Abstract
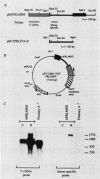
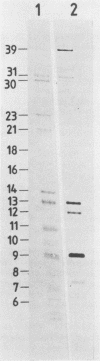
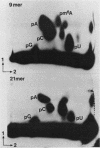
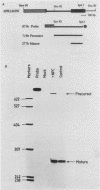
N6-methyladenosine (m6A) residues occur at internal positions in most cellular and viral RNAs; both heterogeneous nuclear RNA and mRNA are involved. This modification arises by enzymatic transfer of a methyl group from S-adenosylmethionine to the central adenosine residue in the canonical sequence G/AAC. Thus far, m6A has been mapped to specific locations in eucaryotic mRNA and viral genomic RNA. We have now examined an intron-specific sequence of a modified bovine prolactin precursor RNA for the presence of this methylated nucleotide by using both transfected-cell systems and a cell-free system capable of methylating mRNA transcripts in vitro. The results indicate the final intron-specific sequence (intron D) of a prolactin RNA molecule does indeed possess m6A residues. When mapped to specific T1 oligonucleotides, the predominant site of methylation was found to be within the consensus sequence AGm6ACU. The level of m6A at this site is nonstoichiometric; approximately 24% of the molecules are modified in vivo. Methylation was detected at markedly reduced levels at other consensus sites within the intron but not in T1 oligonucleotides which do not contain either AAC or GAC consensus sequences. In an attempt to correlate mRNA methylation with processing, stably transfected CHO cells expressing augmented levels of bovine prolactin were treated with neplanocin A, an inhibitor of methylation. Under these conditions, the relative steady-state levels of the intron-containing nuclear precursor increased four to six times that found in control cells.
Full text
PDF
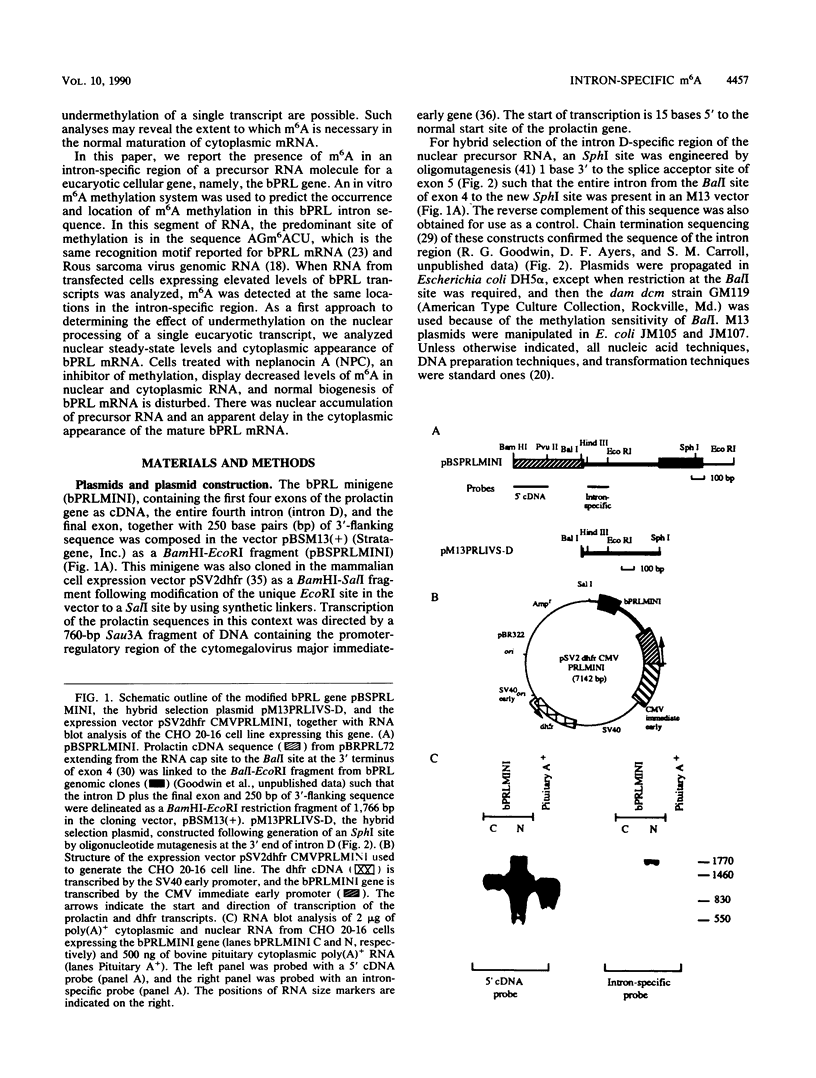




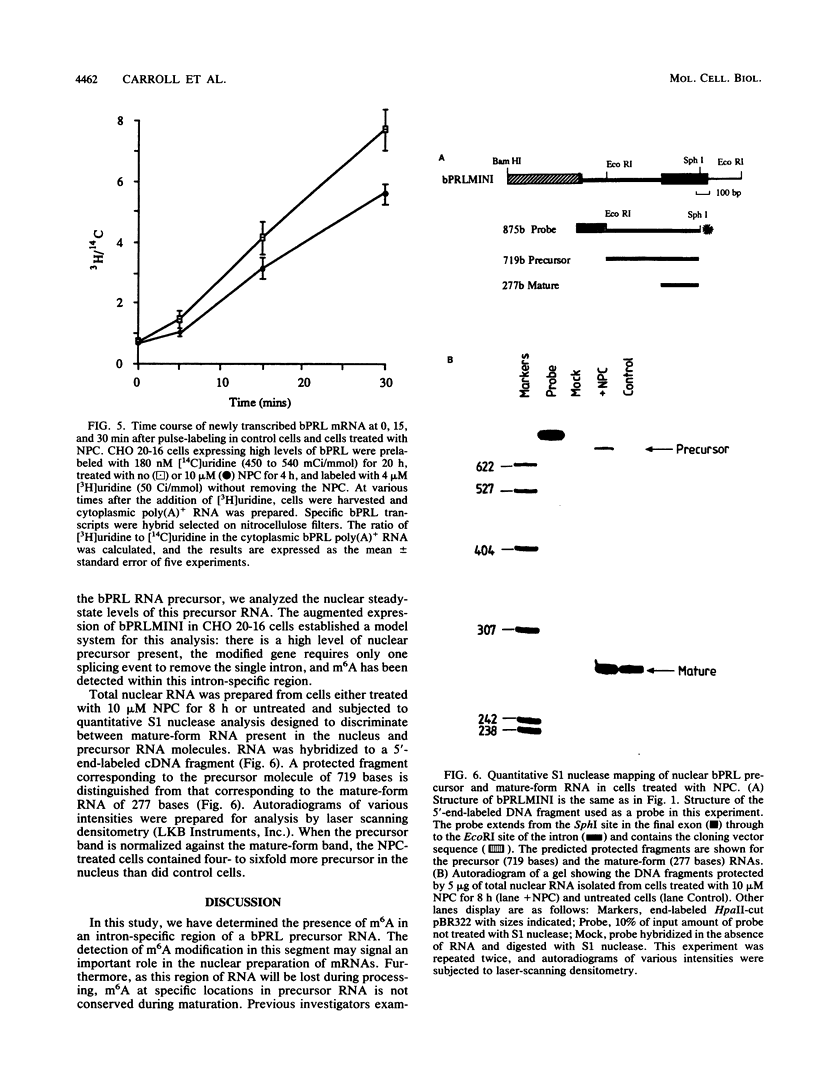



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. J., Coffin B., Rottman F. M. Analysis of mRNA 5'-terminal cap structures and internal N6-methyladenosine by reversed-phase high-performance liquid chromatography. Anal Biochem. 1981 May 1;113(1):118–123. doi: 10.1016/0003-2697(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Amalric F., Caboche M. Biosynthesis and utilization of extensively undermethylated poly(A)+ RNA in CHO cells during a cycloleucine treatment. Nucleic Acids Res. 1978 Aug;5(8):2927–2943. doi: 10.1093/nar/5.8.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977 Jun 15;113(1):165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Albers R. J., Coward J. K., Rottman F. M. Effect of undermethylation on mRNA cytoplasmic appearance and half-life. Mol Cell Biol. 1984 Mar;4(3):538–543. doi: 10.1128/mcb.4.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper S. A., Yao Y. A., Rottman F. M. Hormonal regulation of the bovine prolactin promoter in rat pituitary tumor cells. J Biol Chem. 1985 Oct 5;260(22):12246–12251. [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975 Oct;2(10):1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer R. I., Knode M. C. Neplanocin A. A cyclopentenyl analog of adenosine with specificity for inhibiting RNA methylation. J Biol Chem. 1984 Nov 10;259(21):12964–12969. [PubMed] [Google Scholar]
- Grabowski P. J., Padgett R. A., Sharp P. A. Messenger RNA splicing in vitro: an excised intervening sequence and a potential intermediate. Cell. 1984 Jun;37(2):415–427. doi: 10.1016/0092-8674(84)90372-6. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Horowitz A., Nilsen T. W., Munns T. W., Rottman F. M. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5667–5671. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. E., Beemon K. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J Biol Chem. 1987 Mar 5;262(7):3422–3427. [PubMed] [Google Scholar]
- Kane S. E., Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol. 1985 Sep;5(9):2298–2306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi U., Fernandez-Muñoz R., Darnell J. E., Jr Content of N-6 methyl adenylic acid in heterogeneous nuclear and messenger RNA of HeLa cells. Nucleic Acids Res. 1977 Jan;4(1):63–69. doi: 10.1093/nar/4.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Ayers D. F., Rottman F. M., Maroney P. A., Nilsen T. W. Unequal distribution of N6-methyladenosine in influenza virus mRNAs. Mol Cell Biol. 1987 Apr;7(4):1572–1575. doi: 10.1128/mcb.7.4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Rottman F. M. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988 Nov 25;242(4882):1159–1162. doi: 10.1126/science.3187541. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell. 1975 Apr;4(4):387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Methylated constituents of heterogeneous nuclear RNA: presence in blocked 5' terminal structures. Cell. 1975 Sep;6(1):13–19. doi: 10.1016/0092-8674(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M., Narayan P., Ayers D. F., Rottman F. M., Nilsen T. W. Priming of influenza mRNA transcription is inhibited in CHO cells treated with the methylation inhibitor, neplanocin A. Antiviral Res. 1987 Jul;7(6):317–327. doi: 10.1016/0166-3542(87)90014-3. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasavage N. L., Nilson J. H., Horowitz S., Rottman F. M. Nucleotide sequence of bovine prolactin messenger RNA. Evidence for sequence polymorphism. J Biol Chem. 1982 Jan 25;257(2):678–681. [PubMed] [Google Scholar]
- Schibler U., Kelley D. E., Perry R. P. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol. 1977 Oct 5;115(4):695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- Sommer S., Salditt-Georgieff M., Bachenheimer S., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. J. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976 Mar;3(3):749–765. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dane R. W. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982 Jun;42(3):918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. 5'-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976 Jan 27;15(2):397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975 Apr;4(4):379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Efstratiadis A. In vivo splicing products of the rabbit beta-globin pre-mRNA. Cell. 1984 Dec;39(3 Pt 2):589–602. doi: 10.1016/0092-8674(84)90466-5. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]