Abstract
Irritable bowel syndrome (IBS) is the most common functional bowel disease that affects up to 15% of the US population. The majority of patients with IBS have significant bloating and gas. Recent evidence is beginning to suggest that patients with IBS may have an alteration in the gastrointestinal flora. Specifically, findings suggest that patients with IBS have excessive bacteria in the small bowel, referred to as bacterial overgrowth. Therefore there may be benefits of antibiotic-based therapies for IBS. Rifaximin is a nonabsorbable antibiotic that demonstrates no clinically relevant bacterial resistance. Some studies have demonstrated the efficacy and durable improvement of IBS symptoms after treatment with rifaximin. In this review we explore the current data showing the association of small intestinal bacterial overgrowth (SIBO) and IBS as well as review the available data on the clinical use of rifaximin in the treatment of SIBO in patients with IBS.
Keywords: irritable bowel syndrome, lactulose breath test, microbiome, rifaximin, small intestinal bacterial overgrowth
Introduction
The complex communities of microorganisms that colonize the human gastrointestinal tract play an important role in human health. The development of culture-independent molecular techniques has provided new insights in the composition and diversity of the intestinal microbiota. There are up to a trillion microbes per milliliter of luminal contents in the distal gut [Levitt, 1969]. Our relationship with colonizing gut microbes begins at birth. The specific constitution of this microbiome varies between individuals and between the various regions of the gastrointestinal tract [Kim and Lin, 2007]. Evidence confirms that the human host and biology of gut bacteria are inseparable, so what happens if the relationship between the human host and gut microbiota is disturbed? Recent evidence suggests that a shift in the host–gut microbial relationship as seen in small intestinal bacterial overgrowth (SIBO) may contribute to the pathogenesis of irritable bowel syndrome (IBS). The overgrowth of microbiota in the small intestine can cause excessive gas production and malabsorption with a variety of nonspecific symptoms, such as diarrhea, gas bloating, abdominal pain and constipation [Quigley and Quera, 2006]. Recent evidence suggests a role for gut bacteria and antibiotics in the pathophysiology and treatment of IBS respectively. There may be benefits of the antibiotic rifaximin demonstrated by efficacy and durable improvement in symptoms over 3 months [Pimentel et al. 2011b]. Rifaximin is a rifamycin derivative with a broad range of gastrointestinal-specific antibiotic effects that demonstrates no clinically relevant bacterial resistance because less than 0.5% of the oral dose is absorbed. It has low bioavailability, therefore there is a low risk of systemic toxicity, side effects and drug interactions. These features give rifaximin an importance when considering long-term or repeated courses of treatment [Majewski et al. 2007]. In this review we explore the data on the clinical use of rifaximin in relieving the symptoms of SIBO in patients with IBS.
An alteration in host–gut microbial relationship may cause irritable bowel symptoms
Most of our effort in treating IBS has been based on a symptom approach. Recent evidence is beginning to suggest that patients with IBS may have an alteration in gastrointestinal flora. Specifically, findings suggest that patients with IBS have excessive bacteria in the small bowel, known as bacterial overgrowth. Up to one half of patients with IBS may have SIBO.
One subset of patients with IBS have a history of symptoms beginning after a gastroenteritis event. They have been termed ‘postinfection IBS’. The pathogenesis of the syndrome could be explained by ongoing gut inflammation and mucosal-immune reactivity [Azpiroz et al. 2007]. Other factors hypothesized to contribute to SIBO include an incompetent ileocecal valve, a gastric hypochlorhydric state, impaired small bowel motility and reduced migrating motor complex, concurrent use of pharmacological agents including anticholinergics, narcotics and proton pump inhibitors, as well as small bowel diverticuli. Follow-up work in this area has begun to demonstrate associated factors between gut bacteria and IBS that may explain the different types of IBS [Pimentel and Lezcano, 2007]. For example, a diarrhea-dominant pattern may be a result of bacteria predominately producing hydrogen while for a constipation-dominant pattern the bacteria may produce more methane. Recent reports in patients with IBS that is diarrhea dominant showed that the altered composition of the intestinal microbiota is associated with a significant increase in detrimental bacterial groups and a decrease in beneficial ones, and a reduction in microbial richness [Carroll et al. 2012]
Glucose/lactulose breath test: an approach to diagnose small intestinal bacterial overgrowth in patients with irritable bowel syndrome
More than 80% of a gut microbial strain cannot be cultured by standard means [Eckburg et al. 2005], therefore detecting the gas production by these strains has gained importance as a simple and practical clinical test. The basic principle of the breath test is that the bacteria in the bowel ferment the administrated carbohydrates and produce gases in different quantities from the natural gases produced by human metabolism. Humans produce carbon dioxide, hydrogen and methane, and even hydrogen sulfide. Detecting measured concentrations of hydrogen and methane in the breath is the goal of the breath test. One clinical approach for diagnosing bacterial overgrowth is to combine the test results with the patient’s outcome to the treatment. For example, if the lactulose breath test (LBT) is positive and the patient improves symptomatically with antibiotics and the breath test becomes normal, this suggests that SIBO was the cause of some of the symptoms. Glucose can be used or lactulose. One limitation is that glucose is absorbed in the proximal portion of the small intestine and it may not present to the distal portion of the small bowel. Therefore, it is possible to have a negative glucose breath test in patients with SIBO in the ileum. The gold standard test for diagnosing SIBO is a small bowel aspirate. However, the small intestine is up to 20 ft long and it is unlikely that a culture of the fluid obtained from within the reach of an upper endoscopy is representative of the entire small bowel. Enteroscopy has been used to sample the bowel and cultures are considered positive for SIBO if the total bacterial count is at least 1 × 105 colony-forming units (CFU) per ml of fluid.
In a recent publication, duodenal aspirates were collected for quantitative cultures from 162 patients with IBS and from 24 healthy controls. The results showed that 43% and 12% of patients had more than 5 × 103 CFU/ml of bacteria in the duodenal lumen respectively. It was also found that 24% and 4% of patients had more than 1 × 104 CFU/ml of bacteria in the lumen of the duodenum respectively. When the detection threshold was increased up to 1 × 105 CFU/ml, no differences could be found between patients with IBS and the control group. These findings indicate that the threshold of intestinal overgrowth may need to be reassessed. Hydrogen and methane breath tests using various substrates like glucose, lactulose, lactose and fructose are being used more and more to diagnose SIBO as well as lactose or fructose malabsorption and intolerance. Though quantitative culture of jejunal aspirate is considered the gold standard for the diagnosis of SIBO, hydrogen and methane breath tests, in spite of their low sensitivity, are popular since they are noninvasive. Glucose breath test and LBT are utilized in the clinical setting for the diagnosis of SIBO as well as lactulose. False positives can occur for lactulose because of its rapid small bowel transit [Ghoshal, 2011]. The accepted breath test interpretation is that if the hydrogen peak exceeds 20 ppm when the baseline is below 10 ppm, then it is a positive result. For methane, exceeding 10 ppm is considered positive if the baseline is less than 5 ppm at the start. When the patient starts with a baseline above10 ppm, a further increase of more than 12 ppm indicates a positive breath test [Majewski et al. 2007]. Another challenge of breath test interpretation with lactulose is the occurrence of two peaks during testing. The first peak is the abnormal presence of bacteria in the small intestine and the second peak is indicative of entry of lactulose into the colon. Hydrogen and methane are released when lactulose mixes with the luminal bacteria. The first peak is usually seen within 90 min, depending on the rate of small bowel transit. An example of breath testing is seen in Figure 1.
Figure 1.
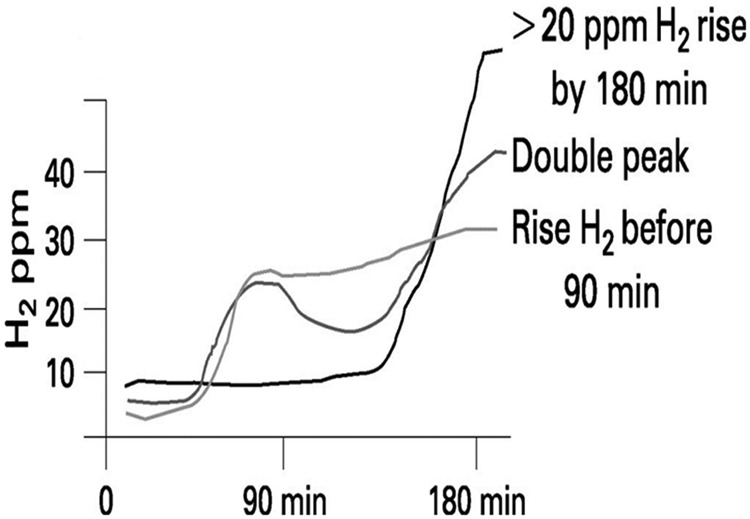
Measurement of hydrogen levels during a breath test.
Treating and retreating small intestinal bacterial overgrowth and irritable bowel syndrome with antibiotics
IBS is the most common functional bowel disease affecting up to 15% of the population, with women accounting for 70–75% of this group. The criteria used to diagnose IBS are the presence of symptoms that meet the Rome criteria [Longstreth et al. 2006]. There is no biological marker or unifying framework available to explain the different symptoms of IBS [Talley, 2006]. The majority of patients with IBS also have significant bloating and gas as part of their presentation, in addition to a degree of constipation, diarrhea and pain [Talley, 2006]. Patients with IBS who present with bloating and gas usually have the perception of abdominal distention. Recent data suggest that abnormalities in gas production and its transit through the small intestine could explain these symptoms. Whether SIBO contributes to some of this gas and bloating in IBS remains an area of active investigation. A recent study was designed to test the effect of treatment with a nonabsorbable antibiotic for IBS in a double-blind design. Patients with IBS underwent a LBT with the results blinded. All patients were subsequently randomized into two treatment groups (neomycin or placebo). One week after completion of treatment, patients returned for repeat LBT. A symptom questionnaire was administered on both days. After exclusion criteria were met, 111 patients (55 on an antibiotic, 56 on placebo) entered the study, with 84% having an abnormal LBT compared with 20% of healthy controls [Pimentel et al. 2003]. Antibiotic administration resulted in a 35% improvement in symptoms compared with 11.4% in those on placebo. In addition, bowel normalization was reported in 35.3% of patients after starting antibiotics compared with 13.9% for those on placebo. The study concluded that an abnormal LBT is common in patients with IBS. Normalization of LBT with an antibiotic leads to a significant reduction in IBS symptoms. This study provided more evidence to suggest the gut microbial origin of IBS [Pimentel et al. 2003].
A more recent study that included patients who had IBS without constipation were assigned to either rifaximin at a dose of 550 mg or placebo, three times daily for 2 weeks. These patients were followed for an additional 10 weeks [Pimentel et al. 2011a]. The primary endpoint was adequate relief of IBS symptoms. The proportion of patients who had adequate relief of IBS-related bloating and gas was assessed weekly. Adequate relief was defined as self-reported relief of symptoms for at least 2 of the first 4 weeks of treatment. The improvement was reported as 40.8% versus 31.2% for placebo (p = 0.01). Secondary endpoints included the proportion of patients who had a response to treatment as assessed by daily self-ratings of global IBS symptoms and individual symptoms of bloating, abdominal pain and stool consistency during the 4 weeks after treatment. The outcome of treatment with rifaximin was that significant relief of IBS symptoms, including bloating, abdominal pain and loose or watery stools, was provided for 2 weeks after treatment among patients who had IBS without constipation. One limitation of this study was that no breath test was performed at baseline to define the percentage of patients who had SIBO, or after the course of rifaximin to assess symptom correlation. Hence the study did not specifically treat patients with SIBO but included all patients with IBS without constipation.
Another recent study comprised 106 of 150 patients with IBS (71%) who were LBT positive and treated with rifaximin. Assessment at week 4 following commencement of therapy showed that rifaximin provided significant improvement of the following IBS-associated symptoms: bloating, flatulence, diarrhea, pain. The authors concluded that rifaximin treatment alleviated symptoms in patients with IBS who were LBT positive and this improvement was observed for a period of 3 months after 2 weeks of treatment with rifaximin [Schoepfer, 2012].
Therefore, if IBS symptoms are caused by an antibiotic-sensitive mechanism, would the improvement in symptoms persist even after rifaximin is withdrawn? A retrospective chart review examined the efficacy of rifaximin in both the treatment and retreatment of IBS compared with neomycin [Yang et al. 2008]. Out of 98 patients, 84 received one course of rifaximin. Fifty (60%) had a follow-up breath test and, of these, 31 (62%) were clinical responders and 19 (38%) were nonresponders. Of the 31 responders, 25 (81%) had a normal follow-up breath test compared with only 3 of the 19 (16%) nonresponders. Of the patients given rifaximin, 69% (58 out of 84) had a clinical response compared with only 38% (9 out of 24) of those on neomycin and 44% (27 out of 61) of those on all nonrifaximin antibiotics, suggesting that rifaximin seems to be more effective than other antibiotics in the treatment and retreatment of IBS.
Recurrence of symptoms after the treatment course
What is the likelihood of SIBO recurrence after completing a course of rifaximin treatment? To help address this question, Lauritano and colleagues treated 80 consecutive patients with IBS and SIBO with rifaximin 400 mg three times daily for 10 days. The presence of SIBO was followed up at 3, 6 and 9 months after the end of treatment. Recurrence of SIBO was documented in 12.6% of patients at 3 months, in 27.5% of patients at 6 months and in 43.7% of patients at 9 months. These results suggest that there is the need for further treatment courses in many patients [Lauritano et al. 2008].
Rifaximin dosing ranges for treatment of irritable bowel syndrome
A dose-finding study evaluating different doses of rifaximin showed that a higher dose of 1200 mg/day is associated with better therapeutic efficacy in terms of SIBO eradication than 600 and 800 mg/day for 7 days. Some investigators suggest using even higher doses of rifaximin in patients who are not responding to 1200 mg/day. Patients who received rifaximin 1200 mg/day experienced a mean improvement of 52% in global IBS symptoms at the end of rifaximin treatment. Similarly, patients who initially did not respond to treatment and who received additional rifaximin at a dose of 2400 mg/day experienced a 53% mean improvement in IBS symptoms. Forty-nine percent of patients who received initial rifaximin and 47% of patients who received high-dose rifaximin achieved at least a 50% global symptom improvement during at least one follow-up visit. High-dose rifaximin successfully normalized LBT results in 11% of patients. Rifaximin was found to be well tolerated. The conclusion was that rifaximin at a dose of 1200 mg/day for 10 days reduced gastrointestinal symptoms in patients with IBS. Patients with incomplete symptom resolution and with positive LBT may respond to increased doses of rifaximin [Jolley, 2011; Majewski et al. 2007].
Recent studies showed that treatment with rifaximin at a dose of 550 mg three times daily for 14 days provides better relief of IBS symptoms than placebo for up to 10 weeks after completion of therapy [Pimentel et al. 2011a].
Rifaximin and irritable bowel syndrome: present and future
There is accumulating evidence pointing towards the benefit of a short course of treatment with rifaximin in the global improvement of patients with IBS. The United States Food and Drug Administration, however, requires further data about the dose of rifaximin and whether patients need another course of rifaximin if their symptoms recur. A new trial addressing this question has commenced.
Acknowledgments
Il J. Paik, MD and Mohamed Othman, MD assisted with the drafting and reviewing of this manuscript. However, the author is responsible for the final document.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no conflicts of interest.
Contributor Information
Mohammed Saadi, Department of Medicine, Texas Tech University Health Science Center – Paul L. Foster School of Medicine, El Paso, TX, USA.
Richard W. McCallum, Texas Tech University Health Sciences Center/Paul L. Foster School of Medicine, Department of Medicine, Division of Gastroenterology, 4800 Alberta Ave , El Paso Texas 79905, USA
References
- Azpiroz F., Bouin M., Camilleri M., Mayer E., Poitras P., Serra J., et al. (2007) Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 19: 62–88 [DOI] [PubMed] [Google Scholar]
- Carroll I., Ringel-Kulka T., Siddle J., Ringel Y. (2012) Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg P., Bik E., Bernstein C., Purdom E., Dethlefsen L., Sargent M., et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal U. (2011) How to interpret hydrogen breath tests. J Neurogastroenterol Motil 2011; 17(3): 312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley J. ( 2011) High-dose rifaximin treatment alleviates global symptoms of irritable bowel syndrome. Clin Exp Gastroenterol 4: 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lin H. (2007) Contribution of gut microbes to GI motility disorder. Pract Gastroenterol. GI motility, A series from AMS [Google Scholar]
- Lauritano E., Gabrielli M., Scarpellini E., Lupascu A., Novi M., Sottili S., et al. (2008) Small intestinal overgrowth recurrence after antibiotic therapy. Am J Gastroenterol 103: 2031–2035 [DOI] [PubMed] [Google Scholar]
- Levitt M. (1969) Production and excretion of hydrogen gas in man. N Engl J Med 281: 122–127 [DOI] [PubMed] [Google Scholar]
- Longstreth G., Thompson W., Chey W., Houghton L., Mearin F., Spiller R. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491 [DOI] [PubMed] [Google Scholar]
- Majewski M., Reddymasu S., Sostarich S., Foran P., McCallum R. (2007) Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci 333: 266–270 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Chow E., Lin H. (2003) Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 98: 412–419 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Lembo A., Chey W., Zakko S., Ringel Y., Yu J., et al. TARGET Study Group (2011a) Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 364: 22–32 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Lezcano S. (2007) Irritable bowel syndrome: bacterial overgrowth – what’s known and what to do. Curr Treat Options Gastroenterol 10: 328–337 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Morales W., Chua K., Barlow G., Weitsman S., Kim G., et al. (2011b) Effects of rifaximin treatment and retreatment in non-constipated IBS subjects. Dig Dis Sci 56: 2067–2072 [DOI] [PubMed] [Google Scholar]
- Quigley E., Quera R. (2006) Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology 130(2 Suppl. 1): S78–S90 [DOI] [PubMed] [Google Scholar]
- Schoepfer A. (2012) Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther 2012; 36(11): 1084–1093 [DOI] [PubMed] [Google Scholar]
- Talley N. (2006) Irritable bowel syndrome. Intern Med J 36: 724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lee H., Low K., Chatterjee S., Pimentel M. (2008) Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci 53: 169–174 [DOI] [PubMed] [Google Scholar]


