Abstract
Quantification of acute brain injury in basal ganglia is essential for mechanistic and therapeutic studies in experimental intracerebral hemorrhage (ICH). Using conventional counting of degenerating cells based on morphological or immunohistochemical criteria, it is hard to define the boundary of the whole lesion area. Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa (DARPP-32) is a cytosolic protein highly enriched in medium-sized spiny neurons of the striatum. We developed new methods for quantifying lesion area by detecting the difference of the DARPP-32 negative area and the hematoma clot, and by measuring DARPP-32 protein level for semi-qualification in rat model of ICH. We found that DARPP-32 negative area around hematoma was present at day-1, peaked at day-3, and decreased at day-14 after ICH, a time course paralleled by DARPP-32 Western blots. The DARPP-32 negative area matched well with the necrotic area determined using propidium iodide. Treatment with an iron chelator, deferoxamine, attenuated the ICH-induced reduction in DARPP-32 protein levels. These results suggest that DARPP-32 is a simple and quantifiable indicator of ICH-induced neuronal death in basal ganglia.
Keywords: DARPP-32, intracerebral hemorrhage, iron, neuronal death
Intracerebral hemorrhage (ICH) is a subtype of stroke with high morbidity and mortality. Ganglionic (putamen, caudate, and thalamus) hemorrhages are the most common forms of ICH (1). Experimental ICH models are essential to study the pathophysiology of ICH, and necrotic, apoptotic and autophagic brain cell death has been found adjacent to the hematoma (1-4). However, there is a lack of a clearly demarcated lesion. A simple and specific method to quantify lesions in the basal ganglia would have great value for mechanistic and therapeutic studies in experimental ICH models.
Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa (DARPP-32) is a cytosolic protein highly enriched in medium-sized spiny neurons of the striatum and is identified as a major target for dopamine-activated adenylyl cyclase in the striatum (5, 6). At the ultrastructural level, DARPP-32 has been found in most subcellular compartments (7). On Western blots, DARPP-32 appears as one band, with a molecular weight of 32 kDa. DARPP-32 has been used as a selective neuronal marker in striatum (8, 9). In the current study, we quantified brain DARPP-32 levels in a rat model of ICH using Western blotting and immunofluorescent staining.
Materials and Methods
Animal Preparation and Intracerebral Injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. Male Sprague-Dawley rats (weighed 275–300g, Charles River Laboratories, Portage, MI) were used in this study. They were anesthetized with pentobarbital (40-50 mg/kg, intraperitoneally) and a polyethylene catheter (PE-50) was inserted into the right femoral artery to monitor arterial blood pressure and determine blood pH, PaO2, PaCO2, hematocrit, and glucose. It was also the source for the intracerebral autologous blood injection. Rectal temperature was maintained at 37.5°C using a feedback-controlled heating pad. Rats were positioned in a stereotactic frame (Kopf Instruments) and received an injection into the right basal ganglia. The coordinates were 0.2mm anterior to bregma, 5.5mm ventral, and 3.5mm lateral to midline. After intracerebral injection, the needle was removed and the skin incision closed with suture.
Experimental Groups
This study was divided into three parts. In the first part, rats received either a needle insertion (Sham) or an intracerebral infusion of 100μl autologous whole blood. Rats were euthanized at 1, 3 or 14 days later for Western blot analysis and immunofluorescent staining. In the second part, propidium iodide (50 mg/mL, 2μL) together with 100μl autologous whole blood was injected into the right basal ganglia and animals were euthanized 3 days later for immunofluorescent staining. In the third part, rats had an intracerebral infusion of 100μl autologous whole blood and were treated with either vehicle (saline) or an iron chelator, deferoxamine (DFX; 100 mg/kg, i.p., 2 h after ICH and then at 12-hour intervals for 3 days). Brains were used for Western blot analysis and immunohistochemistry.
Immunofluorescent Staining
Immunofluorescent staining was performed as described previously (10). Briefly, rats were anesthetized and subjected to intracardiac perfusion with 4% paraformaldehyde in 0.1mol/L phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4% paraformaldehyde for 12 h, then immersed in 25% sucrose for 3 to 4 days at 4°C. Brains were then placed in embedding compound and sectioned on a cryostat (18μm thick). Coronal sections from 1mm posterior to the blood injection site were stained with DARPP-32. The primary antibody was rabbit anti-DARPP-32 antibody (Cell Signaling Technology, 1:800 dilution). Alexa Fluro 488-conjugated donkey anti-rabbit mAb (Invitrogen, 1:500 dilution) was used as the secondary antibody.
For experiments in which damaged cells were examined with propidium iodide injection in vivo PI and was DARPP-32 immunohistochemistry ex vivo, propidium iodide labeling was photographed using emission and excitation wavelengths of 568 and 585nm, respectively.
Western Blot Analysis
Western blot analysis was performed as previously described (10). The brains were perfused and removed under pentobarbital anesthesia, and a coronal brain slice (about 3 mm thick) 4 mm from the frontal pole was cut with a blade. The clot was moved, and the ipsilateral and contralateral basal ganglia were sampled. Brain tissue was immersed in Western sample buffer and sonicated. Protein concentration was determined by Bio-Rad protein assay kit, and 40μg protein from each sample was separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham). Membranes were probed using a DARPP-32 (1:10000 dilution) primary antibody followed by a goat anti-rabbit IgG (Bio-Rad; 1:2,500 dilution) secondary antibody. Antigen-antibody complexes were visualized with the enhanced chemiluminescence system (Amersham) and were exposed to Kodak X-OMAT film. The relative densities of bands were analyzed using Image J.
Statistical Analysis
All data in this study are presented as means ± SD. Comparison of mean values was conducted using unpaired student's t-tests or ANOVA analysis of variance. Significance levels were measured at p<0.05.
Results
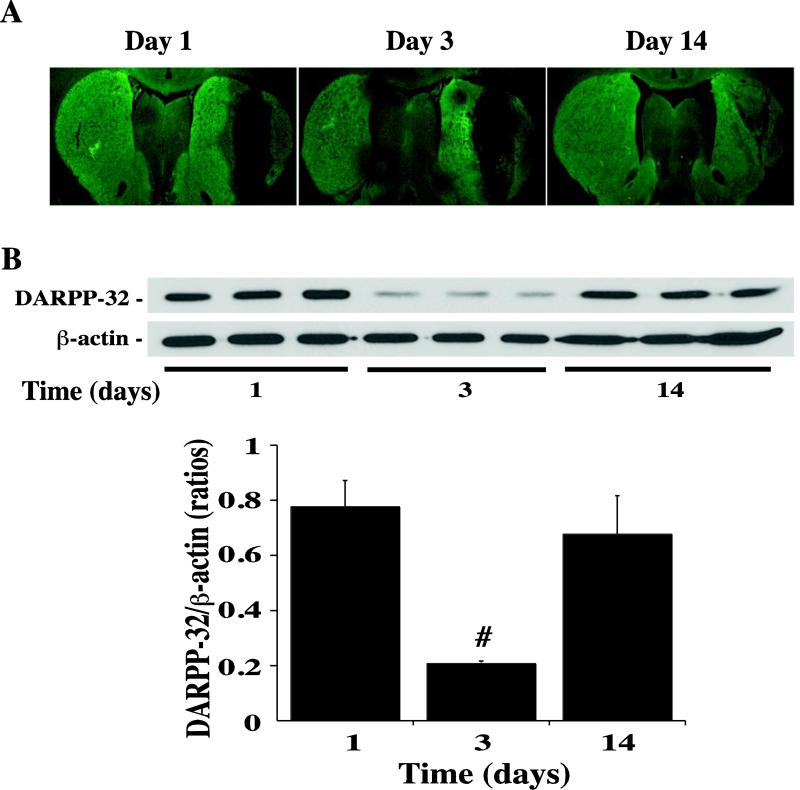
DARPP-32, a specific marker of GABAergic neurons in basal ganglia (9), is expressed in neuronal cell bodies and dendrites. There was marked DARPP-32 immunofluorescent staining bilaterally in the basal ganglia in coronal sections compared to cortical areas (Fig. 1). However, there was a fluorescence-negative perihematomal area in the ipsilateral caudate at day-1 after autologous blood injection (Fig. 1).
Figure 1.
Coronal sections of rat brain one day after injection of 100μl blood into the right caudate. Sections were used for hematoxylin and eosin staining (A) or DARPP-32 immunohistochemistry (B). While DARPP-32 immunohistochemistry showed bilateral staining in the caudate there was a perihematomal DARPP-32 negative area in the ipsilateral basal ganglia. The arrow indicates the hematoma. Scale bar = 2mm.
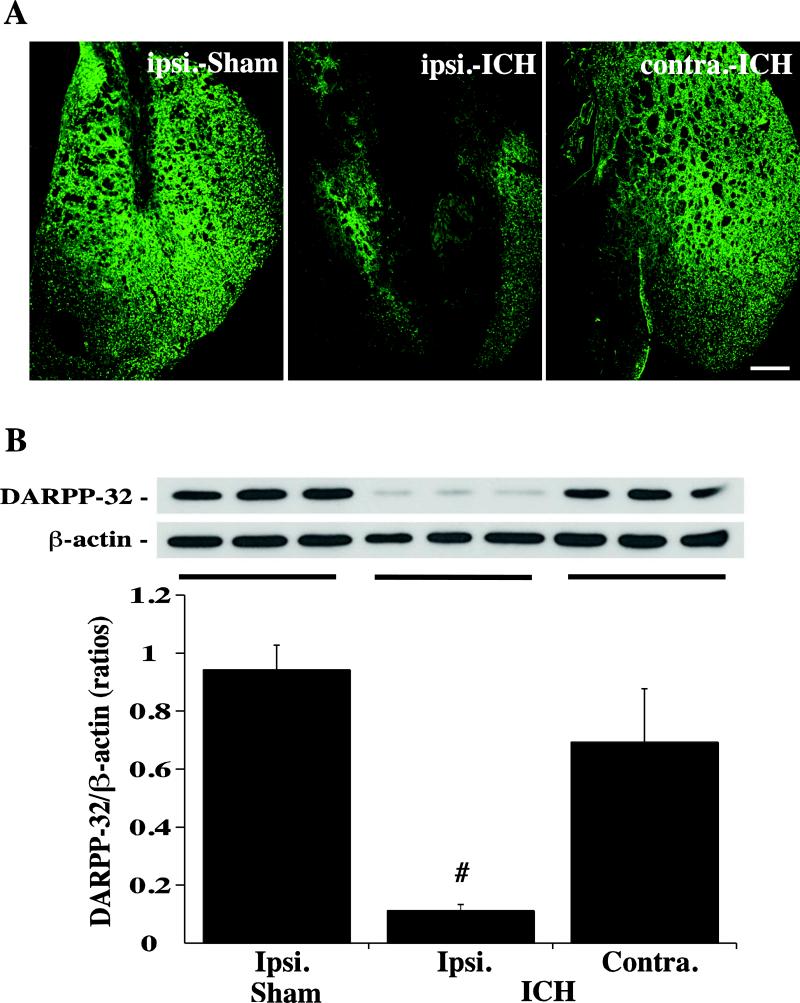
By measuring the difference of immunoflurescent negative area and clot, the time course of DARPP-32 staining demonstrated that the ipsilateral lesion area was increased at day-1, peaked at day-3, and decreased markedly at day-14 after ICH (6.2 ± 0.4mm2 at day-3 vs. 4.6 ± 1.0mm2 at day-1 and 3.5 ± 0.7mm2 at day-14, p<0.05, Fig. 2A). Using Western blot analysis, we found that DARPP-32 levels in the ipsilateral basal ganglia were decreased significantly at day 3 after ICH (Fig. 2B). Compared with Sham and the contralateral basal ganglia, ICH resulted in a large DARPP-32 negative area in the ipsilateral basal ganglia at day-3 after ICH (Fig. 3A). DARPP-32 levels in the ipsilateral basal ganglia were significantly decreased as determined by Western blot analysis (DARPP-32/ actin: 0.11 ± 0.02 versus 0.94 ± 0.08 in the Sham group and 0.69 ± 0.18 in the contralateral basal ganglia, p<0.01; Fig. 3B).
Figure 2.
Immunohistochemistry showing DARPP-32 negative areas (A) and Western blots of the DARPP-32 protein levels (B) in the ipsilateral basal ganglia at 1, 3 and 14 days after ICH. Values are expressed as means ± SD, n=3 per group, # p<0.01 vs. days 1 and 14.
Figure 3.
Immunoreactivity (A) and protein levels (B, Western blot) of DARPP-32 in the ipsi- and contralateral basal ganglia at day-3 after a needle (Sham) or 100μl blood (ICH) injected into the right caudate. β-actin was examined as a protein loading control for Western blots and DARPP-32 levels expressed a ratio to β-actin levels. Values are means ± SD, n=3, # p<0.01 vs. in Sham and in contralateral. Scale bar = 500μm.
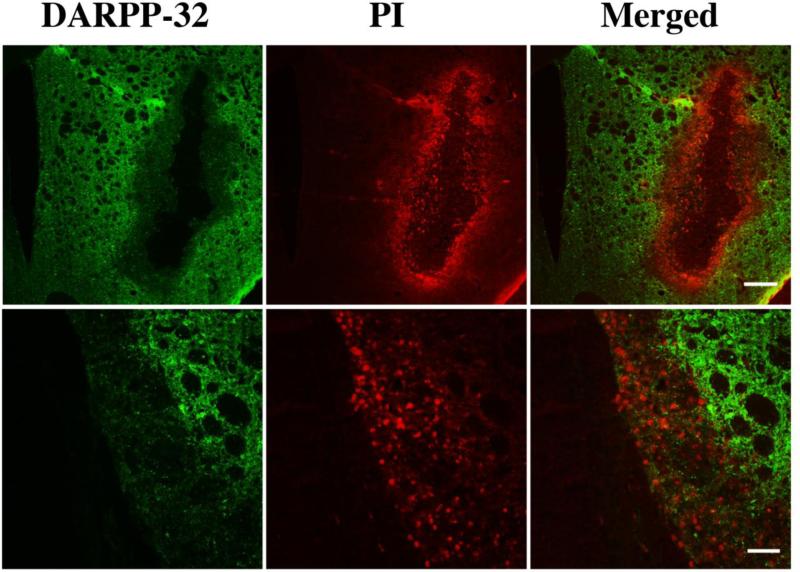
To determine whether the DARPP-32 negative area is the area with significant neuronal death, propidium iodide (PI) staining was used. Plasmalemma permeability to PI is associated with markers of cell death as shown in ischemic, traumatic brain injury and ICH models (11-13). By using in vivo PI co-labeled with DARPP-32 ex vivo at day-3 after autologous blood injection, we found that there was no overlap between PI-positive cells and DARPP-32 positive cells around the hematoma (Fig. 4). This result indicates that the DARPP-32 negative area could precisely represent the lesion area in the basal ganglia.
Figure 4.
Alexa Fluro 488-labeled DARPP-32 (green) and positive staining for propidium iodide (PI; red) in the ipsilateral basal ganglia at day-3 after ICH. Scale bar = 500μm (upper panel) and 100μm (lower panel). Please note that the DARPP-32 negative area superimposes the PI-positive area.
Erythrocyte lysis and hemoglobin toxicity contribute to brain edema and cell death after ICH (14). Iron is one of the hemoglobin degradation products and plays a key role in ICH-induced brain injury (15). DFX is an iron chelator which reduces ICH-induced brain injury (16, 17). In the current study, we found that the effects of DFX on ICH-induced brain injury can be determined by the DARPP-32 measurement. Western-blot analysis showed that systemic treatment with DFX largely prevents the ICH-induced DARPP-32 reduction in the ipsilateral basal ganglia (DARPP-31/β–actin: 0.73 ± 0.02 vs. 0.18 ± 0.03 in the vehicle-treated group, p<0.01, Fig. 5).
Figure 5.
DARPP-32 staining (A) and protein levels (B) in the ipsilateral basal ganglia of rats after ICH. Rats were treated with either deferoxamine (DFX) or vehicle for 3 days after ICH. β-actin was examined as a protein loading control for Western blots and DARPP-32 levels expressed a ratio to β-actin levels. Values are expressed as means ± SD, # p<0.01 vs. the vehicle-treated group. Scale bar = 500 μm.
Discussion
It has been difficult to quantify neuronal death after ICH because of the lack of a clearly defined infarct around the hematoma. Two common methods for detecting brain cell death after ICH are terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin in situ nick end-labeling (TUNEL), used to detect DNA damage (18), and Fluoro-Jade used to detect neuronal degeneration (19). Although both are sensitive measures of cell death, it is hard to quantify the lesion size using those methods. In the current study, we demonstrated that DARPP-32 lesion size and DARPP-32 protein levels are two quantifiable markers of neuronal damage in the basal ganglia after ICH.
DARPP-32 is a cytosolic protein highly enriched in medium-sized spiny neurons and it is a major target for dopamine-activated adenylyl cyclase in striatum (5, 6, 20). The striatum is a central component of the basal ganglia (21). DARPP-32 is expressed at very high concentrations (~50μM) in virtually all medium spiny neurons (22), which constitute the major cell type (95%) in striatum. At the ultrastructural level, DARPP-32 is found in most subcellular compartments (7) with immunoreactivity being observed throughout the cytoplasm and in dendrites. In our study, we have shown that DARPP-32 is a simple and precise tool to detect neurons in basal ganglia.
In this study, we used propidium iodide in vivo to determine cell injury following ICH. We found that propidium iodide-positive area matches the DARPP-32 negative area around the clot. Propidium iodide is a 668 Da membrane impermeable nucleic acid stain that emits bright red fluorescence when bound to RNA or DNA. Ischemic, hemorrhagic and traumatic brain injury can induce plasmalemma permeability to propidium iodide and increased propidium iodide permeability is a marker of necrosis (11-13). Our results indicate that DARPP-32 can be a sensitive marker of neuronal damage in the basal ganglia following ICH.
Measuring the difference between the DARPP-32 negative area and the clot area, there was a significant perihematomal lesion at ipsilateral lesion area at day 1. The lesion area peaked at day 3 and decreased markedly by day 14 after ICH. Lesion semi-quantification by Western Blotting also supported this result with a recovery of DARPP-32 levels by day 14. Behavioral studies in the rat show acute neurological deficits followed by a progressive recovery of function between day 3 and 14 (23). Temporally, the loss of DARPP-32 containing neurons may contribute to the initial neurological deficits, while the recovery of DARPP-32 levels may contribute to the restoration of function. The nature of the recovery in DARPP-32 levels in the caudate awaits elucidation (e.g. is it related to a recovery in neurons that were damaged but not killed, and/or upregulation in neurons more distant from the hematoma).
Iron has a major role in brain damage following ICH. Brain iron overload causes brain edema and neuronal death in the acute phase and brain atrophy later after ICH (4, 24, 25). We have demonstrated that deferoxamine, an iron chelator, reduces ICH-induced brain edema, neuronal death, brain atrophy and neurological deficits in different animal models (1, 4). In the current study, deferoxamine treatment also attenuated the ICH-induced reduction in DARPP-32, reconfirming the ability of this iron chelator to attenuate ICH-induced brain injury.
In conclusion, DARPP-32 can be used as a simple and reliable marker of neuronal injury in basal ganglia after experimental ICH.
Acknowledgments
Sources of Funding
This study was supported by grants NS-039866, NS-057539, NS-073595 and NS-079157.
Footnotes
Disclosures
None
References
- 1.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006 Jan;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000 Feb;20(2):396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 3.He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2008 May;28(5):897–905. doi: 10.1038/sj.jcbfm.9600578. [DOI] [PubMed] [Google Scholar]
- 4.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012 Aug;11(8):720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005 Aug 1;65(15):6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 6.Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983 Jan 6;301(5895):69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 7.Ouimet CC, Greengard P. Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J Neurocytol. 1990 Feb;19(1):39–52. doi: 10.1007/BF01188438. [DOI] [PubMed] [Google Scholar]
- 8.Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006 Jun 30;140(2):607–22. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka H, Niizuma K, Katsu M, Okami N, Sakata H, Kim GS, et al. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J Cereb Blood Flow Metab. 2011 Mar;31(3):868–80. doi: 10.1038/jcbfm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. [Research Support, U.S. Gov't, P.H.S.] 1999 Jun;30(6):1247–55. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 11.Unal-Cevik I, Kilinc M, Can A, Gursoy-Ozdemir Y, Dalkara T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke. 2004 Sep;35(9):2189–94. doi: 10.1161/01.STR.0000136149.81831.c5. [DOI] [PubMed] [Google Scholar]
- 12.Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, et al. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008 Mar;28(3):490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012 Feb;43(2):524–31. doi: 10.1161/STROKEAHA.111.635672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002 Feb;96(2):287–93. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003 Dec;34(12):2964–9. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004 Apr;100(4):672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009 Jun;40(6):2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001 Apr;48(4):875–82. doi: 10.1097/00006123-200104000-00037. discussion 82-3. [DOI] [PubMed] [Google Scholar]
- 19.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000 Aug 25;874(2):123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 20.Anderson KD, Reiner A. Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 1991 Dec 24;568(1-2):235–43. doi: 10.1016/0006-8993(91)91403-n. [DOI] [PubMed] [Google Scholar]
- 21.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 22.Ouimet CC, Langley-Gullion KC, Greengard P. Quantitative immunocytochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res. 1998 Oct 12;808(1):8–12. doi: 10.1016/s0006-8993(98)00724-0. [DOI] [PubMed] [Google Scholar]
- 23.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002 Oct;33(10):2478–84. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 24.Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, et al. NeuN: a useful neuronal marker for diagnostic histopathology. The journal of histochemistry and cytochemistry. 1996 Oct;44(10):1167–71. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Xi G, Keep RF, Hua Y. Iron enhances the neurotoxicity of amyloid beta. Transl Stroke Res. 2012 Jan 1;3(1):107–13. doi: 10.1007/s12975-011-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]