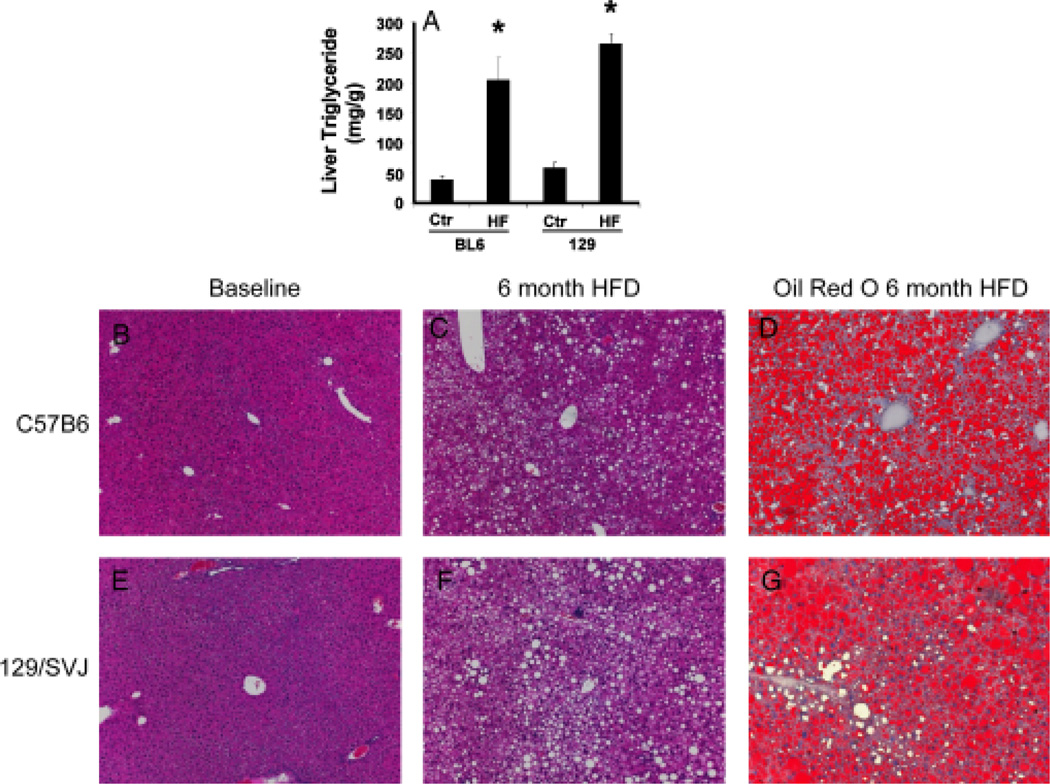
Healthy, adolescent C57BL6 mice and 129/SVJ mice were fed HF diets for 6 months. Chronic consumption of these high-caloric diets resulted in comparable levels of obesity and hyperglycaemia in both strains, but serum triglcyceride levels were higher in 129/SVJ mice (Table 2). On the other hand, hepatic triglyceride content was comparable in the two strains before HF diet initiation, and increased similarly in both strains after high-fat feeding (Fig. 1A). Hence, both C57BL6 mice (Fig. 1B, C, D) and 129/SVJ mice (Fig. 1E, F, G) developed significant hepatic steatosis when subjected to this protocol.
Table 2.
Body weights, liver weights and metabolic parameters of C57BL6 and 129/SVJ mice after 6-months of feeding with the high-fat or control diets
| C57BL6 |
129/SVJ |
|||
|---|---|---|---|---|
| Control | HF | Control | HF | |
| BW (g) | 30.1 ± 2.45 | 53.9 ± 1.09* | 27.04 ± 1.00 | 48.63 ± 5.57 |
| LW (g) | 1.24 ± 0.05 | 2.80 ± 0.29* | 0.95 ± 0.05 | 1.86 ± 0.31 |
| LW/BW ratio (%) | 4.15 ± 0.16 | 5.18 ± 0.48 | 3.50 ± 0.09 | 3.78 ± 0.19 |
| Serum glucose (mg/dl) | 413.25 ± 106.63 | 310.2 ± 69.84 | 477 ± 128.22 | 365.75 ± 67.85 |
| Serum insulin (ng/ml) | 1.48 ± 1.25 | 5.3 ± 0.79* | 0.93 ± 0.54 | 4.65 ± 3.08 |
| Insulin resistance index (mg/dl)2 | 0.07 | 0.19 | 0.038 | 0.14 |
| Serum triglyceride (mg/dl) | 78.47 ± 12.4 | 61.71 ± 10.17 | 174.1 ± 37.79 | 114.83 ± 10.99# |
P < 0.05 vs. control;
P < 0.05 vs. BL6 HF group.
LW, liver weight; BW, body weight.
Fig. 1.
C57BL6 mice and 129/SVJ mice developed similar degrees of hepatic steatosis after 6 months of high-fat (HF) diets. Hepatic triglyceride content in C57BL6 mice (BL6) and 129/SVJ mice (129) after ingesting regular chow (control, Ctr) (n = 11/group) or HF diet (n = 26/group) for 6 months (A). Histology in representative mice (B–G). Haematoxylin and eosin-stained liver sections in BL6 mice (B, C) and 129/SVJ mice (E, F) at baseline (B, E) and after 6 months of HF diets (C, F). Oil red O staining demonstrates neutral lipids in livers of BL6 mice (D) and 129/SVJ mice (G) after 6 months of HF diets. *P<0.05 vs Ctr.
C57BL6 and 129/SVJ mice exhibit similar degrees of liver injury despite differences in free radical (25) production
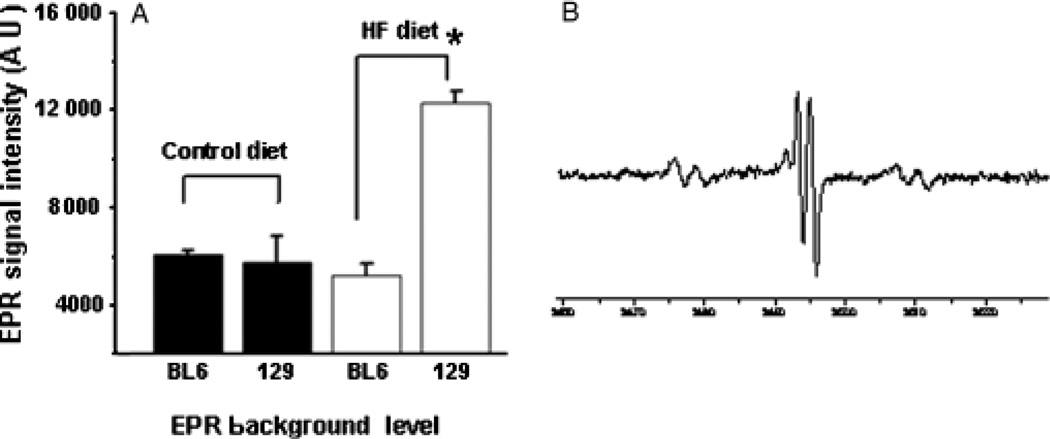
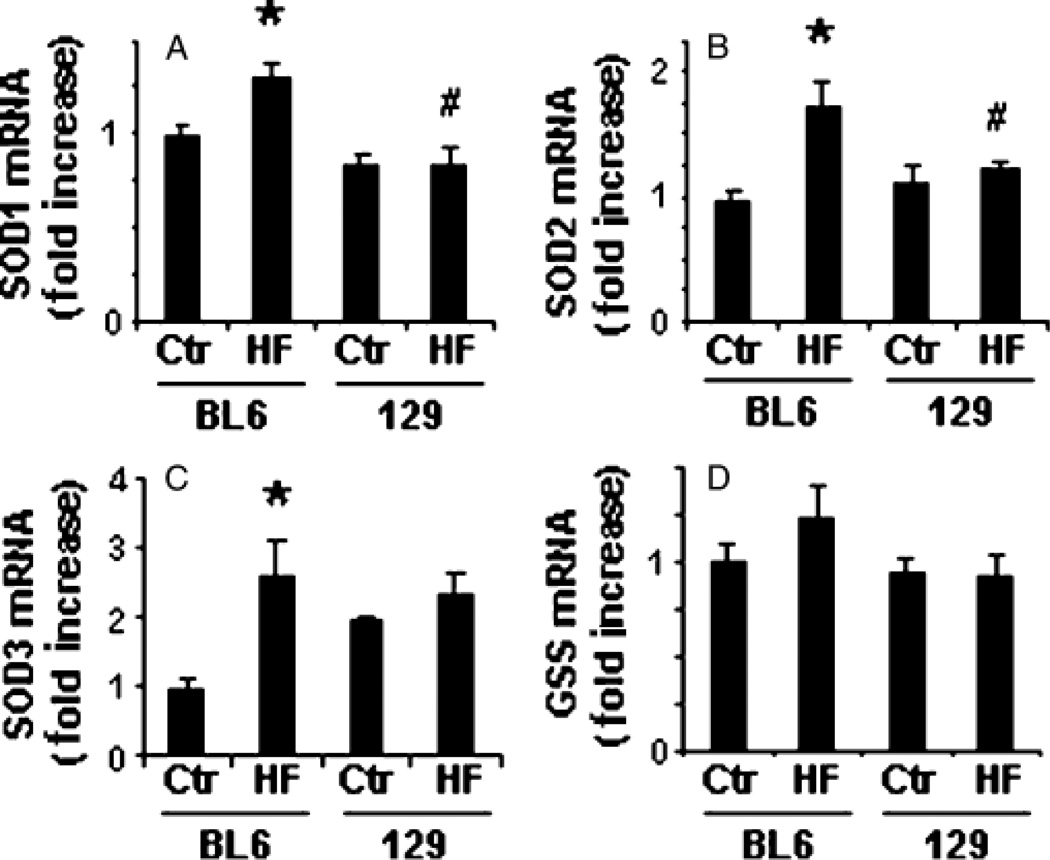
Interestingly, spin trap analysis of biliary free radical (25) content demonstrated that HF diets induced significant hepatic free radical production only in 129/SVJ mice (Fig. 2). Analysis of antioxidant gene expression in liver RNA samples from the two groups suggested a possible explanation for this difference. 129/SVJ mice expressed somewhat lower hepatic levels of superoxide dismutase (SOD)1 mRNA than C57BL6 mice at baseline, and unlike C57BL6 mice, failed to upregulate hepatic mRNA expression of SOD1, SOD2 or SOD3 in response to HF diets (Fig. 3). Hence, fatty liver cells in obese 129/SVJ mice were exposed to higher levels of oxidative stress than those of equally obese and steatotic BL6 mice.
Fig. 2.
Electron spin resonance (ESR) analysis demonstrates strain-related differences in hepatic free radical adduct (reactive oxygen species) (25) production during high-fat (HF) diet treatment. Bile was obtained from randomly selected BL6 and 129/SVJ mice after 6 months of feeding control chow or HF diets (n = 4 mice/group) and analysed for free radical adduct (25) content by ESR. Mean (SEM) results are graphed (A). Electron paramagnetic resonance (EPR) spectrum demonstrates typical reactive oxygen species peaks in a representative 129/SVJ mouse (B). *P<0.05 vs BL6 HF-fed group.
Fig. 3.
Differential induction of hepatic antioxidant enzyme expression in C57BL6 and 129/SVJ mice after high-fat (HF) diet feeding. RNA was isolated from the livers of BL6 and 129/SVJ mice after a 6-month treatment with control diet (Ctr, n = 11/group) or HF diets (n = 26/group) and quantitative reverse transcriptase polymerase chain reaction analysis was performed to assess effects on hepatic expression of superoxide dismutase (SOD) 1 (Mn-SOD, cytoplasmic) (A), SOD 2 (Cu/Zn-SOD, mitochondrial) (B), SOD 3 (Cu, Zn-SOD, extracellular) (C) and glutathione synthetase (D). Results are mean (SEM). *P<0.05 vs respective Ctr, #P<0.05 vs BL6-HF group.
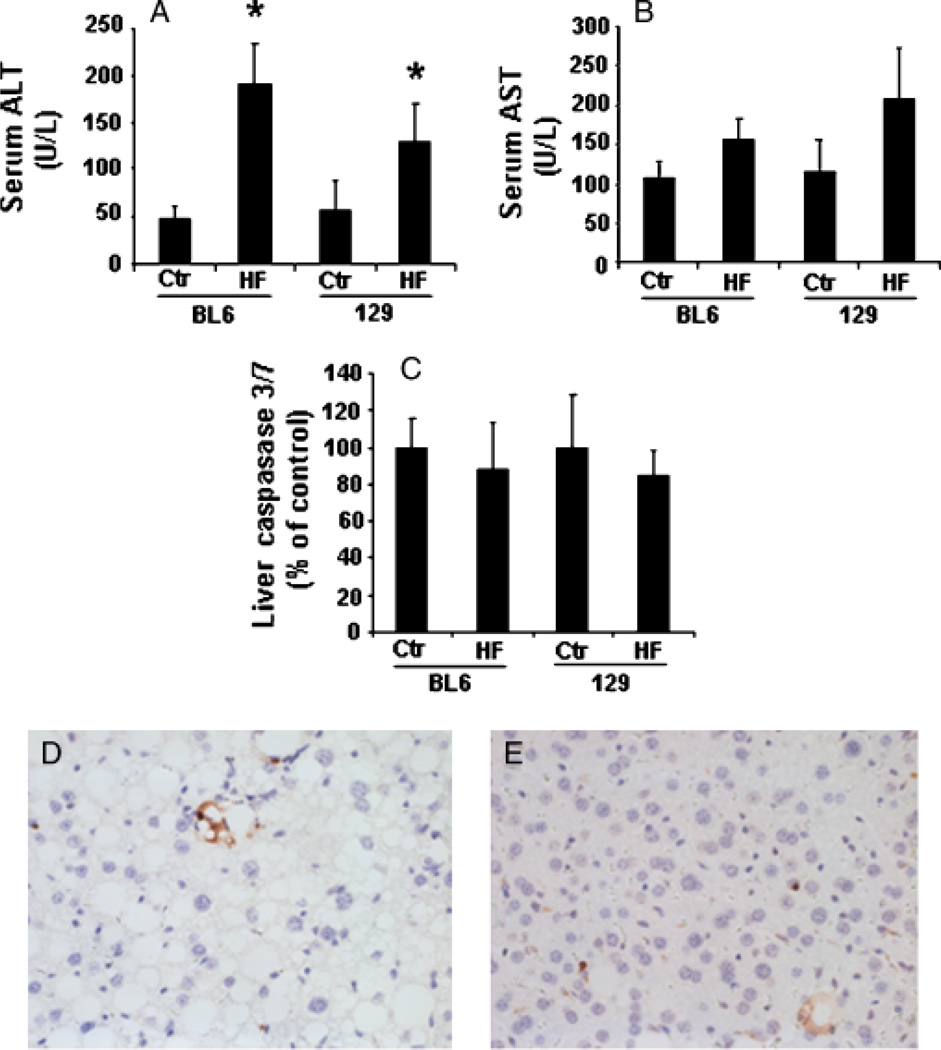
Because oxidant stress is thought to be a risk factor for steatohepatitis (26–28), we next compared various measures of liver injury in the two groups to determine whether the enhanced free radical (25) generation in 129/SVJ mice exacerbated NAFLD progression. Although HF diets increased serum ALT and AST in both groups, aminotransferase values in HF-diet-fed 129/SVJ mice were not greater than in C57BL6 mice (Fig. 4A, B). Tissue caspase 3/7, an important regulator of hepatocyte apoptosis (29, 30) and marker of NASH (31), was also comparable in both groups (Fig. 4C). Similar levels of apoptosis were observed by the TUNEL assay (Fig. 4D, E). On the other hand, 129/SVJ mice tended to accumulate more ballooned hepatocytes than BL6 mice (Table 3). Hepatocyte ballooning has been identified as an important marker of fatty liver damage (24, 32).
Fig. 4.
Markers of liver injury were similar in high-fat (HF)-diet-fed C57BL6 and 129/SVJ mice. Serum and liver tissues were harvested from BL6 and 129/SVJ mice after a 6-month treatment with control diet (Ctr, n = 11/group) or HF diet (HF, n = 26/group). (A) Serum alanine aminotransferase (ALT); (B) aspartate aminotransferase (AST); (C) hepatic caspase 3/7 activity. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling -positive cells in BL6 (D) and 129/SVJ (E). Results are mean (SEM). *P<0.05 vs respective Ctr.
Table 3.
Necroinflammatory scores after 6 months of high-fat treatment
| C57BL6 |
129/SVJ |
|
|---|---|---|
| HF (mice = 15) | HF (mice = 17) | |
| Fat grade | ||
| 0 | 0 | 0 |
| I | 2 | 3 |
| II | 1 | 7 |
| III | 12 | 7 |
| Inflammation | ||
| 0 | 1 | 0 |
| I | 2 | 9 |
| II | 12 | 8 |
| III | 0 | 0 |
| Ballooning | ||
| 0 | 3 | 0 |
| I | 8 | 6 |
| II | 4 | 11 |
| III | 0 | 0 |
HF, high fat.
T helper type 1-polarization of hepatic cytokine expression in C57BL6 mice is associated with increased inflammatory cell infiltrates
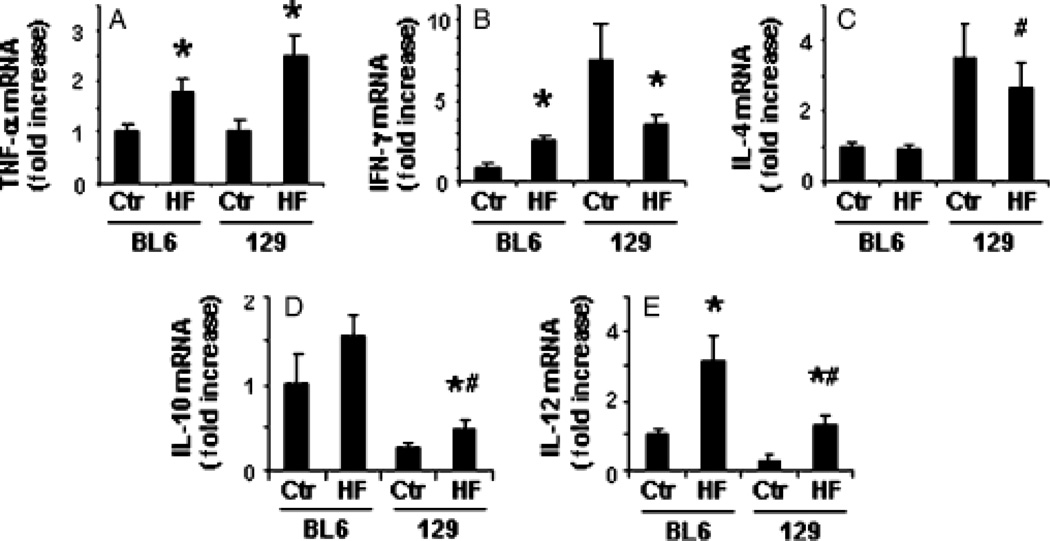
Because overproduction of Th1 (proinflammatory) cytokines has been linked to both oxidant stress and hepatocyte injury in NAFLD (13, 33), we performed RT-PCR analysis of various Th1 and Th2 cytokines in the two strains before and after HF diet exposure (Fig. 5). Compared with C57BL6 mice, 129/SVJ mice expressed comparable mRNA levels of tumour necrosis factor (TNF)-α, higher levels of interferon (IFN)-γ and interleukin (IL)-4, and lower levels of IL-10 and IL-12 before HF feeding. The cytokine response to HF feeding also differed between the two groups. C57BL6 mice tended to upregulate proinflammatory cytokines (e.g. TNF-α, IFN-γ and IL-12), but exhibited little, if any, induction of anti-inflammatory cytokines (IL-10 and IL-4). Thus, as reported by others (34), cytokine production in BL6 mice became Th1 polarized during steatohepatitis. Although 129/SVJ mice also increased their expression of TNF-α and IL-12 after HF feeding, they downregulated IFN-γ and upregulated IL-10 while maintaining a high expression of IL-4. This pattern of gene expression is consistent with relative Th2 polarization of hepatic cytokine production. Consistent with evidence that Th2 cytokines are anti-inflammatory (35, 36), hepatic inflammatory cell infiltrates were somewhat less severe in HF-diet-fed 129/SVJ mice than in comparably treated BL6 mice (Table 3).
Fig. 5.
Strain-related differences in hepatic expression of T helper (Th) 1 and Th2 cytokines. Quantitative reverse transcriptase polymerase chain reaction analysis was performed to compare hepatic expression of pro-inflammatory (Th1) and pro-fibrogenic (Th-2) cytokines in BL6 and 129/SVJ mice after a 6-month treatment with control diet (Ctr, n = 11/group) or high-fat diets (HF, n = 26/group). Mean (SEM) results are graphed. *P<0.05 vs respective Ctr; #P<0.05 vs BL6-HF group. IFN, interferon; IL, interleukin; TNF, tumour necrosis factor. [Correction added after online publication, 27 May 2009: on the Y axis of 5B, TNF-γ was replaced with IFN-γ.]
Dissociation between fibrogenic markers and liver fibrosis in C57BL6 and 129/SVJ mice
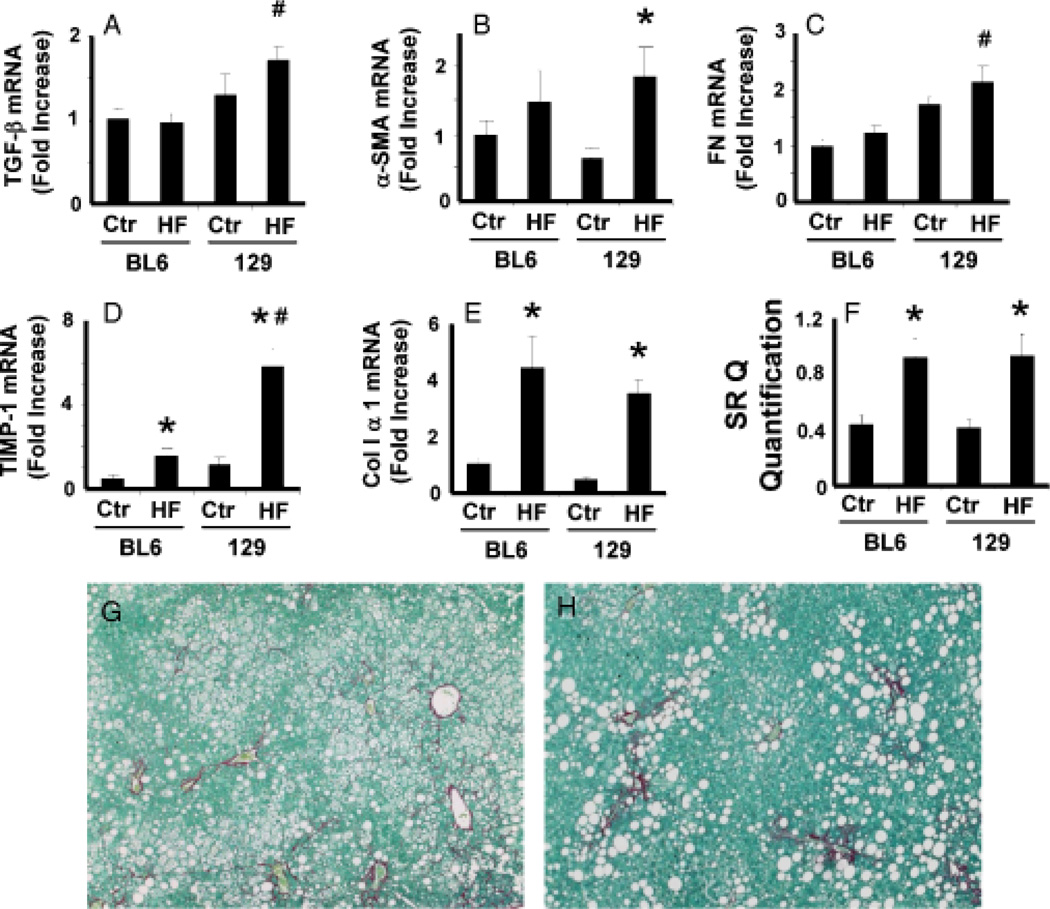
Although Th2 cytokines are anti-inflammatory, they are profibrogenic (37, 38). Therefore, we assessed various fibrosis markers to determine whether the relative over-production of Th2 cytokines in the 129/SVJ strain exacerbated progression of steatohepatitis to liver fibrosis (Fig. 6). mRNA levels of transforming growth factor-1, a major profibrogenic Th2 cytokine, increased after HF feeding only in the 129/SVJ strain (Fig. 6A). While unanticipated initially, this finding is consistent with the aforementioned evidence that 129/SVJ mice, but not BL6 mice, induced other Th2 cytokines during steatohepatitis. Several other fibrosis-related genes, including α-smooth muscle actin (Fig. 6B), a marker of liver myofibroblasts (39), fibronectin (40) (Fig. 6C) and tissue inhibitor of metalloproteinase-1 (41) (Fig. 6D), were also significantly more induced in 129/SVJ mice than BL6 mice with steatohepatitis. Despite these differences, however, hepatic content of collagen α mRNA (Fig. 6E), Sirius red-stained fibrils (Fig. 6G–H) and hydroxyproline (C57BL6: control 0.578±0.008 mg/g of liver vs HF 0.385±0.07, P = 0.13; 129/SVJ: control 0.508±0.057 mg/g of liver vs HF 0.413±0.03, P = 0.24) were virtually identical in the two groups at the end of the 6-month treatment period, demonstrating a surprising disassociation between various well-accepted fibrosis markers and the actual severity of liver fibrosis in this model.
Fig. 6.
Differential induction of fibrosis markers in high-fat (HF) diet-fed C57BL6 and 129/SVJ mice. Expression of various fibrosis-related genes, including (A) transforming growth factor-β (TGF-, (B) α-smooth muscle actin (α-SMA), (C) fibronectin (FN), (D) tissue inhibitor of metalloproteinase (TIMP)-1 and (E) collagen 1α1, was evaluated by quantitative reverse transcriptase polymerase chain reaction analysis of liver RNA from BL6 and 129/SVJ mice fed either control diet (Ctr, n = 11/group) or HF diets (HF, n = 26/group) for 6 months. Liver sections were stained with picrosirius red, and collagen content was assessed by morphometry (F). Photomicrographs from a representative BL6 mouse (G) and a representative 129/SVJ mouse (H) after 6 months HF diet treatment. *P<0.05 vs respective Ctr; #P<0.05 vs BL6-HF group.
Discussion
Liver damage and fibrosis accrue gradually in NAFLD, as in most other types of chronic liver disease. Hence, most individuals die of other causes before developing cirrhosis. However, all known chronic liver disease populations are heterogeneous and harbour subgroups that become cirrhotic relatively rapidly. Because individuals who develop cirrhosis are at high risk for liver-related morbidity and mortality, efforts are focussed on identifying these patients at precirrhotic stages in order to implement effective therapies to arrest/reverse liver damage. Such work is leading to the identification of various genetic and environmental risk factors for progressive liver disease.
Nonalcoholic fatty liver disease is an obesity-related liver disease, and it was quickly recognized that hepatic triglyceride accumulation identified a subset of obese individuals with an increased risk for more advanced liver damage (6, 42). Nevertheless, most obese individuals with fatty livers appear to follow an indolent course, manifesting little liver damage or fibrosis even after decades of hepatic steatosis (2). Significant liver fibrosis/cirrhosis develops mainly in the subset of fatty liver patients with conspicuous histological evidence of hepatocyte injury and death (i.e. steatohepatitis) at the time of diagnosis (31, 43). Cross-sectional analysis of groups with steatohepatitis, as opposed to simple steatosis, demonstrated that the former had more evidence of oxidant stress and higher levels of proinflammatory cytokines (13, 44). Moreover, treatments that corrected these abnormalities tended to lessen liver damage (45, 46), engendering consensus that oxidant and inflammatory stress are the major determinants of NAFLD progression (26).
Results from the current study of two inbred mouse strains raises some doubt about the validity of the latter assumptions, and emphasize the importance of prospectively validating putative risk factors for disease progression in genetically diverse populations. Neither inbred mouse strain developed obesity, NAFLD or liver fibrosis spontaneously. However, when subjected to environmental factors that promote adiposity (HF diets), both developed comparable levels of obesity and hepatic triglyceride accumulation. One of the two strains (129/SVJ) responded to hepatic steatosis with a significant increase in hepatic free radical (25) production. Current dogma predicts that such oxidant stress would incite production of proinflammatory cytokines that would re-enforce reactive oxygen species (ROS) production and promote progression to steatohepatitis (26, 28). Although the latter occurred in 129/SVJ mice, similar disease progression was observed in the C57BL6 strain, which was able to constrain ROS production at pretreatment levels by upregulating various antioxidant enzymes. Given historical correlations between production of ROS and proinflammatory cytokines (44), it was also surprising that hepatic expression of proinflammatory cytokine genes and hepatic inflammatory cell infiltrates were actually greater in C57BL6 mice (which had lower net levels of oxidants). The severity of hepatic inflammation is thought to dictate eventual hepatic fibrosis (47). Hence, more extensive fibrosis might have been expected in the C57BL6 group. However, C57BL6 mice actually exhibited less induction of various fibrosis markers than 129/SVJ mice. The paradoxical enhancement of fibrosis-related gene induction in 129/SVJ mice might have been due, at least in part, to the tendency for their hepatic cytokine production to become Th2 polarized during steatohepatitis. This finding, in turn, suggests that generation of Th2 cytokines might be a more important r iskfactor for hepatic fibrosis than hepatic inflammation. On the other hand, 129/SVJ mice had not developed bridging fibrosis or cirrhosis despite suffering diet-induced overweight/obese since adolescence. Hence, Th2 polarization of hepatic cytokines and upregulation of various mesenchymal genes per se were not sufficient to cause liver fibrosis in the context of HF-diet-induced steatohepatitis. Conceivably, disease progression might eventually ensue if obesity is maintained as these mice continue to age. Indeed, this possibility is supported by a recent publication from another group who reported that 6–9 months of HF diet can induce NASH with fibrosis in Sprague–Dawley rats (48). It is doubtful, however, that such a time-consuming, expensive, and labour-intensive model will be widely adopted for elucidating mechanisms driving NAFLD progression.
The current results complement and extend a sobering body of evidence that none of the currently recognized risk factors for NAFLD-related cirrhosis, including obesity, insulin resistance, diabetes, chronic inflammatory states, oxidant stress, steatohepatitis or activation of fibrogenic genes, are sufficient to cause cirrhosis, even when exposure to one or more of these insults is very prolonged. Clinically relevant experimental models of liver fibrosis are needed to obtain a better understanding of mechanisms that cause some, but not most, individuals with steatohepatitis to develop cirrhosis. Such knowledge is essential to expedite development of diagnostic biomarkers, and to refine treatments for NAFLD.
Acknowledgement
The authors thank Dr Jiawen Huang for his help with animal care and Mr Carl Stone for administrative assistance.
Funding: This work was supported by the National Institutes of Health Grants RO1DK53792 to Anna Mae Diehl.
Abbreviations
- 129
129/SVJ
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ESR
electron spin resonance
- HF
high-fat diet
- IFN-γ
interferon-γ
- IL
interleukin
- NAFLD
nonalcoholic fatty liver disease
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase polymerase chain reaction
- α-SMA
α-smooth muscle actin
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- TIMP-1
tissue inhibitor of metalloproteinase 1
- TNF-α
tumour necrosis factor-α.
References
- 1.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 2.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi S, Hamaguchi K, Seike M, et al. Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. Exp Biol Med (Maywood) 2004;229:486–493. doi: 10.1177/153537020422900606. [DOI] [PubMed] [Google Scholar]
- 9.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem. 2005;38:203–217. doi: 10.1016/j.clinbiochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 13.Sahai A, Malladi P, Pan X, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 14.Schoonjans L, Kreemers V, Danloy S, et al. Improved generation of germline-competent embryonic stem cell lines from inbred mouse strains. Stem Cells. 2003;21:90–97. doi: 10.1634/stemcells.21-1-90. [DOI] [PubMed] [Google Scholar]
- 15.Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome. 1997;8:390–393. doi: 10.1007/s003359900453. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Corton C, Dix DJ, et al. Genetic background but not metallothionein phenotype dictates sensitivity to cadmium- induced testicular injury in mice. Toxicol Appl Pharmacol. 2001;176:1–9. doi: 10.1006/taap.2001.9262. [DOI] [PubMed] [Google Scholar]
- 17.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 18.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 20.Knecht KT, Bradford BU, Mason RP, Thurman RG. In vivo formation of a free radical metabolite of ethanol. Mol Pharmacol. 1990;38:26–30. [PubMed] [Google Scholar]
- 21.Towner RA, Qian SY, Kadiiska MB, Mason RP. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic Biol Med. 2003;35:1330–1340. doi: 10.1016/j.freeradbiomed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 23.Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022–1030. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 24.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 25.Burant CF, Sreenan S, Hirano K, et al. Troglitazone action is independent of adipose tissue. J Clin Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzawa N, Takamura T, Kurita S, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Otogawa K. Role of oxidative stress and Kupffer cells in hepatic fibrosis. J Gastroenterol Hepatol. 2007;22(Suppl. 1):S85–S86. doi: 10.1111/j.1440-1746.2006.04661.x. [DOI] [PubMed] [Google Scholar]
- 28.Kojima H, Sakurai S, Uemura M, et al. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31:S61–S66. doi: 10.1111/j.1530-0277.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 30.Bantel H, Lugering A, Poremba C, et al. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34:758–767. doi: 10.1053/jhep.2001.28229. [DOI] [PubMed] [Google Scholar]
- 31.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 32.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 33.Guebre-Xabier M, Yang S, Lin HZ, et al. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 34.Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 35.Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017–7026. doi: 10.4049/jimmunol.167.12.7017. [DOI] [PubMed] [Google Scholar]
- 36.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 37.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldstein AE, Papouchado BG, Angulo P, et al. Hepatic stellate cells and fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2005;3:384–389. doi: 10.1016/s1542-3565(04)00616-0. [DOI] [PubMed] [Google Scholar]
- 40.Matsui S, Takahashi T, Oyanagi Y, et al. Expression, localization and alternative splicing pattern of fibronectin messenger RNA in fibrotic human liver and hepatocellular carcinoma. J Hepatol. 1997;27:843–853. doi: 10.1016/s0168-8278(97)80322-4. [DOI] [PubMed] [Google Scholar]
- 41.Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005;19:136–138. doi: 10.1096/fj.04-2291fje. [DOI] [PubMed] [Google Scholar]
- 42.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- 43.Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin Liver Dis. 2007;27:327–338. doi: 10.1055/s-2007-991510. [DOI] [PubMed] [Google Scholar]
- 44.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 45.Yalniz M, Bahcecioglu IH, Kuzu N, et al. Amelioration of steatohepatitis with pentoxifylline in a novel nonalcoholic steatohepatitis model induced by high-fat diet. Dig Dis Sci. 2007;52:2380–2386. doi: 10.1007/s10620-006-9194-1. [DOI] [PubMed] [Google Scholar]
- 46.Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Sanyal AJ. Mechanisms of Disease: pathogenesis of non-alcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46–53. doi: 10.1038/ncpgasthep0084. [DOI] [PubMed] [Google Scholar]
- 48.Omagari K, Kato S, Tsuneyama K, et al. Effects of a long-term high-fat diet and switching from a high-fat to low-fat, standard diet on hepatic fat accumulation in Sprague-- Dawley Rats. Dig Dis Sci. 2008;53:3206–3212. doi: 10.1007/s10620-008-0303-1. [DOI] [PubMed] [Google Scholar]