Abstract
The toxicity of chronic immunosuppressive agents required for organ transplant maintenance has prompted investigators to pursue approaches to induce immune tolerance. We developed an approach using a bioengineered mobilized cellular product enriched for hematopoietic stem cells (HSC) and tolerogenic CD8+/TCR− graft facilitating cells (FC) combined with nonmyeloablative conditioning that allows engraftment, durable chimerism, and tolerance induction in highly mismatched related and unrelated donor-recipient pairs. Eight recipients of HLA-mismatched kidney and FC/HSC transplants underwent conditioning with fludarabine, 200 cGy total body irradiation, and cyclophosphamide followed by post-transplant immunosuppression with tacrolimus and mycophenolate mofetil. Subjects ranged in age from 29 to 56 years. HLA match ranged from 5 of 6 related to 1 of 6 unrelated. The absolute neutrophil counts nadired approximately one week after transplant, with recovery by two weeks. Multilineage chimerism at one month was 6% to 100%. The conditioning was well tolerated with outpatient management after postoperative day two. Two subjects exhibited transient chimerism and have been reduced to low-dose tacrolimus monotherapy. One subject developed viral sepsis two months after transplant and experienced renal artery thrombosis. Five subjects have durable chimerism, with immunocompetence and donor-specific tolerance by in vitro proliferative assays and were successfully weaned off all immunosuppression one year after transplant. None of the recipients produced anti-donor antibody or exhibited engraftment syndrome or graft-versus-host disease. These results suggest that manipulation of a mobilized stem cell graft and nonmyeloablative conditioning represents a safe, practical, and reproducible means of inducing durable chimerism and donor-specific tolerance in solid organ transplant recipients.
Keywords: chimerism, tolerance, immunosuppression, hematopoietic stem cells, facilitating cells, kidney transplantation
Introduction
The disappointing long-term results for solid organ transplantation have motivated investigators to pursue the clinical induction of immunologic tolerance. The toxicities associated with non-specific immunosuppressive agents remain major causes of morbidity in organ transplant recipients, including hypertension, opportunistic infections, diabetes, and renal compromise (1). Moreover, these agents do not prevent chronic rejection, the primary cause of late graft loss. Chimerism has been shown to prevent chronic rejection and induce drug-free graft survival in several experimental animal models (2,3). Two recent studies demonstrated the feasibility of this approach in humans (4–6). In one study, while not achieving durable chimerism, the majority of recipients were successfully tapered off immunosuppression (5,6). The other study was limited to HLA-identical related living donor/recipient transplants (4,7).
In this report of an FDA-approved phase 2 study, we tested the hypothesis that a tolerance-promoting facilitating cell (FC)-based (8,9) hematopoietic stem cell (HSC) graft (together designated as FCRx) would promote the establishment of durable chimerism and tolerance in HLA-mismatched living donor renal allograft recipients. CD8+/TCR− bone marrow-derived FC potently enhance engraftment of allogeneic HSC (8) and limiting numbers of syngeneic (10) HSC in conditioned recipients. FC are comprised predominantly of a plasmacytoid precursor dendritic cell subpopulation (p-preDC FC) (9). While removal of ppreDC FC abrogates facilitation, p-preDC FC do not replace FC in function. FC induce the generation of antigen-specific regulatory T cells (Treg) in vitro (11) and in vivo (12) and potently prevent graft-versus-host disease (GVHD) in the mouse (13). As such, FC may represent a clinically relevant cell-based therapy for tolerance induction. We report here the translation of this work to the clinic, resulting in induction of durable high levels of chimerism, stable renal function, and avoidance of GVHD in HLA-mismatched kidney/FCRx recipients who are now off all immunosuppression for periods ranging from 4 to 18 months. A safe approach to induce graft/host tolerance in mismatched donor and recipient combinations could be transformational not only in sold organ and cell transplant recipients, but for applications of hematopoietic stem cell transplantation (HSCT) in general, including hemoglobinopathies, inherited metabolic disorders, and autoimmune diseases.
Results
Subject clinical course
The demographics of the study subjects and composition of the FCRx infused are shown in Table 1. The conditioning consisted of two doses of cyclophosphamide (days +3 and −3), 200 cGy total body irradiation (TBI), three doses of pre-operative fludarabine (days −5, −4, −3), with followed by renal transplantation (day 0) and FCRx infusion (day +1) as detailed in Figure 1A. Immunosuppression after transplant consisted of mycophenolate mofetil (MMF) and tacrolimus. Subjects were discharged on day 2 after renal transplant and subsequently managed as outpatients. The characteristic nadir of absolute neutrophil counts (ANC) occurred approximately one week following kidney/stem cell transplant, with median recovery of ANC to more than 500 per cubic milliliter at 9 days (range 2–14). This was managed with neutropenic precautions. Multilineage chimerism was achieved in all subjects at one month after transplant (Table 1). It has persisted in 5 of the 8 subjects (Tables 2 and 3). None of the subjects demonstrated engraftment syndrome (14) or GVHD. The primary endpoint for discontinuing immunosuppression was donor whole blood and T cell macrochimerism. Secondary end points included a normal protocol biopsy, normal renal function, and absence of GVHD. At the time of publication, five of the eight subjects have been weaned from immunosuppression. Two of the other three are on low-dose monotherapy. A detailed course for each patient is included in the Supplementary Materials.
TABLE 1.
PATIENT CHARACTERISTICS
| Product Composition | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | PRA† | KD Tx Date | Source | HLA match | Donor Relationship | Cause of Renal Failure | αβ T cells# | CD34# | FC# TOTAL | % Chimerism at 1 month†† | Anti-donor Alloantibody | Time off all Immunosuppression |
| NW1 | 50 | M | 0 | 2/09 | IC* | 5/6 | Related | Membranous | 0.963 | .896 | .467 | 30 | None | N/A |
| NW2 | 56 | M | 6 | 4/09 | MPB** | 3/6 | Related | Hypertension | 3.80 | 2.53 | 6.65 | 95 | None | N/A |
| NW3 | 43 | M | 19 | 5/09 | MPB | 1/6 | Unrelated | Polycystic kidney disease | 3.80 | 3.60 | 3.96 | 100 | None | 20 months |
| NW4 | 29 | M | 0 | 6/09 | MPB | 3/6 | Related | Alports Syndrome | 1.94 | 1.00 | 1.55 | 6 | None | N/A |
| NW5 | 40 | M | 0 | 2/10 | MPB | 1/6 | Unrelated | Chronic GN | 3.80 | 3.94 | 3.68 | 100 | None | 11 months |
| NW6 | 39 | F | 14 | 3/10 | MPB | 2/6 | Unrelated | Reflux:2nd Tx | 3.80 | 8.59 | 12.30 | 100 | None | 10 months |
| NW7 | 35 | M | 0 | 4/10 | MPB | 3/6 | Related | Hypertension | 3.80 | 16.90 | 12.20 | 100 | None | 9 months |
| NW8 | 46 | F | 16 | 7/10 | MPB | 1/6 | Unrelated | Polycystic kidney disease | 3.80 | 12.60 | 8.06 | 100 | None | 6 months |
Iliac crest
Mobilized peripheral blood
PRA=panel reactive antibody
Chimerism testing was performed by molecular analysis of short tandem repeats (STR) Sensitivity 2% – 5%.
All cell dosing is # × 106/kg recipient body weight.
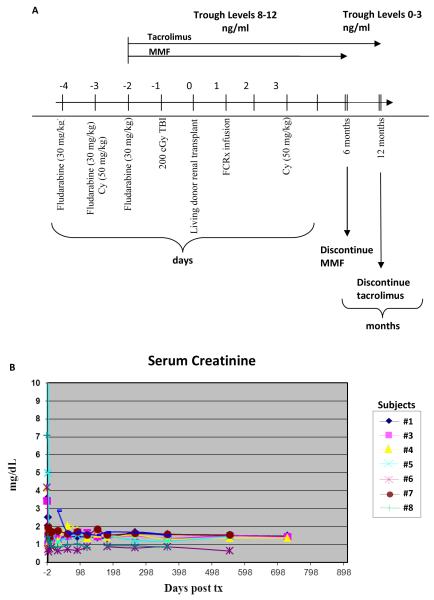
Fig. 1.
Algorithm for conditioning, kidney and FCRx transplant, and maintenance immunosuppression. A) Fludarabine was administered day −4, −3, −2 (30 mg/kg/dose) relative to the living donor kidney transplant (day 0). Dialysis was performed at least 6 hours after each dose pre-transplant. Two doses of cyclophosphamide (50 mg/kg) were given day −3 and day +3. FCRx was administered day +1. If the recipient was chimeric and/or tolerant to their donor at 6 months, and if the standard of care protocol biopsy showed no evidence for rejection, the MMF was discontinued. At 9 months, if renal function remained stable, the tacrolimus was reduced (trough levels 0–3ng/ml). At 12 months, the tacrolimus was discontinued if donor chimerism was present, the repeat protocol biopsy was normal, and renal function stable. B) Serum creatinine levels (mg/deciliter) for all active subjects vs. time.
TABLE 2.
PERCENTAGE OF WHOLE BLOOD CHIMERISM AT SELECTED MONTHS AFTER TRANSPLANT†
| MONTHS * | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 9 | 12 | 13 | 14 | 15 | 18 | 24 | 30 | |
| Subject | ||||||||||||||
| 1 | 30% | 13% | 3% | 4% | 4% | 0% | 0% | 0% | NT | NT† | NT | 0% | 0% | NT |
| 2 | 95% | 100% | 0% | 0% | 0% | 0% | NT | NT | NT | NT | NT | NT | NT | NT |
| 3 | 100% | 100% | 98% | 65% | 100% | 100% | 100% | 100% | NT | 100% | NT | 100% | 100% | 100% |
| 4 | 6% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | NT | NT | NT | 0% | 0% | |
| 5 | 100% | 100% | 92% | NT | 94% | 100% | 100% | 100% | 100% | NT | 100% | 100% | ||
| 6 | 100% | 100% | 100% | NT | 100% | 100% | 100% | 100% | NT | 100% | 100% | 100% | ||
| 7 | 100% | NT | 100% | 100% | 100% | 100% | 98% | 100% | 100% | NT | 100% | 100% | ||
| 8 | 100% | 100% | 99% | NT | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |||
NT = not tested
months post-transplant
TABLE 3.
PERCENTAGE OF T CELL CHIMERISM AT SELECTED TIME POINTS AFTER TRANSPLAN†
| MONTHS* | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 9 | 12 | 13 | 14 | 15 | 18 | 24 | 30 | |
| Subjects | ||||||||||||||
| 1 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| 2 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| 3 | NT | NT | NT | NT | NT | 100% | 100% | 100% | NT | 100% | NT | 100% | 100% | 100% |
| 4 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| 5 | NT | NT | NT | NT | NT | 100% | 100% | 100% | 100% | NT | 100% | 100% | ||
| 6 | NT | NT | NT | NT | 100% | 100% | 100% | 100% | NT | 100% | 100% | 100% | ||
| 7 | NT | NT | NT | 100% | NT | 100% | 100% | 100% | 100% | NT | 100% | 100% | ||
| 8 | 100% | 100% | 100% | NT | NT | 100% | 100% | 100% | 100% | 100% | 100% | |||
Subject #1 is a 50-year-old male with renal failure secondary to membranous nephropathy who received a 5 of 6 HLA-matched living related donor (LRD) kidney and FCRx transplant in February 2009. Mobilized peripheral blood stem cell (MPBSC) collection from the donor was complicated by high granulocyte contamination and resultant impaired product viability. The FCRx product was not administered. A subsequent iliac crest bone marrow harvest was performed intraoperatively during the donor nephrectomy and processed for FCRx. The post-transplant dose of cyclophosphamide was held due to safety concerns about the early nadir period in this patient population. Chimerism was 30% at one month, but gradually decreased and was lost after 5 months. Renal function was excellent with a normal 6 month protocol biopsy and no evidence of GVHD. MMF was discontinued at 6 months, and tapering of tacrolimus initiated in light of donor-specific hyporesponsiveness in cell mediated cytolysis assays in vitro. The subject presented with new onset proteinuria at his one year standard of care visit. A kidney transplant biopsy revealed recurrent membranous nephropathy. Tapering of tacrolimus was halted and the subject was treated with Rituximab (375 mg/m2 weekly for one month). The proteinuria subsequently resolved. A protocol biopsy at 24 months after transplant was free of acute or chronic rejection and confirmed disease quiescence. At the time of publication, he remains on tacrolimus monotherapy with normal renal function (Fig. 1B).
Subject #2 is a 56-year-old male with end-stage renal disease (ESRD) due to hypertension who underwent a 3 of 6 HLA-matched living donor renal/stem cell transplant in April 2009. He tolerated the conditioning and transplant well and was discharged on postoperative day 2. He experienced immediate renal allograft function and the expected nadir for WBC and platelets of less than 2 weeks. He achieved 95% chimerism at month 1 and 100% at month 2. He had no evidence of GVHD or engraftment syndrome. Three months after transplant, the subject developed a febrile illness of uncertain etiology that progressed to intercurrent sepsis and hypotension, requiring inotropic support and ventilation. Bone marrow chimerism testing showed 100% donor chimerism at onset of the illness. After several days, the patient was weaned from inotropic support and extubated. Serum creatinine was noted to be increasing and a renal transplant biopsy showed extensive hemorrhagic necrosis. A nuclear medicine scan of the transplant showed absence of blood flow through the renal artery. He underwent renal transplant nephrectomy. He subsequently underwent a living related renal transplant and continues to do well, with stable renal function on standard immunosuppression. A careful and thorough review by the investigators and Data Safety Monitoring Board (DSMB) for the study concluded that occult viral sepsis was the most likely explanation.
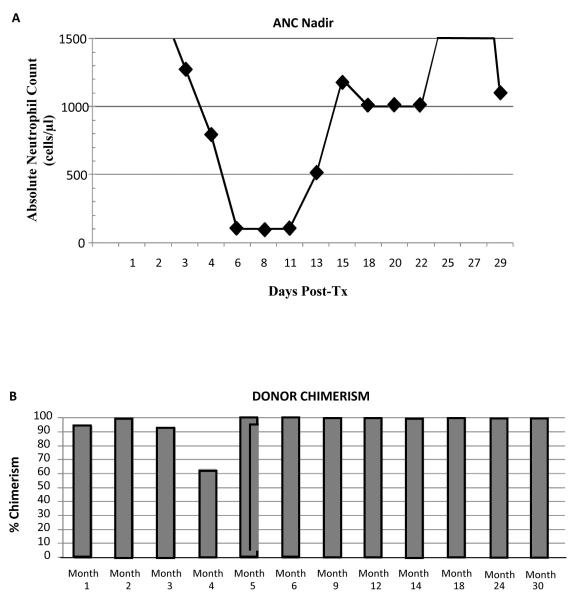
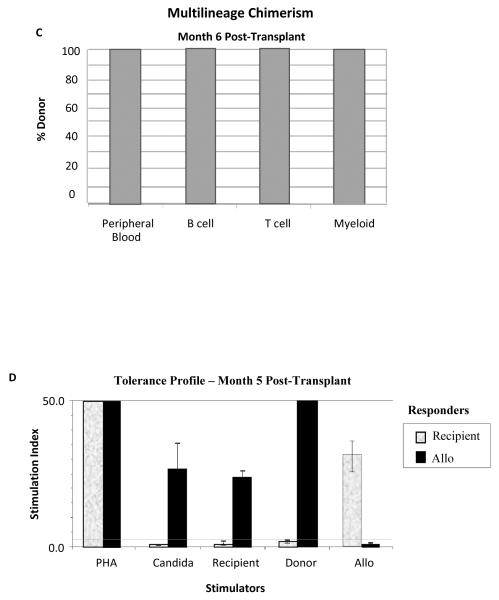
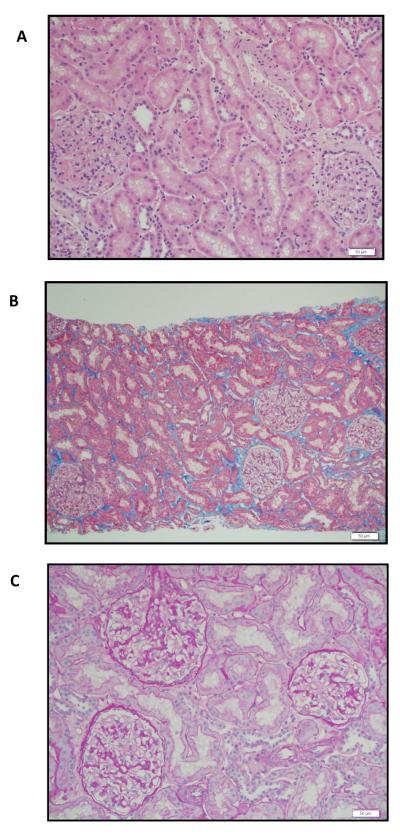
Subject #3 is a 43-year-old male who developed ESRD due to polycystic kidney disease. A detailed clinical course is presented as he represents our first complete success in this trial. He received a 1 of 6 HLA-matched living unrelated kidney/FCRx transplant in May 2009. The characteristic nadir, which is similar for all recipients, is shown in Figure 2A. He had 95% donor chimerism at one month after transplant. Chimerism has fluctuated between 63% and 100% with no evidence of GVHD or engraftment syndrome (Fig. 2B, Table 2). At 6 months, peripheral blood multilineage chimerism testing revealed the presence of 100% donor B cell, T cell, and myeloid cell production (Fig. 2C). T cell chimerism has consistently been 100% (Table 3). Flow cytometric crossmatch for anti-donor antibodies was negative at 1 month, 6 months, 1 year, and 2 years after transplant. At month 3, the recipient began to exhibit donor-specific hyporesponsiveness but regained immunocompetence to respond to HLA-disparate third-party alloantigen in in vitro mixed lymphocyte reaction (MLR) proliferative assays which has persisted (Fig. 2D). Renal function has remained stable and within normal limits (Fig. 1B). Protocol biopsies at 6 and 12 months after transplant were histologically normal. MMF was discontinued at 6 months after transplant. Tacrolimus was reduced to subtherapeutic dosing at 9 months (trough 0–3 ng/ml) and discontinued at one year. A subsequent protocol graft biopsy at 24 months after transplant (12 months of all immunosuppression) was histologically normal (Fig. 3A–C). Donor chimerism has remained 100% more than one year after immunosuppression was withdrawn. Adverse events in this subject include an exanthema at one month after transplant. GVHD was included in the differential diagnosis, but a biopsy revealed a sulfa-based drug rash. The rash resolved spontaneously and has not recurred. In addition, the subject developed a single dermatome varicella zoster reactivation at 9 months following transplant, which resolved and has not recurred. At the time of publication, he was 32 months after transplant with stable renal function.
Fig. 2.
Summary of nadir, donor chimerism, and clinical outcomes in Subject #3. A) Kinetics of nadir (absolute neutrophil count [ANC]) ≤ 500. The horizontal axis represents days after kidney transplant and vertical axis ANC. B) Whole blood mononuclear cell chimerism using the STR molecular assay (LabCorp). The sensitivity and specificity for these assays was 1–2%. C) Whole blood mononuclear cells were isolated and sorted for B cells (CD19+), T cells (CD3+), or myeloid-derived cells (CD66B+). D) Fresh recipient peripheral blood lymphocytes were exposed to PHA, Candida, recipient stimulators, donor simulators, and third-party allo-stimulators in one-way MLR proliferative assays. Third-party allo-responder controls were performed. A stimulation index ≥ 3 is positive. The stimulation index is calculated as the ratio of antigen-specific proliferation to unstimulated recipient lymphocytes. Error bars are mean ± SD for triplicate assays.
Fig. 3.
Representative sections of kidney transplant biopsy at one year after all immunosuppression was withdrawn. Staining with A) hematoxylin-eosin (20×), B) Masson trichrome (10×), and C) PAS (20×) shows no acute or chronic rejection, minimal tubular atrophy, and minimal interstitial fibrosis.
Subject #4 is a 29-year-old male with ESRD due to Alports Syndrome who received a LRD kidney/FCRx transplant (3 of 6 HLA-match) June 2009. He received a deliberately reduced αβ-T cell dose (Table 1) because of unresolved concerns regarding the etiology of the rash in Subject #3, described above. His course after transplant was complicated by a wound infection successfully treated with intravenous antibiotics. Donor chimerism was 6% at one month but was gradually lost by 3 months (Table 2). Donor-specific hyporesponsiveness persisted as assessed by MLR, and a protocol biopsy at 6 months was histologically normal. MMF was discontinued at 6 months and tapering of tacrolimus was initiated. However, a protocol biopsy at 12 months showed subclinical Banff 1A rejection despite a normal serum creatinine. Staining for C4d and donor-specific antibody was negative. He was treated with a short course of intravenous corticosteroids and has been maintained on therapeutic tacrolimus monotherapy (trough 5–8 ng/ml). Renal function has remained stable (Fig. 1B), no donor-specific antibody has been detected, and a follow-up allograft biopsy at 17 and 24 months after transplant were histologically normal.
The first four subjects represent a learning curve in this pilot study. Because of the loss of chimerism in Subjects #1 and #4, who both received lower numbers of αβ T cells, FC, and CD34+ cells, all subsequent subjects in our study have received processed stem cell infusions modeled after the product administered in Subject #3 (Table 1), as well as both doses of cyclophosphamide. This has resulted in durable high levels of whole blood and CD3 cell chimerism in Subjects #5 through #8 as shown in Tables 2 and 3. No subject has exhibited engraftment syndrome, none has developed anti-donor antibody, and none has exhibited acute or chronic GVHD. All have stable renal function (Fig. 1B).
Immunologic monitoring
We conducted serial immunophenotypic analyses of lymphocyte and monocyte subpopulations in all subjects. The figures are provided in Supplemental Materials (Fig. S1A – S1H). We observed a reduction in CD3+ T cells/αβ-TCR+ lymphocytes early after transplant (Fig. S1A). Immunologic reconstitution during the first year after transplant was notable for a blunted return of CD4+ T cells (Fig. S1B) compared to CD8+ T cells (Fig. S1C), with an inversion of the CD4/CD8 ratio (Fig. S1D). In addition, immunologic reconstitution was characterized by robust recovery and/or increase in the numbers of CD19+ B cells (Fig. S1E), CD14+ monocytes (Fig. S1F), and CD56+ natural killer (NK) cells (Fig. S1G). A marked reduction in CD19+ B cells was seen at 12 and 18 months after transplant in Subject #1, reflecting the administration of the anti-B cell monoclonal antibody rituximab as therapy for his recurrent membranous nephropathy. Furthermore, although we noted an initial decrease in the absolute numbers of CD4+/CD25+/FoxP3+/CD127− phenotypic regulatory T cells (Treg), an increase in the Treg to T effector (Teff) cell ratio was frequently observed in durably chimeric recipients compared to the two recipients who exhibited only transient macrochimerism (Subjects 1 and 4) (Fig. S1H). In the context of full donor chimerism without GVHD, this observation suggests that dynamic immune regulation by Treg likely contributes to the prevention of GVHD in our subjects.
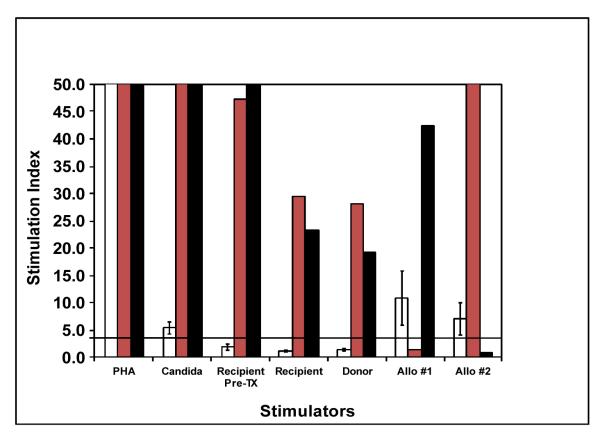
In vitro proliferation assays performed in our subjects identified the development of donor-specific hyporesponsiveness and the ability to respond to third-party alloantigen. Of interest, donor-specific hyporesponsiveness was evident during the first year after transplant in Subjects 1 and 4 even after donor chimerism was lost, and in Subject #4 at the time that subclinical rejection occurred. The presence of full donor chimerism without GVHD was associated with tolerance to donor alloantigen as reflected by the absence of in vitro proliferative responses of the chimeric recipient peripheral blood lymphocytes against archived pre-transplant recipient stimulators in Subjects 3, 5, 6, 7, and 8 by MLR. Figure 4 for Subject #5 is representative of this effect. In striking contrast, peripheral blood lymphocytes obtained from the living donor demonstrated robust proliferation against recipient stimulators (Fig. 4). These data support the notion that the donor chimeric lymphocytes are rendered tolerant to recipient alloantigens after transplant.
Fig. 4.
Non-responsiveness of chimeric recipients to archived pre-transplant recipient stimulators. Subject 5 is shown as a representative MLR. Y-axis denotes the mean stimulation index; x-axis denotes the antigenic challenge used. White bar demonstrates response of the transplant recipient; gray and black bars show response of third-party MHC-disparate individuals allo#1 and allo#2. Please note that allo #1 and #2 were also used as stimulators in these experiments.
Composition of FC total (CD8+/TCR−) subpopulations
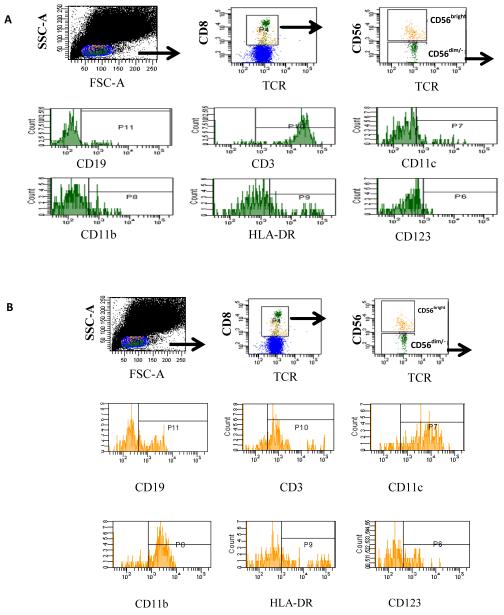
We previously reported that in the mouse FC subpopulations include NK FC, p-preDC FC, and CD19+ FC (8). Multiparamater flow cytometry was performed to analyze the phenotype of FC subpopulations in human mobilized HSCT product. Two major FC subpopulations are CD56dim/− and CD56bright. Using multicolor flow cytometric analysis we found that the majority of CD56dim/− FC are also positive for CD3ε and HLA-DR and negative for the dendritic cell markers CD11c and CD123 (Fig. 4A). CD56dim/− FC comprise approximately 49% of FC total (n=4 experiments). The majority of the CD56bright FC subpopulation is CD19+, CD11c+, CD11b+ and CD3ε− and comprises approximately 46% of FC total (Fig. 4B).
Discussion
Transplantation of organs and cellular grafts has become widely accepted therapies. However, the toxicity of the immunosuppressive agents required for graft maintenance is substantial, resulting in end organ damage and failure (15,16), opportunistic infections, hypertension, diabetes, and an increased rate of malignancy (15,16). Chronic rejection remains the primary cause of late graft loss (17). Therefore, strategies to induce donor-specific tolerance are being actively pursued. One approach to achieve tolerance is through cell-based therapies resulting in chimerism. First described in mice (2), mixed chimerism has been shown to induce donor-specific tolerance in a number of species, including humans, and is the only approach that appears to be generalizable to all species in which it has been tested (18–20).
A number of reports from clinical protocols using combined HSC and renal transplantation have recently demonstrated the feasibility of using cell-based therapies to induce tolerance. One significant variable in these approaches is whether the researchers only achieved microchimerism, defined as very low levels of donor chimerism vs. macrochimerism, where donor cells are typically present as at least 1% of peripheral blood cells. In a series of ten subjects with end-stage renal failure who underwent conditioning with cyclophosphamide, Rituximab, 700 cGy of thymic irradiation, and anti-CD2 monoclonal antibody followed by transplantation of unmodified donor marrow, very low levels of donor chimerism were present for up to 21 days in the peripheral blood (5,6,20). Thereafter chimerism was undetectable. A capillary leak syndrome associated with production of anti-donor antibody and elevated creatinine levels, recently termed “engraftment syndrome” (21), developed in 9 of the 10 subjects (5,20). Two subjects lost their kidneys to acute rejection (14). Seven of the ten total subjects were successfully tapered off immunosuppression. However, one of these seven subjects developed acute rejection 7 weeks after immunosuppression was discontinued, and 6 months following reinstitution of immunosuppression renal function declined to a level where dialysis was required (6). Another of these recipients has developed C4d positivity on biopsy and anti-class II HLA donor antibody, indicating antibody and complement deposition which has been associated with an increased risk of chronic allograft nephropathy and graft loss (20). All seven recipients reportedly show donor-specific hyporeactivity in vitro. GVHD did not occur, perhaps because sustained engraftment was not achieved (22). The impact of antibody-mediated rejection on long-term renal allograft function remains a concern, as it has historically been associated with impaired graft survival (23).
A second report described the outcomes in 12 HLA-identical kidney/CD34-selected peripheral blood stem cell transplants (4,24). Recipients were conditioned with 800 to 1200 cGy total lymphoid irradiation and transplanted with 8 × 106 CD34+ cells enriched with 1 × 106 CD3+ T cells/kg recipient body weight. One of the first six recipients treated engrafted durably with macrochimerism and was successfully tapered from immunosuppression (24). The others did not demonstrate macrochimerism, but were recently tapered from immunosuppression (24). Eight of twelve subjects enrolled in the study were successfully tapered from immunosuppression. Three of the four who remain on immunosuppression experienced rejection episodes during the tapering process, and one subject developed recurrent disease (24). However, this approach did not achieve durable chimerism when it was expanded to HLA-mismatched recipients (7). The requirement for HLA matching would preclude the application of this approach to the majority of solid organ recipients who are not HLA-matched to their donor, especially deceased donor transplants. Collectively, these studies demonstrate the feasibility and efficacy of using stem cell-based therapies to induce tolerance in the clinic.
The phase 2 study reported here is a translational research protocol based on the tolerogenic features of CD8+/TCR− graft FC that were first discovered in the mouse (8). FC are a heterogeneous population comprised predominantly of p-preDC FC (9). Removal of p-pre-DC FC completely abrogates FC function in vivo and in vitro (9,12). However, p-preDC FC do not completely replace CD8+/TCR− FC in function. CD8+/TCR− FC enhance clonogenicity of HSC in vitro (9,25) and robustly prevent GVHD in vivo (13). They induce FoxP3+/CD4+/CD25+ Treg in vitro (26) and antigen-specific Treg in vivo (12). As such, FC may address the primary challenge in translating cell-based therapies to the clinic: to maintain the tolerogenic features after transplantation while avoiding GVHD (27). In the present study, we tested whether a stem cell-based cellular product engineered to be enriched for FC plus HSC while depleting cells that predispose to GVHD would allow the induction of reciprocal graft/host tolerance in renal allograft recipients. We report here a nonmyeloablative conditioning HSCT approach to establish chimerism and tolerance, while avoiding GVHD and engraftment syndrome, in renal transplant recipients. To our knowledge, this is the first report of durable macrochimerism without GVHD in HLA-mismatched related and unrelated stem cell/renal transplant recipients. We have observed that both HSCT composition and conditioning significantly influence outcome.
A recent large series of haploidentical (3 of 6 HLA match) transplants in subjects with hematologic malignancies and co-morbidities that precluded ablative HSCT who were similarly conditioned with fludarabine, 200 cGy TBI and 2 or 4 doses of cyclophosphamide pre- and post-transplant demonstrated that the pre- and post-transplant cyclophosphamide was required for durable engraftment (22). This approach was first developed in a mouse model of nonmyeloablative conditioning then translated to the clinic (28). Building on this experience, we found that both doses of cyclophosphamide in the renal tolerance protocol were required for durable chimerism in this patient population. The cyclophosphamide is hypothesized to eliminate newly activated host-versus-graft and graft-versus-host lymphoid populations while sparing immunologic memory, FC, and HSC.
It is noteworthy that despite the high levels of donor chimerism and the degree of HLA mismatch in our subjects, neither GVHD nor production of anti-donor antibodies has occurred in any of our chimeric recipients. This absence of GVHD was associated with a lack of in vitro proliferative responses of donor peripheral blood lymphocytes from chimeric recipients against archived pre-transplant recipient stimulator PBMC. In striking contrast, fresh donor-derived PBMC mounted a robust response against recipient alloantigen. This would indicate that the chimeric donor lymphocytes were tolerized to the recipient, perhaps by tolerogenic features of the FCRx product itself in combination with the after transplant cyclophosphamide (22). The FC is tolerogenic, and induces antigen-specific Treg in vivo (12). A significant increase in CD4+ Treg/Teff populations was noted to be present in the peripheral blood of those subjects who remained durably chimeric compared to the two subjects who lost chimerism. However, in the human subjects, a direct comparison of FC-depleted vs. FC-replete or unmodified grafts was deemed inadvisable by the investigators and the data safety monitoring board. As such, a direct role for FC in outcomes cannot be definitely concluded.
A clinical trial of mismatched recipients using unmodified bone marrow cells did not show the GVHD-sparing effect experienced in our subjects. In similarly conditioned haploidentical recipients of unmodified mobilized peripheral blood mononuclear cells who underwent HSCT for hematologic malignancy, the incidence of grade II–IV and II–III acute GVHD by day 200 was 34 and 6%, respectively (22). Chronic GVHD occurred in 25%, with the majority presenting by 100 days after transplantation and all by 300 days (22). The major difference in our study is the removal of effector GVHD-causing cells, the presence of FC, and the renal transplant itself. Since GVHD normally occurs within 300 days after HSCT and while the recipients are still on tacrolimus/MMF GVHD prophylaxis, it is highly unlikely that our subjects will develop de novo GVHD in the future. Luznik et al. concluded that the post-transplant cyclophosphamide deleted alloreactive host-vs-graft and graft-vs-host reactive cells while sparing the HSC and host and donor memory immune responses, resulting in reciprocal self/allo-tolerance and superior immunologic recovery (22).
The patterns of immune reconstitution observed in our combined FCRx/kidney recipients resemble those previously described in adult recipients of HSCT (29). We observed a rapid recovery and increase in CD56+ NK cells after FCRx in our subjects, consistent with previous published studies (30). Initial recovery of the T cell compartment has been shown to reflect peripheral expansion of memory T cells, driven by cytokines and the presence of alloreactive antigens, before the production of naive T cells in the thymus begins. This is especially true for CD4+ T cells that reconstitute later than CD8+ T cells and rely more on thymic production of naive T cells after HSCT, leading to an inversion of the CD4/CD8 ratio as we observed in our FCRx/kidney patients. While initial expansion of T cells in our fully donor chimeric recipients may reflect proliferation of T cells contained in the FCRx product, recovery in our subjects who lost chimerism likely derives from cells that escaped the effects of the low intensity conditioning regimen. Moreover, the fact that the PBL from the chimeric recipients who were 100% donor do not respond to archived pre-transplant recipient alloantigen would suggest that these donor-derived T cells are newly produced and/or have been tolerized. T cell receptor rearrangement excision DNA circles (TREC), as well as CD31 expression, have been used as markers for naive T cell reconstitution occurring in the thymus. TREC levels have been reported to remain low for months, even years, after allogeneic HSCT in adults. We plan to investigate the kinetics of thymic repopulation and the contribution of new thymic emigrants to repopulation of different T cell subsets in future studies.
We observed a relative increase in numbers of CD19+ B cells in nearly all of our FCRx/kidney subjects during the first year after transplant. Recent studies in operationally tolerant kidney transplant recipients who have been tapered off immunosuppression following conventional organ transplantation have identified a particular blood B cell phenotype, with an expansion of memory activated B cells and increased expression of inhibitory molecules, suggesting a role for B cells in maintaining graft tolerance (31). Whether so-called regulatory B cells contribute to immune modulation, helping to control graft-versus-host responses in our chimeric subjects, and/or operational tolerance and graft acceptance in subjects where chimerism is lost, will require future studies.
Chimeric recipients exhibited responses to PHA and third-party alloantigen in vitro. It is not surprising that standard immunocompetence testing using PHA and alloantigens revealed immunocompetence, as these responses are polyclonal and a crude measure of immune function. Previous experiments using chimeric animals suggested that the thymic epithelium was imprinting MHC restriction. Viral challenge in ablatively conditioned fully chimeric mice was lethal, while mixed chimeras eliminated the virus and survived (32). That would lead one to question whether 100% donor chimeric humans would be immunologic cripples. In recent studies in mice, this has not proven to be the case. Elegant studies using transgenic mice prepared as tetraparental aggregation chimeras have demonstrated that TCR:antigen APC interactions are more important than MHC class I or class I, and that T cell repertoire selection is independent of thymic MHC (33). These studies demonstrated that the MHC of nonthymic epithelial cells efficiently selects a functional T cell repertoire. We would therefore hypothesize that nonmyeloablatively conditioned chimeric humans would be immunocompetent to respond to infectious challenge. It is likely that there are sufficient numbers of professional and nonprofessional antigen-presenting cells remaining to allow robust immunocompetence and tolerance in the nonmyeloablatively conditioned subjects with high levels of donor chimerism (33,34). It is of note that all severe adverse events experienced by the 8 subjects occurred while they were on conventional maintenance immunosuppression.
Studies that empirically wean immunosuppression in non-chimeric individuals rely on the concept of “operational tolerance,” a term that has evolved to refer to subjects who have been off immunosuppression for at least 1 year. One major limitation in studies of operationally tolerant recipients is that there is a paucity of biomarkers or assays that predict which subjects will do well with minimization or cessation of immunosuppression and which are prone to chronic (35) or even acute rejection (35). Clearly, standard in vitro proliferative responses are not reliable, as reflected by the one subject in our study who lost chimerism, remained tolerant to his donor in proliferative assays, yet experienced a subclinical rejection episode (Banff 1A) on protocol biopsy that responded to increased immunosuppression. It is of interest that this subject has since gone on to lose donor specific hyporesponsiveness at 2 years after transplant (Fig. S2). Similarly, one subject reported in the Massachusetts General Hospital tolerance study experienced acute rejection 7 weeks after cessation of immunosuppression despite in vitro evidence for tolerance (20). Levitsky et al. reported that although 80% of liver transplant recipients can be successfully weaned from immunosuppression, only 20% are successfully maintained off immunosuppression long-term without experiencing rejecting (35). Notably, there was no reliable biomarker/endpoint identified as a predictor of success vs. failure in tapering and maintaining rejection-free graft survival off immunosuppression. Some subjects were off immunosuppression for well over 1 year before rejection occurred. The persistence of high levels of donor chimerism observed in the present study appears to represent a reliable, easily evaluable biomarker for donor-specific tolerance induction and the ability to safely wean subjects from immunosuppression. The chimerism testing was performed by an independent CLIA and FDA approved laboratory (LabCorp) as per standard of care for HSCT (36). It has been shown in mice (37) and humans that T cell chimerism exceeding 50% for more than 6 months reliably predicts durable graft acceptance after HSCT (38). In our own renal/FCRx recipients, T cell chimerism was associated with persistent donor chimerism and the ability to successfully wean subjects from immunosuppression while maintaining stable renal function. This has now been adapted as our primary clinical endpoint.
The ability to establish high levels of donor multilineage chimerism in haploidentical and highly mismatched unrelated donor/recipient pairs without GVHD or engraftment syndrome could have revolutionary therapeutic implications for treatment of disorders for which HSCT can provide a “functional cure,” including inherited metabolic disorders, hemoglobinopathies, and autoimmune diseases (39). It could also address the significant numbers of individuals who are candidates for a bone marrow transplant for hematologic malignancy but do not have a suitably matched donor.
Materials and Methods
All protocols are approved by the Northwestern University and University of Louisville Institutional Review Boards and the FDA (IDE 13947). Informed consent was obtained for all donors and recipients. Donor and recipient eligibility criteria are detailed in supplementary materials.
Cell dosing algorithm
Most bone marrow cell processing devices leave residual T cells after depletion. A dose-escalation for maximum allowable αβ-T cell content was utilized in phase I of this protocol, with the intent to administer as many FC and HSC as possible in that context. Historically, beginning in 1996, the starting maximum allowable αβ-T cell dose was 2 × 105/kg recipient body weight. If engraftment and GVHD did not occur, the T cell dose was increased by 4 × 105 αβ-T cells/kg in the next recipient. Macrochimerism did not occur in any subjects transplanted until the current conditioning and cell dosing was adopted for the subjects reported here. All non-engrafting subjects resumed endogenous hematopoiesis and were not tapered from immunosuppression.
Mobilized peripheral blood stem cell collection and FCRx preparation
At least two weeks prior to the renal transplant, donors were mobilized with granulocyte colony stimulating factor (10 mcg/kg bid), and apheresis was performed on day +4. The product was diluted in nutrient-rich ex vivo cell medium (BioWhittaker) and immediately transported by courier, in a controlled temperature container, to the Institute for Cellular Therapeutics at the University of Louisville and processed in a Foundation for the Accreditation of Cellular Therapy (FACT)-accredited GMP facility by a proprietary approach under FDA IDE 13947 using the CliniMACS (Miltenyi Biotec) to remove mature GVHD-producing and antigen-presenting cells while retaining HSC, FC, and progenitor cells. The product was then shipped back to Northwestern University for intravenous infusion on the day following living donor kidney transplant. Seven of eight subjects received cryopreserved product. Recipients underwent similar mobilization, and autologous cryopreserved mPBSC were stored in case hematologic rescue was required.
Immunologic monitoring
Recipient responses to PHA, Candida, tetanus toxoid, donor, and third-party alloantigens were tested monthly as previously described (40). Flow crossmatch assays were performed at 1 and 6 months. Protocol biopsies were performed at 6 months and 1 year after transplant per standard of care.
Chimerism testing
Chimerism was determined by genotyping of simple sequence-length polymorphisms encoding short tandem repeats (STR) at an independent laboratory (LabCorp) (36). For lineage chimerism testing, CD19+ (B cells), CD3+ (T cells), and/or CD66B+ (myeloid) cells were sorted from whole blood, then analyzed by molecular STR typing. This assay is sensitive to approximately 1–2%. Internal controls to define the sensitivity are performed for each assay.
Weaning of immunosuppression and clinical end points
Tacrolimus and MMF were continued at therapeutic levels until 6 months after transplant (Fig. 1). If renal function and protocol biopsy were normal, and if chimerism or donor-specific hyporesponsiveness were present, the MMF was discontinued. The tacrolimus was tapered at 9 months to subtherapeutic trough levels (0–3 ng/ml). Immunosuppression was discontinued at 1 year if the following criteria were met: durable whole blood macrochimerism, T cell chimerism, stable renal function, no anti-donor antibody, and a normal protocol renal allograft biopsy.
Supplementary Material
Fig. 5.
Representative 10 color flow cytometric analysis of FC subpopulations in the transplanted stem cell product. CD8+/TCR− FC Total were sorted and re-stained for CD56, CD19, CD3, CD11c, CD11b, HLA-DR, fox p3 and CD123. A) CD56dim/− FC were analyzed for co-expression of CD19, CD3ε, CD11c, CD11b, HLA-DR, and CD123. B) Flow cytometric analysis of CD56bright FC.
Acknowledgments
We thank Dr. Haval Shirwan for review of the manuscript and helpful comments; Carolyn DeLautre for manuscript preparation; Irma Dixler and Laura Coleman for clinical trial assistance.
Funding: Supported by NIH R01 DK069766; The Department of the Army, Office of Army Research: The National Foundation to Support Cell Transplant Research; the W. M. Keck Foundation; and The ASTS Collaborative Scientist Award.
Footnotes
Author Contributions: JRL contributed to study design, conduct of the clinicaltrial, data analysis, and writing of the paper; MMA contributed to conduct of the clinical trial and writing of the paper; JM contributed to data analysis and writing of the paper; LG contributed to conduct of the clinical trial and writing of the paper; K.R. protocol design and implementation, data analysis; RH protocol design, data interpretation; DJT statistical design, protocol development and implementation; BK study design, data interpretation, product development; MJE performed, supervised, and analyzed the immunophenotyping and immunologic function assays; GH protocol design, data interpretation; and STI protocol design, regulatory oversight, data analysis, product development.
Competing Interests: Dr. Suzanne Ildstad has equity interest in Regenerex, LLC, a start-up biotech company. David Tollerud and Brad King are officers of Regenerex LLC. The company has not been capitalized. All other authors have no conflict of interest to declare.
Publisher's Disclaimer: This manuscript has been accepted for publication in Science Translational Medicine. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencetranslationalmedicine.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS
Reference List
- 1.Ildstad ST, Shirwan H, Leventhal J. Is durable macrochimerism key to achieving clinical transplantation tolerance? Curr. Opin. Organ Transplant. 2011;16:343–344. doi: 10.1097/MOT.0b013e328348e67a. [DOI] [PubMed] [Google Scholar]
- 2.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 3.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr., Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J. Clin. Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scandling JD, Busque S, jbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman HA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin. Immunol. 2011;23:165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, Tan JC, Salvatierra O, Hoppe RT, Strober S. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman CL, Colson YL, Wren SM, Watkins SL, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone-marrow derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 9.Fugier-Vivier I, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, Chilton PM, Ildstad ST. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J. Exp. Med. 2005;201:373–383. doi: 10.1084/jem.20041399. PMCID: PMC2213023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimes HL, Schanie CL, Huang Y, Cramer D, Rezzoug F, Fugier-Vivier I, Ildstad ST. Graft facilitating cells are derived from hematopoietic stem cells and functionally require CD3, but are distinct from T lymphocytes. Exp. Hematol. 2004;32:946–954. doi: 10.1016/j.exphem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Taylor KN, Shinde-Patil VR, Cohick E, Colson YL. Induction of FoxP3+CD4+25+ regulatory T cells following hemopoietic stem cell transplantation: role of bone marrow-derived facilitating cells. J. Immunol. 2007;179:2153–2162. doi: 10.4049/jimmunol.179.4.2153. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Bozulic LD, Miller T, Xu H, Hussain LR, Ildstad ST. CD8a+ plasmacytoid precursor DC induce antigen-specific regulatory T cells that enhance HSC engraftment in vivo. Blood. 2011;117:2494–2505. doi: 10.1182/blood-2010-06-291187. PMC3062412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colson YL, Christopher K, Glickman J, Taylor KN, Wright R R, Perkins DL. Absence of Clinical GVHD and the In Vivo Induction of Regulatory T cells following Facilitating Cell Transplantation. Blood. 2004;104:3829–3835. doi: 10.1182/blood-2004-01-0393. [DOI] [PubMed] [Google Scholar]
- 14.Farris AB, Taheri D, Kawai T. T, Fazlollahi L, Wong W, Tolkoff-Rubin N, Spitzer TR, Iafrate AJ, Preffer FI, LoCascio SA, Sprangers B, Saidman S, Smith RN, Cosimi AB, Sykes M, Sachs DH, Colvin RB. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am. J. Transplant. 2011;11:1464–1477. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 16.Senechal M, Dorent R, du Montcel ST, Ghossou JJ, Pavie A, Petitclerc T, Dubois M, Isnard R, Gandjbakhch I. End-stage renal failure and cardiac mortality after heart transplantation. Clin. Transplant. 2004;18:1–6. doi: 10.1111/j.1399-0012.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat. Rev. Nephrol. 2009;5:513–519. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 18.Orloff MS, DeMara EM, Prehn J, Jordan SC. Alterations of the interleukn-4 pathway in production of tolerance by mixed hematopoietic chimerism. Surgery. 1995;118:212–219. doi: 10.1016/s0039-6060(05)80326-5. [DOI] [PubMed] [Google Scholar]
- 19.Buhler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, Saidman SL, Sackstein R, McAfee S, Dey B, Colby C, Cosimi AB. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism. Curr. Opin. Organ Transplant. 2011;16:366–371. doi: 10.1097/MOT.0b013e3283484b2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Sachs DH, Spitzer T, Tolkoff-Rubin N, Colvin RB, Saidman S, Ko DSC, Wong W, Williams W, Sykes M, Cosimi AB. Tolerance of Renal Allografts across HLA barriers: Follow-up on the First Trial and Initial Results of the Second. XXIII International Congress of The Transplantation Society; Vancouver, Canada. August 15–19, 2010; Ref Type: Abstract. [Google Scholar]
- 22.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolanos-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodeky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Q, Terasaki PI, Cai J, Briley K, Catrou P, Haisch C, Rebellato L. Extremely high association between appearance of HLA antibodies and failure of kidney grafts in a five-year longitudinal study. Am. J. Transplant. 2007;7:864–871. doi: 10.1111/j.1600-6143.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 24.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N. Engl. J. Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier I, Ildstad ST. TNFa is critical to facilitation of hematopoietic stem cell engraftment and function. J. Immunol. 2008;180:49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- 26.Taylor KN, Laszkowska M, Cohick E, Colson YL. Induction of FoxP3+CD4+CD25+ regulatory T cells by a bone marrow population distinct from plasmacytoid-DC. Cell. Immunol. 2008;251:43–49. doi: 10.1016/j.cellimm.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: Clinical application of T regulatory cells. Semin. Immunol. 2011;23:304–313. doi: 10.1016/j.smim.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colson YL, Wren SM, Schuchert MJ, Patrene DK, Johnson PC, Boggs SS, Ildstad ST. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J. Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 29.Gress RE, Emerson SG, Drobyski WR. Immune reconstitution: how it should work, what's broken, and why it matters. Biol. Blood Marrow Transplant. 2010;16:S133–S137. doi: 10.1016/j.bbmt.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 31.Pallier A, Hillion S, Danger R, Giral M, Racape M, Degauque N, Dugast E, Ashton-Chess J, Pettre S, Lozano JJ, Bataille R, Devys A, Cesbron-Gautier A, Braudeau C, Larrose C, Soulillou JP, Brouard S. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 32.Ruedi E, Sykes M, Ildstad ST, Chester CH, Althage A, Hengartner H, Sachs DH, Zinkernagel RM. Antiviral T cell competence and restriction specificity of mixed allogeneic (P1 + P2 -> P1) irradiation chimeras. Cell. Immunol. 1989;121:185–195. doi: 10.1016/0008-8749(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 33.Martinic MM, Rulicke T, Althage A, Odermatt B, Hochli M, Lamarre A, Dumrese T, Speiser DE, Kyburz D, Hengartner H, Zinkernagel RM. Efficient T cell repertoire selection in tetraparental chimeric mice independent of thymic epithelial MHC. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1861–1866. doi: 10.1073/pnas.252641399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinic MM, van den Broek MF, Rulicke T, Huber C, Odermatt B, Reith W, Horvath E, Zellweger R, Fink K, Recher M, Eschli B, Hengartner H, Zinkernagel RM. Functional CD8+ but not CD4+ T cell responses develop independent of thymic epithelial MHC. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14435–14440. doi: 10.1073/pnas.0606707103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitsky J. Operational tolerance: past lessons and future prospects. Liver Transpl. 2011;17:222–232. doi: 10.1002/lt.22265. [DOI] [PubMed] [Google Scholar]
- 36.Akpinar E, Keary JM, Kurlander R, Hale DA. Measurement of chimerism in cynomolgus monkeys using human-specific short tandem repeat-based assay. Transplantation. 2005;79:236–239. doi: 10.1097/01.tp.0000148916.95656.93. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Chilton PM, Huang Y, Schanie CL, Ildstad ST. Production of donor T cells is critical for induction of donor-specific tolerance and maintenance of chimerism. J. Immunol. 2004;172:1463–1471. doi: 10.4049/jimmunol.172.3.1463. [DOI] [PubMed] [Google Scholar]
- 38.Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB, Maloney DG, Heimfeld S, Oparin D, Zellmer E, Radich JP, Grumet FC, Blume KG, Chauncey TR, Little MT. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 39.Colson YL, Shinde Patil VR, Ildstad ST. Facilitating cells: Novel promoters of stem cell alloengraftment and donor-specific transplantation tolerance in the absence of GVHD. Crit. Rev. Oncol. Hematol. 2007;61:26–43. doi: 10.1016/j.critrevonc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Muluk SC, Clerici M, Via CS, Weir MR, Kimmel PL, Shearer GM. Correlation of in vitro CD4+ T helper cell function with clinical graft status in immunosuppressed kidney transplant recipients. Transplantation. 1991;52:284–291. doi: 10.1097/00007890-199108000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.