Abstract
The primary objective was to compare the evoked K-complex response to salient versus non-salient auditory stimuli in combat-exposed Vietnam veterans with and without Post-Traumatic Stress Disorder (PTSD). Three categories of auditory stimuli (standard 1000Hz tones, trauma-related combat sounds, and affectively neutral environmental sounds) were presented during stage 2 sleep utilizing an oddball paradigm with probabilities of occurrence of 60%, 20% and 20% respectively. Twenty-four combat-exposed Vietnam veterans, 14 with PTSD and 10 without PTSD were studied in a sleep laboratory at the National Center for PTSD in Menlo Park, CA. While significantly fewer K-complexes overall were elicited in patients, there were no differences in the proportion of K-complexes elicited by tones and combat stimuli within either group. Patients produced significantly more K-complexes to neutral stimuli than to tone or combat stimuli. Examination of the N550 component of the evoked K-complex revealed significantly longer latencies in the patient group. Across the entire sample, N550 latencies were longer for combat stimuli relative to tone neutral stimuli. There were no group or stimulus category differences for N550 amplitude. The results suggest that salient information, as defined by trauma-related combat sounds, did not preferentially elicit K-complexes in either the PTSD group or the control group, suggesting that K-complexes function to protect sleep more than to endogenously process meaningful stimuli.
Keywords: K-complex, PTSD
1. Introduction
It is not yet clear to what extent the salience of a stimulus can be detected during sleep. The ability to evaluate the external environment while asleep, to enable waking if a predator or some other danger were detected, would be adaptive in an evolutionary sense. Animal studies demonstrate that during sleep the ability to process environmental information is diminished, although not absent, due to sensory gating at the thalamus (Coenen and Drinkenburg, 2002; Steriade et al., 1993). To assess the sleeping brain’s ability to respond to salient information, evoked K-complex responses to combat sounds were measured in a sample of combat-exposed Vietnam veterans with and without Post-Traumatic Stress Disorder (PTSD).
PTSD is an anxiety disorder characterized by symptoms of re-experiencing, avoidance, and hyperarousal that arise and persist for more than one month after exposure to a traumatic event, defined as experiencing or witnessing of a real or perceived threat of death or injury to one’s self or others, and associated with intense feelings of fear, horror, or hopelessness (American Psychiatric Association2000). Sleep disturbances are a core feature of PTSD (Ross et al., 1989), and sleep-specific mechanisms may contribute to the pathophysiology of the disorder (Germain et al., 2008). Given the role of sleep in emotional mnemonic processes (Born; Stickgold and Walker, 2007; Walker and Stickgold, 2006), it is possible that sleep disruption impact information processing during sleep. Studying evoked K-complexes during sleep in PTSD may provide novel insights into the nature of underlying abnormal information processing that characterize PTSD (Weber, 2008).
The role of stimulus characteristics in eliciting K-complexes during sleep is contentious (Colrain, 2005). However, Oswald and colleagues’ (1960) widely cited paper that observed more K-complexes evoked to participant’s own name versus another name has led many to believe that stimulus salience plays an important role in K-complex production. The associated assumption is that the K-complex can in some way be seen as a reflection of information processing during sleep. The K-complex is an unusual EEG phenomenon, in that it can both occur spontaneously and be evoked by a variety of different types of stimuli, most readily by sounds (Davis et al., 1939; Roth et al., 1956) and inspiratory occlusions (Webster and Colrain, 1998). As with any other evoked EEG response, evoked K-complexes are embedded within the ongoing EEG signal. While their large size relative to the background makes them easily seen in stage 2, they are more difficult to observe in slow wave sleep. Even within stage 2, ongoing EEG activity unrelated to the stimulus can add to or subtract from the K-complex (depending on its phase). This is one of the reasons responsible for their extremely variable morphology (Da Rosa et al., 1991; Halász et al., 1985; Ujszàszi and Halász, 1986, 1988).
Bastien and Campbell (1992) proposed the use of signal averaging to extract the pure K-complex response from the unrelated EEG activity in the background, a technique commonly employed when recording averaged event-related potentials (ERP). This technique assumes the evoked K-complex response to be temporally invariant with respect to the stimulus, whereas unrelated EEG activity, randomly distributed relative to the stimulus, will tend to average to zero. Research using this averaged evoked K-complex technique has led to the conclusion that the K-complex is largely responsible for the N550 component of the non-REM sleep evoked response, with the N550 being prominent in K-complex averages (KC+) and dramatically reduced or absent in the averages of responses not containing a K-complex (KC-) (Afifi et al., 2003b; Bastien and Campbell, 1992, 1994; Colrain et al., 1999; Cote et al., 1999; Crowley et al., 2005; Crowley et al., 2002a; Crowley et al., 2002b; Gora et al., 2001; Harsh et al., 1994; Nicholas et al., 2002a; Nicholas et al., 2002b; Nicholas et al., 2006; Niiyama et al., 1994; Niiyama et al., 1995). Comparing the evoked K-complex response to relevant versus non-relevant stimuli is one method to test whether the K-complex is in fact a marker of information processing during sleep.
In 1960, Oswald and colleagues (1960) found that more K-complexes were evoked by the subject’s own name than by subject’s name backward or other names. In another paper a few years later, Beh and Barrett (1965) reported that K-complexes were produced 60% of the time to a subject’s own name but only 15% of the time when another name was presented. They also found evidence that previously conditioned stimuli (during prior wakefulness) produced more K-complexes than unconditioned stimuli. The study by McDonald and colleagues (1975) only partially replicated these results. They reported more K-complexes evoked by conditioned than unconditioned stimuli; however, K-complexes were produced only 52% of the time to subjects’ own names versus 60% of the time to other names and 38% of the time to tones. Voss and Harsh (1998) recently reported an own name effect, however, a 200 μV criterion was used for K-complex identification, and the overall proportions reported were very small (< 15% in all conditions). In two studies, Perrin and colleagues (2000; 1999) reported no effect of own name versus other name on K-complex elicitation or on N550 amplitude. There was, however, an enhancement of what they referred to as an N2-P3 component earlier in the waveform. Finally, Pratt and colleagues (1999) reported no differences in the waveforms for own names, other words, or clicks.
Rare or deviant auditory tones identified as “target” stimuli during wakefulness produce more K-complexes during subsequent sleep, and the amplitude of the KC+ N550 component is typically reported as being larger than standard stimuli (Colrain et al., 1999; Niiyama et al., 1995). Colrain and colleagues (2000a) however, indicated that this most likely reflects a stimulus probability effect rather than some residual recognition of “target” status during sleep, by showing similar levels of K-complex production and KC+ N550 amplitudes for target and non-target stimuli presented at the same probability level (0.2).
The data relating to the ability of the K-complex to be differentially produced to “relevant” or “salient” stimuli during sleep are therefore ambiguous. In order to address the issue in a different manner, the present paper sought to study responses to trauma-related combat sounds versus non trauma-related neutral sounds in a sample of Vietnam veterans with chronic PTSD, and a control group of combat-exposed Vietnam veterans without PTSD. Individuals with PTSD have been shown to be hyperreactive to traumatic cues during wake, even when those cues are presented outside of awareness (i.e., subliminally processed; for review, see Buckley et al., 2000; McNally, 1995, 1998). By presenting such triggers during sleep, the salience of trauma-based stimuli in this population provides an ideal test of the hypothesis that personally meaningful stimuli preferentially evoke K-complexes.
2. Materials and Methods
2.1 Participants
Data were collected from 24 male Vietnam veterans (mean age = 54 years, range = 48 to 59). All participants had combat experience during their military service (i.e., they were trauma-exposed). Of this sample, 14 participants had current diagnosis of chronic PTSD (patients) and 10 participants (combat controls) were free from psychiatric disorders. An additional patient was withdrawn from the study during the night by the experimenter because he would awaken to every stimulus presented while he was sleeping.
Patients were recruited from a residential treatment program for PTSD run by the National Center for PTSD located at the Palo Alto VA Health Care System, Menlo Park Division. As is common in PTSD populations (Brady et al., 2000; McMillen et al., 2002), there was a large degree of psychiatric comorbidity in the PTSD sample. In particular, depression, alcohol, and other substance abuse were prevalent among the patients. Patients were free from alcohol and substance use/abuse for at least five months prior to their participation in the study. All patients had a history of depression, 10 had a history of alcohol abuse or dependence, 9 had a history of other substance abuse or dependence, and 6 had a history of an anxiety disorder other than PTSD. Controls without current psychiatric disorders and no lifetime diagnosis of PTSD were recruited from the community, and their psychiatric status was confirmed with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) and the Structured Clinical Interview for DSM-IV Axis I Diagnoses (First, 1997). See Table 1 for a listing of participant characteristics.
Table 1.
Participant characteristics.
| PTSD | Control | ||||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Possible range | |
| Age (years) | 52.8 | (2.6) | 56.1 | (2.2) | |
| BDI score | 26.5 | (10.7) | 3.4 | (3.1) | 0 – 63 |
| CES score | 27.5 | (6.6) | 27.0 | (6.4) | 0 – 35 |
| CAPS total score | 59.5 | (15.5) | 6.0 | (5.3) | 0 – 136 |
SD = standard deviation
BDI = Beck Depression Inventory
CES = Combat Exposure Scale
CAPS = Clinician-Administered PTSD Scale
2.2 Procedure
Participants arrived at the sleep laboratory between 7:30 pm and 9:30 pm. Auditory thresholds for each ear were assessed using 1000 Hz and 4000 Hz pure tones using an Beltone Audio Scout Audiometer (Beltone Electronics Corporation, Glenview, IL) in a standardized manner, using the same insert earphones used during the overnight sleep recordings. E-A-RTONE 3A (Aero Corporation, Indianapolis, IN) insert earphones (i.e., placed into the ear canal) were used. Group differences in 1000 Hz and 4000 Hz auditory thresholds between the patient and control group were not significant. Next, physiological sensors were attached. Afterward, participants were free to go to bed when feeling sleepy, which was usually right away. Auditory stimulus presentation commenced once participants achieved at least 15 minutes of consolidated stage 2 sleep. The Stanford University Medical School/Veterans Affairs Palo Alto Administrative Panel for Human Subjects in Medical Research approved the study, and all participants provided informed consent.
2.3 Stimuli
Three types of stimuli were presented to participants during stage 2 sleep: pure tones, combat-related sounds, and environmental sounds. All stimuli were 500 ms in duration and were normalized to be equally loud (73 dB on average) and to have equivalently fast rise times (20 ms), with the exception of tone stimuli, which had faster rise times (5 ms). The stimuli consisted of a 1000 Hz pure tone; the 5 acoustically complex combat sounds consisted of gunshots and explosions; and the 5 acoustically complex non-combat (i.e., neutral) sounds consisted of a crow’s call, telephone ring, piano note, bell, and trumpet note. The stimuli were presented in an oddball paradigm with a randomized inter-stimulus interval (ISI) of 15 to 30 seconds, as the probability of K-complex production has been shown to be maximized at this ISI (Bastien and Campbell, 1994). Tones were most frequent, occurring 60% of the time, while neutral and combat stimuli each occurred 20% of the time. A random order for stimulus presentation was generated, and this same list was used for all participants. Typically, stimuli used in studies investigating the evoked K-complex are brief (i.e., 50 ms) with fast rise times. Stimuli were longer than typically used (i.e., 500 ms), because we wanted to be able to compare recognizable combat sounds to other more neutral sounds and tone stimuli. To investigate whether the long duration of the stimuli evoked K-complexes later than usually seen, ‘late’ K-complexes were also scored using an evoked window of 1000 to 1400 ms post-stimulus. There were far fewer late evoked K-complexes (only about 10% of trials), and no apparent differences between the groups; as a result, these data were not further analyzed. Upon detection of a movement arousal, stage 1 sleep, REM sleep or wakefulness, the presentation of stimuli was halted. Stimulus presentation was resumed once a participant achieved at least five minutes of uninterrupted stage 2 sleep. The few stimuli that were erroneously presented during any other stage of sleep were discarded. Stimuli were presented intermittently throughout the entire night of recording. The total number of stimuli presented to each subject was dependent on their sleep quality and continuity. The mean (standard deviation) number of stimuli presented was 233 (139) for control participants and 243 (103) for patient participants, t=2.07, p=0.85. No participants had an adverse reaction, such as a panic attack or complaint of distress, to any of the stimuli presented during sleep. As stated earlier, stimuli presentations were stopped during the night for one patient participant because he would awaken to every stimulus presented. Another patient woke in the night with significant anxiety, an apparent nocturnal panic attack, but this occurred at a time in which stimuli were not being presented; he was able to return to sleep and complete the experiment.
2.4 Psychophysiological Measures
Electroencephalogram (EEG) activity was measured at F7, F8, F3, F4, Cz, P3, and P4, each referenced to linked ear lobes, A1 + A2 (bandpass filtered 0.1-100 Hz). One electrooculogram (EOG) channel was measured with two bipolar electrodes placed near upper right eye and lower left eye (bandpass filtered 0.1-100 Hz). Chin electromyogram (EMG) activity for sleep stage scoring was recorded using a bipolar reference from among three electrodes attached over the mentalis/submentalis muscles (bandpass filtered 30-300 Hz). Electrocardiogram (ECG) was measured using bipolar leads placed near the right collarbone and lower left rib rage (bandpass filtered 0.1-30 Hz). Blood oxygen levels were measured using an oximeter placed on the index finger to ensure participants were not having significant oxygen desaturations, and was not digitized.
2.5 Recording Apparatus, Data Acquisition, and Data
Reduction Gold cup electrodes were used to collect EEG, EOG, and EMG signals. EEG electrodes were affixed to the scalp with either Grass 10-20 paste or tape (for those sites in which hair was not present) following abrasion. Impedances were measured to ensure they were below 5 kOhms. ECG was collected with disposable electrodes.
Auditory stimuli contained a pulse presented via the second channel of the stereo sound generator. The pulse was sent to a data channel when each stimulus was presented to participants; pulses were later used to extract responses to stimuli from the continuous recording for signal averaging. Signals were amplified using analog amplifiers (Grass-Telefactor, Warwick, RI), and then digitized at sampling rate of 400 Hz. Calibrations were conducted at the beginning of each subject’s recording; these were used to correct the data amplitudes prior to data processing. Continuous sleep data were down-sampled to 200 Hz to facilitate storage. Thirty second per stimulus epochs were extracted, beginning 20 seconds prior to each stimulus onset. Data collection and reduction software was written with VisualBasic (Microsoft Corporation, Redmond, WA) and Matlab software (Mathworks, Inc., Natick, MA), respectively, by one of the authors (S.H.W.).
2.6 Psychophysiological Variables
Evoked K-complex identification and trial averaging
The extracted epochs were evaluated as single trials for the presence/absence of a K-complex. K-complex scoring was done blind to the participant’s PTSD status and order of stimulus presentation. K-complexes were identified in frontal recording leads (i.e., F3 and F4) using the standard Rechtschaffen and Kales (1968) criteria.
To be considered an evoked K-complex, the peak of the negative component also had to satisfy the following additional criteria: the amplitude of the negative peak had to occur between 400 and 1000 ms from stimulus onset and had to have an amplitude of at least -75 μV or greater as per the convention in the majority of evoked K-complex studies (Colrain, 2005). The evoked window in the present study is longer than typical due in part to the long stimulus duration in the present study, as well as the older age of our participants; older individuals have been shown to have both longer N550 latencies and smaller N550 amplitudes (Crowley et al., 2002a). Responses in which a movement arousal, delta burst, or other artifact occurred immediately before or coincident with a stimulus presentation were rejected and not used in the averaging procedure. All responses that were to be included in the averaging process were classified into two response types on the basis of visual inspection of each epoch: those responses that contained K-complexes (KC+) and those which did not (KC-). Grouped responses were averaged relative to stimulus type (tone, neutral, and trauma) for each subject. These produced average evoked potential files for each of the response types.
Amplitude and latency of the signal-averaged N550 component was determined by identifying the most negative value (amplitude) that occurred between 400 ms and 1000 ms on the averaged KC+ files. N550 was measured by averaging F3 and F4 to approximate Fz, as this is where the N550 component has previously been shown to be maximal (Afifi et al., 2003a; Colrain et al., 2010; Colrain et al., 2009; Colrain et al., in press; Colrain et al., 1999; Cote et al., 1999; Crowley et al., 2005; Crowley et al., 2002a; Gora et al., 1999, 2001; McCormick et al., 1997; Nicholas et al., 2006; Niiyama et al., 1995).
2.7 Statistical Analysis
Analyses were conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL). The data were tested for normality of distribution, and analyses of variance (ANOVAs) were used to compare differences between patient and control groups on K-complex proportion, amplitude, and latency in response to the three types of auditory stimuli (tone, neutral, and combat). Greenhouse-Geiser corrections were applied where appropriate, however, original degrees of freedom are reported. Effects were considered significant with an alpha level of .05. Simple effects ANOVAs and post-hoc t-tests were used to assess differences within significant interactions for comparisons of interest.
3 Results
3.1 Probability of K-complex Elicitation
A 2 × 3 (Group X Stimulus Type) repeated measures ANOVA of proportion of K-complex elicitation revealed a significant interaction between Group and Stimulus Type F(2,44)=3.97, p=0.026 (see Figure 1), as well as significant main effects of group, F(1,22)=17.54, p=0.0004 and stimulus type, F(2,44)=4.51, p=0.017. Controls had significantly more evoked K-complexes than patients, on average 55% vs. 35%. There were no differences in rate of K-complex production between the tone and combat stimuli for the patient group (t=-0.63, p=0.538) or the control group (t =0.13, p=0.898), or across any of the other stimuli types in the control group (i.e., between neutral and tone or combat, p=0.966 and p=0.893 respectively). Post-hoc paired sample t-tests demonstrated that within the patient group, K-complex elicitation rate was significantly higher for neutral stimuli than for tone (t=-5.18, p<0.001) or combat stimuli (t=3.94, p=0.002); both results survive Bonferonni correction. Post-hoc independent sample t-test demonstrated no difference between the controls and patients on K-complex elicitation rate for neutral stimuli (t=1.32, p=0.201).
Figure 1.
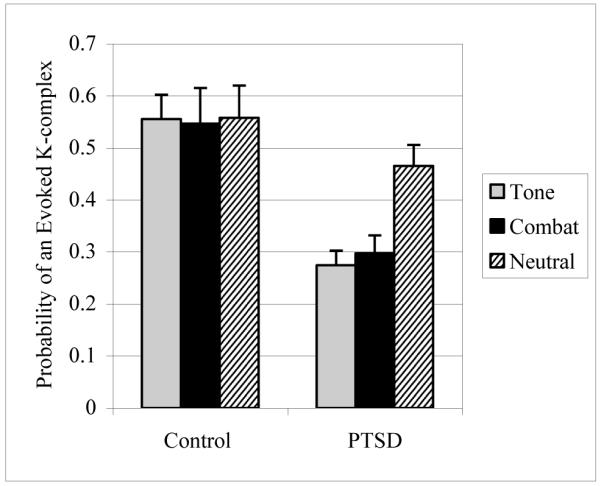
K-complex proportions (mean and standard errors) to the three stimulus categories in patients and controls.
3.2 Evoked K-complex: N550 Amplitude and Latency
Two factor (Group X Stimulus Type) repeated measures ANOVA revealed no significant effects of group, stimulus type, or their interaction for N550 amplitude. For N550 latency, the repeated measures ANOVA revealed a significant effect of group (F(1,22)=4.323, p<0 .05), with the patients having later N550 responses. There was also a main effect of stimulus type (F(2,44)=3.730, p<0.05), with longer N550 latencies to combat sounds compared to neutral sounds; the difference between combat and tone, and neutral and tone were not significant. The interaction of stimulus type with group displayed a trend for significance (p=0.100) (see figure 2).
Figure 2.
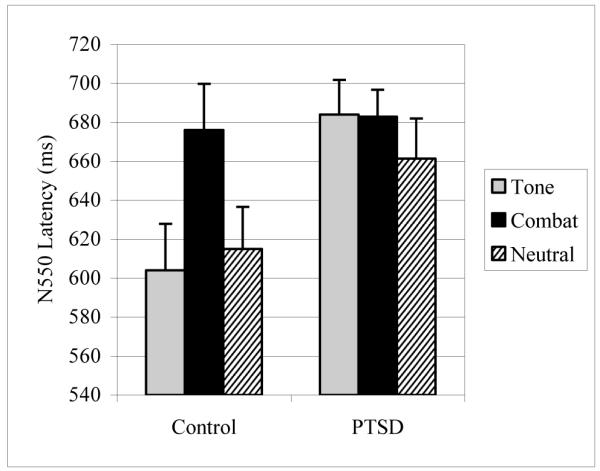
N550 latencies (mean and standard errors) averaged between F3 and F4 in the KC+ averaged evoked responses to the three different stimulus types in patients and controls.
4. Discussion
Oswald and colleague’s (1960) hypothesis that K-complexes are more likely to be evoked by salient stimuli was not supported. Neither PTSD patients nor combat-exposed controls demonstrated within group differences between combat sounds and pure tones in the elicitation of K-complexes. That is, there was no difference in K-complex production for the most stringent comparison: information-rich, highly salient stimuli (combat) versus essentially information-free, meaningless stimuli (pure tones). Results from the present study also highlight the all-or-nothing nature of the K-complex (Bastien and Campbell, 1992). When they were elicited, the N550 of the averaged K-complexes tended to be the same size (i.e., amplitude), regardless of stimulus type or subject group. Later evoked K-complexes (i.e., those later than 1000 ms) were rare in both the PTSD and control groups. If indeed the evoked K-complexes reflected some form of information processing to the different stimuli during sleep, then the processing time necessary to evaluate the content of the relatively long 500 ms stimuli by the sleeping brain should have been expressed as later evoked K-complexes occurring later than 1000 ms, however, this was not the case.
Unexpectedly, while patients generally exhibited fewer evoked K-complexes than did the controls, they were essentially equivalent in their responses to neutral stimuli. Although the neutral condition was originally added to the study design to control for sound complexity and probability of occurrence, in hindsight, it is possible that the neutral sounds were actually more complex and more subjectively rare than the combat stimuli. Condition-wise, neutral and combat stimuli had the same likelihood of occurring (20% each). The neutral stimuli, however, came from different ontological categories. It is therefore possible that the increased response rate to neutral stimuli observed in the PTSD group was a novelty effect (i.e., each neutral stimulus was more rare than the similarly sounding combat stimuli). In this context, it could be argued that the patients were showing the often-reported stimulus probability effect where K-complexes are more likely to rare than to common stimuli (Colrain et al., 2000a; Colrain et al., 1999; Colrain et al., 2000b; Niiyama et al., 1995; Sallinen et al., 1994).
This argument has to be modified by two other data points. First, the differential response of the PTSD group to neutral stimuli was not found in the controls. However, it is possible that this is due to ceiling effect obscuring detectable differences in their responses to the three types of stimuli in the control group. The age regression for K-Complex probability reported in Colrain et al. (2010) would predict a probability of ~0.58 for the control group in the present study, and Colrain et al. (2009) reported a probability of 0.55 in male control subjects with a similar mean age. Second, there is no overall probability effect of less elicitation to tones compared to combat sounds in the patient group or tones compared to stimuli of either rare category in the controls. It is therefore possible that the patients may have been hyper-responsive to either the novelty or rarity of the neutral sounds despite there being no stimulus salience effect.
Alternatively, the observed findings may relate to previous reports of heightened awakening threshold from slow-wave sleep in patients with PTSD relative to control subjects (Dagan et al., 1991; Lavie et al., 1998). These findings were interpreted as indicative of increased sleep depth in patients with PTSD. Combined with the present findings, it is possible that the chronic sleep disruption that characterizes PTSD (Kobayashi et al., 2007) is indeed associated with heightened homeostatic sleep drive, and attenuated monitoring or detection of external stimuli. Additionally, the observation that PTSD subjects were more responsive to novel, neutral stimuli than tones or combat sounds may reflect intact processing of external incoming information during sleep in PTSD. Therefore, the co-existence of an elevated homeostatic drive (attributable to chronic sleep disruption) and heightened information processing of internal relative to external stimuli provides a plausible explanation to reconcile the previous and current observations. Further studies are required to evaluate and provide differential information processing mechanisms that may contribute to or be affected by PTSD, or more broadly, by trauma exposure. Of note, the present findings during sleep are consistent with studies that have reported attentional problems and altered central information processing during wakefulness in patients with PTSD accompanied by deficits in discerning irrelevant information (Felmingham et al., 2002; Metzger et al., 1997; Shucard et al., 2008), and abnormal neutral (Weber, 2008) and novel (Kimble et al., 2000) stimulus processing in PTSD.
Study findings are limited in part by a relatively small sample size, particularly in the control sample. Even though the sample size was small, the PTSD subjects definitely did not show a stimulus saliency effect. The combat stimuli were significantly less likely to elicit K-complexes than neutral stimuli and the difference between combat stimuli and tones was only a small proportion of the difference between combat and neutral stimuli. The present findings are further complicated by a number of factors common to research with psychiatric populations, such as comorbid diagnoses, extensive substance abuse histories, and use of psychotropic medications in the PTSD sample. More homogeneous PTSD samples (e.g., veterans with a current diagnosis of PTSD without substance abuse problems or depressive disorders) are difficult to obtain and may be less clinically relevant. The use of other control samples would have been useful in the design of the present study and merit consideration in future studies, such as age-matched groups of (a) trauma naive individuals, (b) individuals matched for alcohol use/abuse-matched but free from PTSD, and (c) psychopathology comparison groups, such as individuals with depression and/or anxiety.
The issue of alcohol abuse is particularly relevant given the findings of altered K-complex production in those with a history of alcohol dependence (Colrain et al., 2009; Nicholas et al., 2002a). These studies show a reduction in K-complex production in those with alcohol dependence but also a marked reduction in N550 amplitude, thought to be due in part to a loss of cortical gray matter (Colrain et al., 2011). The confound in the present data set between alcohol dependence and PTSD diagnosis does not explain the observed data, as there is no difference between the groups in N550 amplitude and no difference in K-complex production to the neutral stimuli.
The use of a recently traumatized sample would have provided an ideal comparison of acute versus chronic PTSD. For example, a stimulus salience effect might not be found in chronic PTSD patients who have had years of experience using compensatory mechanisms that might dampen or even eliminate that effect. Lastly, we did not assess whether the combat sounds used in this study were individually meaningful for each of the participants, and we did not assess subjective response to the neutral sounds. Future studies could examine whether the salience of sounds differ subject by subject, and to what impact, if any, this has on the evoked K-complex response.
In conclusion, this study did not support the findings of Oswald et al. (1960), Beh and Barrett (1965), or Voss and Harsh (1998) that salient stimuli produce more K-complexes in trauma-exposed military veterans with and without PTSD. Rather it is consistent with the studies of McDonald et al. (1975), Pratt et al. (1999) and Perrin et al. (2000; 1999) who failed to find such an effect in healthy subjects. The differential responses to subjectively diverse neutral stimuli in the PTSD sample, however, serve to temper a conclusion that stimulus “meaning” is irrelevant to K-complex generation. It may be that the PTSD group is hyper-reactive to any type of stimulus that is detected as being different or novel (e.g., that they are particularly sensitive to monitoring the environment for potential threat cues, which similarly occurs in the wake state; Kolb, 1987), while not processing the stimulus to such an extent where judgments of content (e.g., personal relevance) can be made. We conclude that the findings are suggestive of and consistent with theories that propose that K-complexes function to protect sleep more than to endogenously process meaningful stimuli in trauma-exposed military veterans. As outlined in recent studies of evoked K-complexes (Colrain et al., 2010; Colrain et al., 2008), it is more likely that the evoked K-complex is produced via activation of frontal cortex via a non-thalamic pathway, and reflects a modality independent sleep protective mechanism, utilizing a similar burst firing of cortical cells to that underlying delta generation in slow wave sleep (Colrain, 2005).
Highlights.
We examined K-complexes evoked to tone, combat, and neutral auditory stimuli. Overall, fewer K-complexes were evoked in the PTSD group.
However, responses to combat sounds and tones did not differ within either group.
Thus, salient information did not preferentially elicit K-complexes in either group.
Acknowledgments
This work was supported in part by the Department of Veterans Affairs, and the National Center for PTSD, Dissemination and Training Division. The authors would like to thank Lorraine P. Leskin, Wendy K. Stegman, and Ned J. Arsenault for their assistance with this study. Dr. Franzen is supported by NIH grant K01 MH077106. Dr. Colrain is supported by NIH grant AA14211.
Abbreviations
- PTSD
Post-traumatic stress disorder
- ANOVA
Analysis of Variance
- KC+
trials that contained a K-Complex
- KC−
trials that did not contain a K-Complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Diagnostic and statistical manual of mental disorders. 4th Edition, Text Revision. American Psychiatric Association; Washington, DC: 2000. 4th, Text Revision ed. [Google Scholar]
- Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respiratory Physiology and Neurobiology. 2003a;136:221–234. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003b;136:221–234. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Bastien C, Campbell K. The evoked K-complex: all-or-none phenomenon? Sleep. 1992;15:236–245. doi: 10.1093/sleep/15.3.236. [DOI] [PubMed] [Google Scholar]
- Bastien C, Campbell K. Effects of rate of tone-pip stimulation on the evoked K-Complex. J Sleep Res. 1994;3:65–72. doi: 10.1111/j.1365-2869.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Beh HC, Barratt PE. Discrimination and Conditioning During Sleep as Indicated by the Electroencephalogram. Science. 1965;147:1470–1471. doi: 10.1126/science.147.3664.1470. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 11(Suppl 1):16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 7):22–32. [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Drinkenburg WH. Animal models for information processing during sleep. Int J Psychophysiol. 2002;46:163–175. doi: 10.1016/s0167-8760(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Colrain IM. The K-complex: A seven decade history. Sleep. 2005 doi: 10.1093/sleep/28.2.255. In Press. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Afifi L, Baker FC, Padilla M, Turlington SR, Trinder J. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiol Aging. 2010;31:874–883. doi: 10.1016/j.neurobiolaging.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Padilla M, Baker FC. The impact of alcoholism on sleep evoked delta frequency responses. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Padilla M, Baker FC. The impact of alcoholism on sleep evoked Delta frequency responses. Biol Psychiatry. 2009;66:177–184. doi: 10.1016/j.biopsych.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Di Parsia P, Gora J. The impact of prestimulus EEG frequency on auditory evoked potentials during sleep onset. Can J Exp Psychol. 2000a;54:243–254. doi: 10.1037/h0087344. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Sullivan EV, Rohlfing T, Baker FC, Nicholas CL, Padilla ML, Chanraud S, Pitel A-L, Pfefferbaum A. Independent contributions of cortical gray matter, aging and alcoholism to K-complex amplitude evoked during sleep. Sleep Med Rev. 2011 doi: 10.5665/SLEEP.1050. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Sullivan EV, Rohlfing T, Baker FC, Nicholas CL, Padilla ML, Chanraud S, Pitel AL, Pfefferbaum A. Independent contributions of cortical gray matter, aging and alcoholism to K-complex amplitude evoked during sleep. Sleep. doi: 10.5665/SLEEP.1050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8:273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G, Campbell KB. The roles of vertex sharp waves and K-complexes in the generation of N300 in auditory and respiratory-related evoked potentials during early stage 2 NREM sleep. Sleep. 2000b;23:97–106. [PubMed] [Google Scholar]
- Cote KA, de Lugt DR, Langley SD, Campbell KB. Scalp topography of the auditory evoked K-complex in stage 2 and slow wave sleep. J Sleep Res. 1999;8:263–272. doi: 10.1046/j.1365-2869.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Crowley K, Sullivan EV, Adalsteinsson E, Pfefferbaum A, Colrain IM. Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep. 2005;28:865–870. doi: 10.1093/sleep/28.7.865. [DOI] [PubMed] [Google Scholar]
- Crowley K, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002a;11:129–140. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002b;113:1615–1622. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- Da Rosa AC, Kemp B, Paiva T, Lopes da Silva FH, Kamphuisen HA. A model-based detector of vertex waves and K complexes in sleep electroencephalogram. Electroencephalogr Clin Neurophysiol. 1991;78:71–79. doi: 10.1016/0013-4694(91)90021-u. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Lavie P, Bleich A. Elevated awakening thresholds in sleep stage 3-4 in war-related post-traumatic stress disorder. Biol Psychiatry. 1991;30:618–622. doi: 10.1016/0006-3223(91)90031-g. [DOI] [PubMed] [Google Scholar]
- Davis H, Davis PA, Loomis AL, Harvey EN, Hobart G. Analysis of the electrical response of the human brain to auditory stimulation during sleep. Am J Physiol. 1939;126:537–551. [Google Scholar]
- Felmingham KL, Bryant RA, Kendall C, Gordon E. Event-related potential dysfunction in posttraumatic stress disorder: the role of numbing. Psychiatry Res. 2002;109:171–179. doi: 10.1016/s0165-1781(02)00003-3. [DOI] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders SCID-I : clinician version, administration booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora J, Colrain IM, Trinder J. Respiratory-related evoked potentials during the transition from alpha to theta EEG activity in stage 1 NREM sleep. J Sleep Res. 1999;8:123–134. doi: 10.1046/j.1365-2869.1999.00144.x. [DOI] [PubMed] [Google Scholar]
- Gora J, Colrain IM, Trinder J. The investigation of K-complex and vertex sharp wave activity in response to mid-inspiratory occlusions and complete obstructions to breathing during NREM sleep. Sleep. 2001;24:81–89. doi: 10.1093/sleep/24.1.81. [DOI] [PubMed] [Google Scholar]
- Halász P, Pal I, Rajna P. K-complex formation of the EEG in sleep. A survey and new examinations. Acta Physiol Hung. 1985;65:3–35. [PubMed] [Google Scholar]
- Harsh J, Voss U, Hull J, Schrepfer S, Badia P. ERP and behavioral changes during the wake/sleep transition. Psychophysiology. 1994;31:244–252. doi: 10.1111/j.1469-8986.1994.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biol Psychiatry. 2000;47:880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- Kolb LC. A neuropsychological hypothesis explaining posttraumatic stress disorders. Am J Psychiatry. 1987;144:989–995. doi: 10.1176/ajp.144.8.989. [DOI] [PubMed] [Google Scholar]
- Lavie P, Katz N, Pillar G, Zinger Y. Elevated awaking thresholds during sleep: characteristics of chronic war-related posttraumatic stress disorder patients. Biol Psychiatry. 1998;44:1060–1065. doi: 10.1016/s0006-3223(98)00037-7. [DOI] [PubMed] [Google Scholar]
- McCormick L, Nielsen T, Nicolas A, Ptito M, Montplaisir J. Topographical distribution of spindles and K-complexes in normal subjects. Sleep. 1997;20:939–941. doi: 10.1093/sleep/20.11.939. [DOI] [PubMed] [Google Scholar]
- McDonald DG, Schicht WW, Frazier RE, Shallenberger HD, Edwards DJ. Studies of information processing in sleep. Psychophysiology. 1975;12:624–629. doi: 10.1111/j.1469-8986.1975.tb00059.x. [DOI] [PubMed] [Google Scholar]
- McMillen C, North C, Mosley M, Smith E. Untangling the psychiatric comorbidity of posttraumatic stress disorder in a sample of flood survivors. Compr Psychiatry. 2002;43:478–485. doi: 10.1053/comp.2002.34632. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Automaticity and the anxiety disorders. Behav Res Ther. 1995;33:747–754. doi: 10.1016/0005-7967(95)00015-p. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Experimental approaches to cognitive abnormality in posttraumatic stress disorder. Clin Psychol Rev. 1998;18:971–982. doi: 10.1016/s0272-7358(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Lasko NB, Pitman RK. Auditory event-related potentials to tone stimuli in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:1006–1015. doi: 10.1016/s0006-3223(97)00138-8. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. J Sleep Res. 2002a;11:247–253. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Trinder J, Colrain IM. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep. 2002b;25:882–887. [PubMed] [Google Scholar]
- Nicholas CL, Trinder J, Crowley KE, Colrain IM. The impact of slow wave sleep proximity on evoked K-complex generation. Neurosci Lett. 2006;404:127–131. doi: 10.1016/j.neulet.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Niiyama Y, Fujiwara R, Satoh N, Hishikawa Y. Endogenous components of event-related potential appearing during NREM stage 1 and REM sleep in man. Int J Psychophysiol. 1994;17:165–174. doi: 10.1016/0167-8760(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Niiyama Y, Fushimi M, Sekine A, Hishikawa Y. K-complex evoked in NREM sleep is accompanied by a slow negative potential related to cognitive process. Electroencephalogr Clin Neurophysiol. 1995;95:27–33. doi: 10.1016/0013-4694(95)00021-p. [DOI] [PubMed] [Google Scholar]
- Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–453. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- Perrin F, Bastuji H, Mauguiere F, Garcia-Larrea L. Functional dissociation of the early and late portions of human K-complexes. Neuroreport. 2000;11:1637–1640. doi: 10.1097/00001756-200006050-00008. [DOI] [PubMed] [Google Scholar]
- Perrin F, Garcia-Larrea L, Mauguiere F, Bastuji H. A differential brain response to the subject’s own name persists during sleep. Clin Neurophysiol. 1999;110:2153–2164. doi: 10.1016/s1388-2457(99)00177-7. [DOI] [PubMed] [Google Scholar]
- Pratt H, Berlad I, Lavie P. ‘Oddball’ event-related potentials and information processing during REM and non-REM sleep. Clin Neurophysiol. 1999;110:53–61. doi: 10.1016/s0168-5597(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Rechtshaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. BIS/BRI; Los Angeles: 1968. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- Roth M, Shaw J, Green J. The form voltage distribution and physiological significance of the K-complex. Electroencephalogr Clin Neurophysiol Suppl. 1956;8:385–402. doi: 10.1016/0013-4694(56)90004-9. [DOI] [PubMed] [Google Scholar]
- Sallinen M, Kaartinen J, Lyytinen H. Is the appearance of mismatch negativity during stage 2 sleep related to the elicitation of K-complex? Electroencephalogr Clin Neurophysiol. 1994;91:140–148. doi: 10.1016/0013-4694(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Shucard JL, McCabe DC, Szymanski H. An event-related potential study of attention deficits in posttraumatic stress disorder during auditory and visual Go/NoGo continuous performance tasks. Biol Psychol. 2008;79:223–233. doi: 10.1016/j.biopsycho.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujszàszi J, Halász P. Late component variants of single auditory evoked responses during NREM sleep stage 2 in man. Electroencephalogr Clin Neurophysiol. 1986;64:260–268. doi: 10.1016/0013-4694(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Ujszàszi J, Halász P. Long latency evoked potential components in human slow wave sleep. Electroencephalogr Clin Neurophysiol. 1988;69:516–522. doi: 10.1016/0013-4694(88)90163-0. [DOI] [PubMed] [Google Scholar]
- Voss U, Harsh J. Information processing and coping style during the wake/sleep transition. J Sleep Res. 1998;7:225–232. doi: 10.1046/j.1365-2869.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Weber DL. Information Processing Bias in Post-traumatic Stress Disorder. Open Neuroimag J. 2008;2:29–51. doi: 10.2174/1874440000802010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. Multichannel EEG analysis of respiratory evoked-potential components during wakefulness and NREM sleep. J Appl Physiol. 1998;85:1727–1735. doi: 10.1152/jappl.1998.85.5.1727. [DOI] [PubMed] [Google Scholar]


