Abstract
Objective. A shift in the total incidence from left- to right-sided colon cancer has been reported and raises the question as to whether lifestyle risk factors are responsible for the changing subsite distribution of colon cancer. The present study provides a review of the subsite-specific risk estimates for the dietary components presently regarded as convincing or probable risk factors for colorectal cancer: red meat, processed meat, fiber, garlic, milk, calcium, and alcohol. Methods. Studies were identified by searching PubMed through October 8, 2012 and by reviewing reference lists. Thirty-two prospective cohort studies are included, and the estimates are compared by sex for each risk factor. Results. For alcohol, there seems to be a stronger association with rectal cancer than with colon cancer, and for meat a somewhat stronger association with distal colon and rectal cancer, relative to proximal colon cancer. For fiber, milk, and calcium, there were only minor differences in relative risk across subsites. No statement could be given regarding garlic. Overall, many of the subsite-specific risk estimates were nonsignificant, irrespective of exposure. Conclusion. For some dietary components the associations with risk of cancer of the rectum and distal colon appear stronger than for proximal colon, but not for all.
1. Introduction
Global estimates for 2008 indicate that colorectal cancer is the third most common cancer in the world [1]. Reports in several countries have described diverging incidence rates in colorectal cancer by subsite, including, in relative terms, an increasing proportion of proximal tumors [2–15], and thus a shift in absolute incidence from left- to right-sided colon cancers.
The reasons for this trend are not well understood; the subsites differ in physical function, artery supply, histology, and innervation, and they also derive from different segments in the primitive intestinal tract in the embryo [16]. The proximal colon originates from the midgut, whereas the distal colon and the rectum derivate originate from the hindgut. Comparisons have also shown that proximal colon tumors tend to have different molecular characteristics, with a higher proportion of microsatellite instability, and are more likely to have CpG island methylator phenotype and Ki-ras mutations than distal colon and rectal tumors [17].
It has been estimated that 45 percent of all colorectal cancer cases can be prevented in high-risk populations through modifications of diet, physical activity habits, and weight control [18]. According to the recent report from the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), there is convincing evidence that dietary fiber protects against colorectal cancer and that red and processed meat and alcohol (particularly in men) increase the risk of the disease [19]. Further, it is stated that garlic, milk, and calcium probably protect against colorectal cancer. No distinction is, however, made for the different subsites of the colorectum [19]. Also, although meta- [20–23] and pooled analyses [24–26] have provided quantitative synthesis for several of the dietary risk factors, little emphasis has been placed on subsite risks.
Based on the biological differences in the colorectal segments and the reported differences in incidence, we may suggest differences across the segments in their association to lifestyle factors, such as diet. The aim of the present paper is to give an updated overview summarizing the etiological differences between the colorectal subsites with regard to the dietary factors considered to be convincing or probable risk factors for colorectal cancer.
2. Material and Methods
The specific risk factors studied were red meat, processed meat, fiber, garlic, milk, calcium, and alcohol, selected given an a priori assessment of their importance in colorectal cancer etiology following the WCRF/AICR report in 2011 [19], and for which their modification could lead to a reduction in rates of colorectal cancer. The outcome was the risk of primary colorectal cancer according to subsite.
A search for cohort studies published as original articles was conducted via a search of PubMed (http://www.ncbi.nlm.nih.gov), using a search strategy that combined the term “colorectal neoplasms” with the terms “risk factors” and “cohort study” with either the term “diet,” “nutrition,” or “alcohol.” The search was restricted to studies published or available online as of October 8, 2012, in the English language. A detailed description of the search strategy and the resulting papers retrieved is given in Table 1, and the procedure is described in Figure 1. A total of 341 articles were identified and reviewed according to title and abstract. The initial evaluation yielded 108 articles in the study database and underwent a second evaluation based on full-text review. A similar PubMed search for case-control studies nested within a cohort identified 46 articles, of which one underwent full-text review. In addition, further 62 articles were identified by scanning the reference lists of retrieved articles, reviews, meta-, and pooled analyses and underwent full-text review (Figure 1).
Table 1.
Search results as of October 8, 2012.
| Queries | Result | |
|---|---|---|
| 1 | Search colorectal neoplasms | 148374 |
| 2 | Search risk factors | 745969 |
| 3 | Search diet | 332911 |
| 4 | Search nutrition | 259739 |
| 5 | Search alcohol | 718622 |
| 6 | Search cohort study | 1253216 |
| 7 | Search 3 OR 4 OR 5 | 1215492 |
| 8 | Search 2 AND 7 | 81363 |
| 9 | Search 1 AND 8 | 2099 |
| 10 | Search 6 AND 9 | 627 |
| 11 | Search case control study | 622385 |
| 12 | Search review | 2082039 |
| 13 | Search 10 NOT 11 NOT 12 | 356 |
| 14 | Search 13 Limits: Human, English | 341 |
Figure 1.
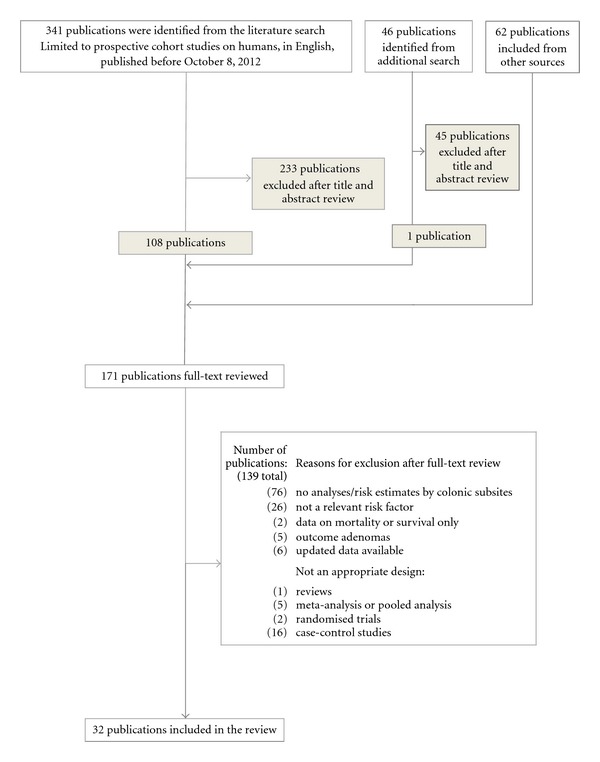
Flow diagram of the study selection.
Studies were included if they provided risk estimates (and corresponding confidence intervals) for both proximal and distal colon cancer. Proximal colon (right sided) includes ceacum, ascending and transverse colon, while distal colon (left-sided) includes descending colon, sigmoid flexure, and sigmoideum. Some studies have not followed the above-mentioned classification [27–29], and this is specified in Table 2. Further, to be included, the cohorts had to be either population based, registry based, or obtained from censuses. Studies on specific subpopulations (e.g., hospital-based cohorts) were not included nor were studies examining second cancers, metastasis, survival, or mortality. Following the full-text evaluation, 139 articles were excluded for the reasons indicated in Figure 1, and 32 articles were included in the review. Of these, seven gave data on (red) meat [30–36], five on processed meat [31–35], eight on fiber [37–44], one on garlic [37], three on milk [45–47], seven on calcium [28, 45, 47–51], and ten on alcohol [27, 29, 36, 52–58].
Table 2.
Characteristics of cohort studies included in the review on subsite specific dietary risk factors for colorectal cancer.
| Exposure | Reference | Cohort | Cases | Sex | Age | Follow-up, yr | Adjustments*** | Study Cohort, Country or Continent | Abbreviation |
|---|---|---|---|---|---|---|---|---|---|
| Red meat |
Shin et al., [36] | 869725 M | M: 3051 CRC, 536 PC, 751 DC, 1535 RC | B | 30–80 | 7 | 1 | Korean National Health System, Korea | KNHS |
| 395501 F | F: 1093 CRC, 236 PC, 225 DC, 451 RC | ||||||||
| Cross et al., [35] | 300948 | 2719 CRC, 1150 PC, 787 DC, 724 RC | B | 50–71 | 7.2 | 2, 3, 5, 6, 7, 12 | NIH-AARP Diet and Health Study, U.S. | NIH-AARP | |
| Sato et al., [34] | 41835 | 396/368 CRC, 123/115 PC, 85/75 DC, 159/155 RC∗$ | B | 40–64 | 11 | 1, 2, 3, 4, 5, 6, 7, 8, 11, 12 | The Miyagi Cohort Study, Japan | MCS | |
| Chao et al., [31] | 148610 | 1667 CRC, 667 PC, 408 DC, 470 RC | B | 50–74 | 8-9 | 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 13 | The Cancer Prevention Study II Nutrition Cohort, U.S. | CPS-II | |
| Larsson et al., [32] | 61433 | 733 CRC, 234 PC, 155 DC, 230 RC | F | 40–75 | 13.9 | 1, 3, 6, 7, 8, 12 | The Swedish Mammography Cohort, Sweden | SMC | |
| Norat et al., [33] | 478040 | 1329 CRC, 351 PC, 391 DC, 474 RC | B | 35–70 | 4.8 | 1, 2, 3, 4, 5, 6, 7, 8, 14 | The European Prospective Investigation into Cancer and Nutrition, Europe | EPIC | |
| Giovannucci et al., [30] | 47949 | 251 CRC, 69 PC, 89 DC, 46 RC | M | 40–75 | 6 | 1, 3, 4, 5, 8, 9, 10, 11, 14 | The Health Professionals Follow-up Study, U.S. | HPFS | |
|
| |||||||||
| Processed meat | Cross et al., [35] | 300948 | 2719 CRC, 1150 PC, 787 DC, 724 RC | B | 50–71 | 7.2 | 2, 3, 5, 6, 7, 12 | NIH-AARP Diet and Health Study, U.S. | NIH-AARP |
| Sato et al., [34] | 41835 | 474 CRC, 142 PC, 100 DC, 198 RC* | B | 40–64 | 11 | 1, 2, 3, 4, 5, 6, 7, 8, 11, 12 | The Miyagi Cohort Study, Japan | MCS | |
| Chao et al., [31] | 148610 | 1667 CRC, 667 PC, 408 DC, 470 RC | B | 50–74 | 8-9 | 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 13 | The Cancer Prevention Study II Nutrition Cohort, U.S. | CPS-II | |
| Larsson et al., [32] | 61433 | 733 CRC, 234 PC, 155 DC, 230 RC | F | 40–75 | 13.9 | 1, 3, 6, 7, 8, 12 | The Swedish Mammography Cohort, Sweden | SMC | |
| Norat et al., [33] | 478040 | 1329 CRC, 351 PC, 391 DC, 474 RC | B | 35–70 | 4.8 | 1, 2, 3, 4, 5, 6, 7, 8, 14 |
The European Prospective Investigation into Cancer and Nutrition, Europe | EPIC | |
|
| |||||||||
| Fiber/ whole grain |
Murphy et al., [44] | 142250 M 335062 F |
4517 CRC, 1298 PC, 1266 DC, 1648 RC | B | 25–70 | 11 | 1, 2, 3, 4, 5, 6, 7, 8, 12, 13, 14 | The European Prospective Investigation into Cancer and Nutrition, Europe | EPIC |
| Egeberg et al., [43] | 26630 M | M: 244 CC, 89 PC, 140 DC, 169 RC | B | 50–64 | 10.6 | 1, 3, 4, 6, 8, 12, 13 | The Danish Diet, Cancer and Health Prospective Cohort study, Denmark | DDCHPC | |
| 29189 F | F: 217 CC, 84 PC, 118 DC, 114 RC | ||||||||
| Kabat et al., [42] | 158800 | 1476 CRC, 798 PC, 351 DC, 303 RC | F | 50–79 | 7.8 | 1, 3, 4, 5, 6, 7, 11, 12, 13, 14 | The Women's Health Initiative, U.S. | WHI | |
| Nomura et al., [40] | 85903 M | M: 1138 CRC, 382 PC, 327 DC, 276 RC | B | 45–75 | 7.3 | 1, 3, 4, 5, 6, 8, 9, 10, 11, 13, 14 | The Multiethnic Cohort Study, Hawaii and Los Angeles, U.S. | MEC | |
| 105108 F | F: 972 CRC, 356 PC, 234 DC, 179 RC | ||||||||
| Schatzkin et al., [41] | 489611 | 2974 CRC, 1139 PC, 914 DC, 858 RC* | B | 50–71 | 5 | 2, 3, 4, 5, 6, 7, 8, 11, 12, 13, 14 | NIH-AARP Diet and Health Study, U.S. | NIH-AARP | |
| Larsson et al., [39] | 61433 | 805 CRC, 249 PC, 170 DC, 252 RC | F | 40–76 | 14.8 | 1, 3, 6, 7, 12 | The Swedish Mammography Cohort, Sweden | SMC | |
| Fuchs et al., [38] | 88757 | 787 CRC, 281 PC, 255 DC, 143 RC* | F | 34–59 | 16 | 1, 3, 4, 5, 6, 7, 8, 9, 10, 11 |
The Nurses' Health Study, U.S. | NHS | |
| Steinmetz et al., [37] | 41837 | 212 CC, 86 PC, 120 DC* | F | 55–69 | 5 | 1, 7 | The Iowa Women's Health Study, U.S. | IWHS | |
|
| |||||||||
| Garlic | Steinmetz et al., [37] | 41837 | 212 CC, 86 PC, 120 DC* | F | 55–69 | 5 | 1, 7 | The Iowa Women's Health Study, U.S. | IWHS |
|
| |||||||||
| Milk | Larsson et al., [47] | 45306 | 449 CRC, 124 PC, 131 DC, 173 RC | M | 45–79 | 6.7 | 1, 3, 4, 5, 6, 7, 8, 10, 11, 12, 14 | The Cohort of Swedish Men, Sweden | COSM |
| Larsson et al., [46] | 60708 | 798 CRC, 246 PC, 170 DC, 249 RC | F | 40–76 | 14.8 | 1, 3, 6, 7, 12 | The Swedish Mammography Cohort, Sweden | SMC | |
| McCullough et al., [45] | 60866 | 421 CRC, 124 PC, 103 DC, 119 RC | M | 50–74 | 4-5 | 1, 3, 4, 5, 6, 7, 11, 12 | The Cancer Prevention Study II Nutrition Cohort, U.S. | CPS-II | |
|
| |||||||||
| Calcium | Ishihara et al., [51] | 35194 | 464 CRC, 129 PC, 183 DC, 146 RC* | M | 45–74 | 7.8 | 1, 3, 4, 5, 6, 7, 8, 14 | The Japan Public Health Center-based Prospective Study, Japan | JPHC |
| Larsson et al., [47] | 45306 | 449 CRC, 124 PC, 131 DC, 173 RC | M | 45–79 | 6.7 | 1, 3, 4, 5, 6, 7, 8, 10, 11, 12, 14 | The Cohort of Swedish Men, Sweden | COSM | |
| Flood et al., [50] | 45354 | 482 CRC, 172 PC, 112 DC, 74 RC | F | 61.9 (mean) | 8.5 | 1, 7 | The Breast Cancer Detection Demonstration Project, U.S. | BCDDP | |
| McCullough et al., [45] | 60866 | 421 CRC, 124 PC, 103 DC, 119 RC | M | 50–74 | 4-5 | 1, 3, 4, 5, 6, 7, 11, 12 | The Cancer Prevention Study II Nutrition Cohort, U.S. | CPS-II | |
| Terry et al., [48] | 61463 | 572 CRC, 164 PC, 121 DC, 191 RC | F | 39–75 | 11.3 | 1, 3, 6, 7, 8, 12 | The Swedish Mammography Cohort, Sweden | SMC | |
| Wu et al., [49] | 87998 F 47344 M |
1025 CC, 426 PC, 411 DC | B | 30–55 F 40–75 M |
16 10 |
1, 3, 4, 5, 6, 8, 10, 11, 13, 14 | The Nurses' Health Study, U.S. and The Health Professionals Follow-up Study, U.S. | NHS & HPFS | |
| Stemmermann et al., [28] | 8006 | 277 CRC, 43 PC, 33 TDC, 113 SC, 88 RC | M | 46–68 | 19–22 | 1 | Japan Hawaii Cancer Study, Japan | JHCS | |
|
| |||||||||
| Alcohol | Razzak et al., [29] | 41836 | 1255 CRC, 633 PC, 594 DCR | F | 55–69 | 12 | 1, 3, 4, 5, 6, 7, 13 | The Iowa Women's Health study, U.S. | IWHS |
| Shin et al., [36] | 869725 M | M: 3051 CRC, 536 PC, 751 DC, 1535 RC | B | 30–80 | 7 | 1 | Korean National Health System, Korea | KNHS | |
| 395501 F | F: 1093 CRC, 236 PC, 225 DC, 451 RC | ||||||||
| Park et al., [58] | 153000 | M: 241 CRC, 78 PC, 65 DC, 81 RC F: 217 CRC, 76 PC, 54 DC, 69 RC |
B | 26–84 | 1, 3, 4, 5, 6, 7, 12, 14 | UK Dietary Cohort Consortium, UK | UKDCC | ||
| Bongaerts et al., [57] | 4118** | M: 881 CRC, 240 PC, 293 DC, 232 RC F: 622 CRC, 254 PC, 189 DC, 108 RC |
B | 55–69 | 13.3 | 1, 2, 3, 4, 6, 7, 11 | The Netherlands Cohort study, The Netherlands | NLCS | |
| Akhter et al., [55] | 21199 | 307 CRC, 78 PC, 78 DC, 131 RC | M | 40–64 | 11 | 1, 3, 4, 5, 6, 11, 12 | The Miyagi Cohort study, Japan | MCS | |
| Ferrari et al., [56] | 478732 | 1833 CRC, 476 PC, 528 DC, 649 RC | B | 35–70 | 6.2 | 1, 2, 3, 4, 5, 7, 12 | The European Prospective Investigation into Cancer and Nutrition, Europe | EPIC | |
| Pedersen et al., [54] | 29132 | 613 CRC, 159 PC, 202 DC, 202 RC | B | 23–95 | 14.7 | 1, 2, 5, 14 | The Copenhagen Centre for Prospective Population Studies, Denmark | CCPPS | |
| Giovannucci et al., [53] | 47931 | 205 CC, 69 PC, 89 DC, 46 RC | M | 40–75 | 6 | 1, 3, 4, 5, 6, 7, 9, 10, 11, 14 | The Health Professionals Follow-up Study, U.S. | HPFS | |
| Klatsky et al., [27] | 106203 | 203 CC, 69 PC, 52 TDC, 77 SC, 66 RC | B | 7 | 1, 2, 5, 6, 12, 14 | Northern California Kaiser Permanente Study, U.S. | NCKPS | ||
| Wu et al., [52] | 11644 | M: 58 CRC F: 68 CRC |
B | 4.5 | 1 | Retirement Community of Los Angeles, U.S. | RCLA | ||
M: male, F: female, B: both sexes, CRC: colorectal cancer, PC: proximal colon cancer, DC: distal colon cancer, RC: rectal cancer, DCR: distal colorectal cancer, TDC: transverse and descending colon cancer, SC: sigmoid colon cancer. *The numbers come, all or partly, from tables, and may thus not include all cases in the cohort. **Subcohort. ***The risk estimates are adjusted for: 1: age, 2: sex, 3: BMI, 4: physical activity, 5: smoking, 6: dietary items, 7: energy intake, 8: alcohol intake, 9: history of polyps, 10: NSAIDS/aspirin, 11: family history of CRC, 12: education, 13: hormone replacement therapy or oral contraceptive use, 14: others. $The numbers vary according to the exposure analyzed.
The number adds up to more than 32 as several papers give data for more than one risk factor. If several papers on the same risk factor were published for a given cohort, all data was retrieved from the most recent paper. The relative risk (RR), hazard rate ratio (HRR), hazard ratio (HR), incidence rate ratio (IRR) or odds ratio (OR), and corresponding 95% confidence intervals (95% CI) for each risk factor are presented (as RR) in Figures 2–8, sorted in ascending order of magnitude by sex. For one study the risk estimates were tabulated according to the lowest versus highest exposure category in the original paper [28] and are presented as the inverse of the value in the corresponding figure (Figure 7).
Figure 2.
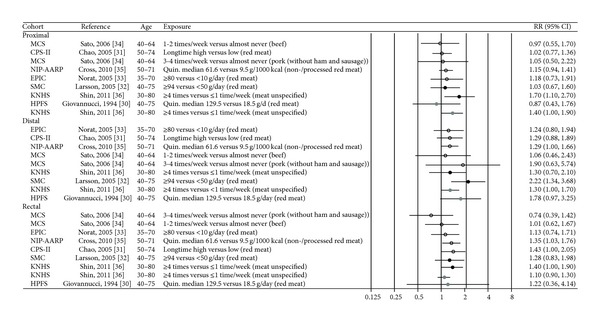
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of red meat. (The results are stratified on sex. Open circles: both gender combined. Closed black circles: females. Closed grey circles: men. All estimates are sorted from the lowest to the highest by subsite and sex.)
Figure 8.
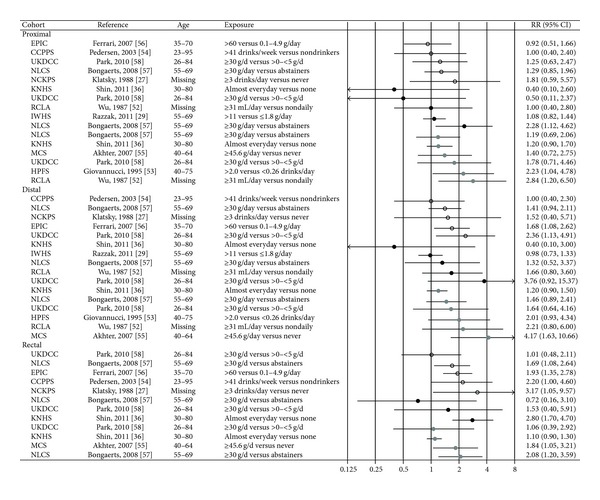
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of alcohol. (The results are stratified on sex. Open circles: both gender combined. Closed black circles: females. Closed grey circles: men. All estimates are sorted from the lowest to the highest by subsite and sex.)
Figure 7.
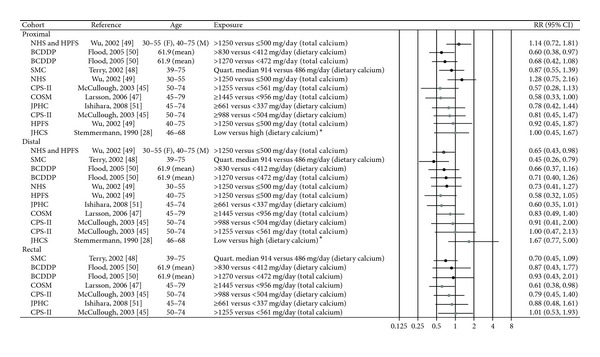
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of calcium. (The results are stratified on sex. Open circles: both gender combined. Closed black circles: females. Closed grey circles: men. All estimates are sorted from the lowest to the highest by subsite and sex, except Stemmermann et al. [28].)
Meta-analyses were not performed as a consequence of the heterogeneity in factors such as exposure measurement, the categorization of risk factor levels, and the confounders adjusted for in the studies [59].
3. Results
Overall, data from 21 cohorts with information on one or more of the risk factors were included in this review. Table 2 gives a detailed description of the studies, with information on cohort size, number of cases, sex, age distribution, follow-up time, and factors adjusted for in the risk analyses. The European Prospective Investigation into Cancer and Nutrition (EPIC) study consists of subcohorts from 10 European countries. The other cohorts were from the USA (10 cohorts), Sweden (2), Denmark (2), Japan (3), Korea (1), UK (1), and the Netherlands (1). The subsite-specific results (Figures 2–8) are described according to the individual risk factors, as presented in the following.
3.1. Red Meat
Seven cohort studies were included in the review, of which five reported on red meat [30–33, 35], one on beef and pork [34], and one on meat (not further specified) [36]. The five studies on red meat reported an increased risk of colorectal cancer with increasing intake [30–33, 35] (Table 2), although most of the risk estimates were not statistically different from unity. In Figure 2, there appears to be somewhat more consistency with the observation of an increased risk of cancers of the rectum and distal colon than there is for proximal colon cancer. This picture remains when restricting the evaluation to studies with adequate statistical power. In a Swedish study, women consuming 94 or more grams of red meat per day had an increased risk of distal colon cancer of 2.22 (95% CI 1.34–3.68) compared to women consuming less than 50 grams/day, and there was a significant trend of increasing risk with increasing consumption (P trend < 0.001) [32]. A long-term high intake of red meat yielded an increased risk of rectal cancer of 43% (95% CI 1.00–2.05) relative to low intake in a US study [31], whereas in another US study there was a significant trend of increased risk of both proximal colon, distal colon and rectal cancer with increasing consumption of red meat (including both processed and nonprocessed red meat) (for all P trend = 0.02) [35].
Neither beef consumption nor pork consumption was significantly associated with risk of cancer of any colorectal subsite in a Japanese study [34], while the frequency of meat consumption (not further specified) was positively associated with proximal and distal colon cancer in South Korean men, and with proximal colon and rectal cancer in South Korean women [36].
3.2. Processed Meat
Four studies have reported on processed meat [31–33, 35] and one has reported on ham and sausages [34] (Table 2). In the EPIC study, consumption of 80 grams or more of processed meat per day conferred a 62% (95% CI 1.04–2.50) increased risk of rectal cancer compared to an intake of less than 10 grams per day [33], and in a US study a high long-term intake of processed meat was associated with a 50% (95% CI 1.04–2.17) increased risk of distal colon cancer [31] (Figure 3). In a more recent American study, consuming 22.3 grams or more of processed meat per 1000 kcal increased the risk of rectal cancer with 30% (95% CI 1.00–1.68) compared to consuming 1.6 gram or less per 1000 kcal [35]. No significant associations were seen in Swedish [32] and Japanese studies [34].
Figure 3.
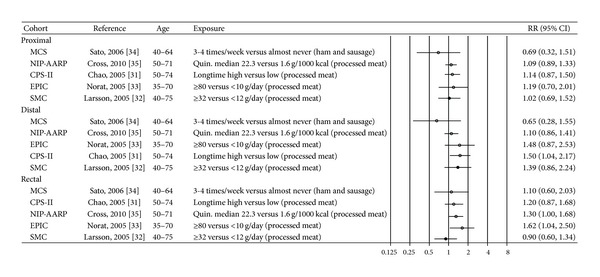
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of processed meat. (The results are stratified on sex. Open circles: both gender combined. Closed black circles: females. All estimates are sorted from the lowest to the highest by subsite and sex.)
3.3. Fiber
Eight cohort studies were included in the review; six have provided estimates for the risk of the colonic subsites in relation to fiber consumption [37, 40–42, 44] and two have provided estimates for the whole grain consumption [39, 43] (Table 2). As for fiber, most of the risk estimates were not statistically different from unity [37, 41, 42] (Figure 4). The EPIC study reported an inverse association between fiber intake and colorectal cancer with no strong evidence of different associations across the subsites: when fiber intake was analyzed as a categorical variable, a significant inverse association was seen for distal colon cancer only (P trend = 0.02), whereas when fiber intake was analyzed as a continuous variable and corrected for measurement errors, significant inverse associations were seen for proximal colon (HR per 10 gram/day increase 0.83, 95% CI 0.75–0.92) and rectum cancer (HR per 10 gram day/increase 0.87, 95% CI 0.79–0.96), but not for distal colon cancer [44]. The Multiethnic Cohort observed a reduced risk for distal colon and rectal cancer among those in the highest compared to the lowest fiber intake group, but the reduction was only significant in men (RR 0.56, 95% CI 0.35–0.90 and RR 0.52, 95% CI 0.32–0.84, resp.) [40].
Figure 4.
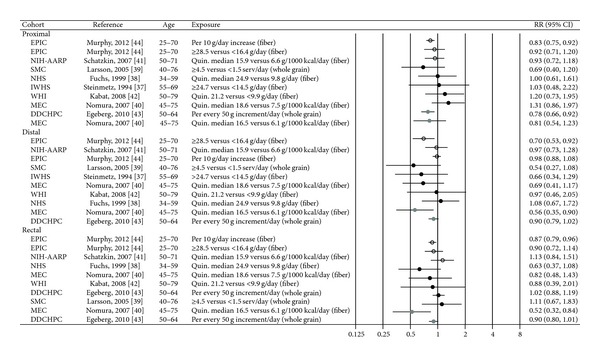
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of fiber and whole grain. (The results are stratified on sex. Open circles: both gender combined. Closed black circles: females. Closed grey circles: men. All estimates are sorted from the lowest to the highest by subsite and sex.)
Two Scandinavian papers have reported on associations between whole grain consumption and cancer risk of colorectal subsites [39, 43]. In a Danish study, which is also included in the EPIC study, total consumption of whole grain products was associated with a significantly lower risk of proximal colon cancer and a borderline significantly lower risk of distal colon cancer and rectal cancer in men (IRR per each increment in intake of 50 gram per day 0.78, 95% CI 0.66–0.92 for proximal colon, 0.90, 95% CI 0.79–1.02 for distal colon, and 0.90, 95% CI 0.80–1.01 for rectum) but not in women (data not given) [43]. No significant associations between whole grain consumption and cancer of any of the colorectal subsites were seen in the Swedish Mammography Cohort [39] (Figure 4).
The width of the confidence intervals for the risk estimates on fiber and whole grain varies markedly between the included studies. However, whether all studies are included or those with the lowest power are excluded, there are rather minimal differences in relative risk across the subsites.
3.4. Garlic
Only one study on garlic is included in the review. The Iowa Women's Health Study reported that having at least one serving per week of garlic was associated with a 48% reduced risk of distal colon cancer compared to zero servings of garlic (RR 0.52, 95% CI 0.30–0.93) [37] (Table 2, Figure 5).
Figure 5.
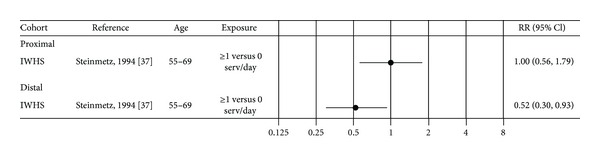
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of garlic. (Closed black circles: females.)
3.5. Milk
Three cohort studies were included in the review [45–47]. Two cohort studies have examined the relation between total consumption of milk and the risk of cancer in colorectal subsites [45, 47] (Table 2, Figure 6). No significant associations were seen for any of the colorectal subsites in a US study that combined both sexes (estimates not given for women) [45]. In a study of Swedish men, a significantly reduced risk for distal colon cancer was seen for those consuming 1.5 glasses or more of milk per day compared to those consuming less than two glasses per week (RR 0.53, 95% CI 0.33–0.87) [47].
Figure 6.
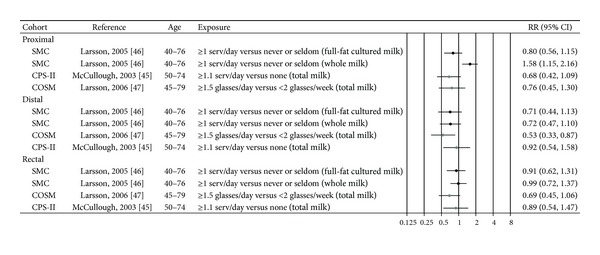
Estimates of relative risk with 95% CI for the highest versus the lowest exposure categories of milk. (The results are stratified on sex. Closed black circles: females. Closed grey circles: men. All estimates are sorted from the the lowest to the highest by subsite and sex.)
In another Swedish study restricted to high fat dairy food and conducted among women only, a significant inverse trend was observed between consumption of full-fat cultured milk and risk of distal colon cancer (P trend = 0.03), whereas a significant increased risk of proximal colon cancer was observed among women who consumed whole milk (1 or more servings per day compared to never/seldom consumers RR 1.58, 95% CI 1.15–2.16) [46].
3.6. Calcium
Seven papers from eight cohorts were included in the review [28, 45, 47–51] (Table 2, Figure 7). In the analyses from the Nurses' Health Study and the Health Professionals Follow-Up Study that studied colon cancer only, non-significant inverse associations were seen between total calcium and distal colon cancer for both men and women, and a pooled analysis of the two cohorts gave a significant inverse trend for distal colon cancer (P trend = 0.01) [49]. Likewise, a significant inverse trend was reported between total calcium and distal colon cancer in the Swedish Mammography Cohort (P trend = 0.02) [48], while in a US female cohort, a significant inverse trend was seen between dietary calcium intake and proximal colon cancer (P trend = 0.01) [50]. No association was seen for total calcium. In a US study examining both sexes, total calcium intake was significantly inversely associated with proximal colon cancer among men (P trend = 0.04), but not dietary calcium, and there were no significant associations seen for women (estimates not given) [45]. In a study of Swedish men, a significant inverse relation was found between total calcium and rectal cancer (P trend = 0.02), whereas nonsignificant inverse associations were seen for cancer of the colon [47]. No significant associations between dietary calcium and cancer of any colorectal subsite were reported in a Japanese study either for men or for women (estimates not given for women) [51]. An earlier study of Hawaiian-Japanese men reported a significant inverse association between dietary calcium and sigmoid colon cancer (P trend = 0.02) [28]. In addition to the eight cohorts included in this paper, an additional cohort study among American women states that their data provided little support for a protective effect of total calcium at either tumor subsite, but does not present risk estimates [60].
3.7. Alcohol
Ten articles [27, 29, 36, 52–58] were included in the review (Table 2). Three analyses for both sexes combined consistently showed a higher risk of rectal cancer with increasing alcohol consumption and no significant associations for any of the colon subsites [27, 54, 57]. In the EPIC study [56] an increased risk was reported both for rectal and distal colon cancer, whereas in the UK dietary cohort consortium (part of which is included in the EPIC study) [58] a significantly increased risk was found for distal colon cancer only (Figure 8). Several sex-specific analyses have been done. Among men, two older studies with limited statistical power reported that alcohol consumption was positively related to proximal colon cancer only [52, 53], whereas four more recent studies reported non-significant associations with proximal colon cancer [36, 55, 57, 58]. A study among Japanese men reported increased risk for distal colon cancer, but the confidence interval for the risk estimate was very wide (consumption of 45.6 grams alcohol or more per day compared to never drinkers HR 4.17, 95% CI 1.63–10.66) [55]. In terms of consumption of alcohol and rectum cancer among men, two of four studies have reported a positive association [55, 57].
For women, fewer significant associations have been reported. In the Iowa Women's Health Study, alcohol was not significantly associated with either proximal colon or distal colorectal cancer, nor did further separation into distal colon and rectal cancer reveal any significant associations (estimates not given) [29]. Furthermore, no significant associations were seen in an earlier study of American women [52] or in the UK dietary cohort consortium [58]. In The Netherlands Cohort Study, however, women consuming 30 grams or more of alcohol per day had HRR of proximal colon cancer of 2.28 (95% CI 1.12–4.62) compared to abstainers, whereas analyses for the other subsites were not sufficiently robust to draw any conclusions [57]. In a Korean study, women who frequently consumed alcohol or who consumed greater amounts of alcohol had a higher risk of rectal cancer [36].
4. Discussion
This review provides an overall and updated synthesis of the results from cohort studies examining the association between dietary factors that are convincingly or probably related to the risk of colorectal cancer and subsite-specific colorectal cancer. Our study indicates that consumption of alcohol is more strongly related to the risk of rectal cancer than to colon cancer, also that meat consumption tends to be somewhat more strongly related to the risk of distal colon cancer and rectal cancer than proximal colon cancer, that there are only minor differences in relative risk for colorectal cancer across the major subsites for fiber, milk, and calcium, and that no statement can be given for garlic due to limited data. It should be noted that for all exposures the majority of the analyses showed nonsignificant associations with cancer risk, the exception being the positive association between alcohol consumption and rectal cancer.
The pathway of colorectal cancer is complex. The subsite etiology is rather poorly understood, and the mechanism for the various dietary factors is likely to differ. Even for red and processed meat, both established risk factors for colorectal cancer, the underlying mechanisms are not well defined. One suggested mechanism for the somewhat stronger association for rectum and distal colon relative to proximal colon cancer relates to the enhanced endogenous formation of carcinogenic N-nitroso compounds with a high intake of meat [61]. The level of markers of N-nitroso compounds appears to be higher in tissue from distal colon and rectum than in that of the proximal colon [62]. This finding is in keeping with a previously meta-analyses which implicated processed meat consumption as a stronger risk factor for cancer occurrence at the distal colon relative to the proximal colon [22].
A suggested mechanism by which dietary fiber may decrease the risk of colorectal cancer is linked to the fermentation of fiber. The fermentation produces short-chain fatty acids and, in particular, acetic, propionic, and butyric acids. Butyrate is particularly of interest, as it has been shown to induce apoptosis and to be cytotoxic to both colorectal adenoma and carcinoma cells [63]. Studies on mice have shown that the concentration of butyrate is highest in the distal colon [64]. In humans, fiber is fermented in the proximal colon, and the total amount of short-chain fatty acids has been estimated to be considerably higher in the proximal site compared to the distal [65]. One study on proximal and distal colonocytes indicates, however, that butyrate is a more important source of energy for the distal, than for the proximal, colonic mucosa [66]. If so, this could be a relevant biological mechanism explaining any differences in risk between the sites. In addition, fiber may dilute the concentration of carcinogenic substances in the distal colon. Despite these findings, a pooled analysis on fiber reported no convincing differences in colorectal risk for the various anatomical subsites [25], in line with our own results published here.
The reduced risk of colorectal cancer with increasing consumption of milk is likely to be at least partly mediated by calcium, which is thought to have a protective effect through its ability to bind bile acids, and its growth-restraining and differentiation- and apoptosis-inducing effect on colorectal cells [67]. No convincing site-specific associations regarding milk and calcium were seen in our review. A former pooled analysis reported an inverse association for milk limited to cancer of the distal colon and rectum [24], while the results from two meta-analysis on site-specific impact of calcium have been conflicting [20, 21].
The consumption of alcohol is associated with increasing risk of cancer in several organs in the digestive tract, including the colorectum [18]. Alcohol is not a carcinogen itself, but acts as a tumor promoter and possibly as a co-carcinogen. Alcohol also acts as a solvent and thus might increase the exposure to other carcinogens by enhancing the penetration of carcinogens into the cell [18]. Acetaldehyde is a metabolite of alcohol and may be the most important agent responsible for the carcinogenic effect as it is highly toxic, mutagenic, and carcinogenic [68]. A pooled analyses on alcohol reported similar risk across all areas of the large bowel [26]. However, a stronger association with alcohol for rectal cancer compared with colon cancer as seen in our paper could possibly be related to a higher degree of epithelial hyperregeneration in rectum [69]. The number of sex-specific analysis is presently too low to suggest any significant interaction by sex at the subsite level.
There are some methodological issues in this study which may have impacted on the findings. There is considerable variability in several key characteristics of the assembled cohort studies: the follow-up time across studies included in this paper varied from 4 to 22 years, while the total number of colorectal cancer cases analyzed ranged from 126 to 2974. Short-term follow-up studies tend to accrue a lower number of cases, and the resulting estimates are subject to greater uncertainty. The substratification of cases by sex, tumor location, and exposure categories, as presented here, inevitably leads to smaller numbers and a greater degree of imprecision in the estimates, even for relatively large studies. Statistically significant associations may thus be more spurious at the subsite-specific level. However, restricting the evaluation to studies with adequate statistical power did not change the overall picture. Long-term follow-up studies will commonly accrue a greater number of cases, but are more prone to measurement error given that an increasing follow-up time raises the possibility that exposure status of the participants will change, leading to misclassification of exposure and under- or overestimation of the risk estimates. However, the natural history of colorectal cancer is on average of long duration, and exposure in the more distant past may be the most relevant when estimating subsequent risk.
Another issue is the difference in risk factor dosages between studies and that the categories compared sometimes vary considerably between studies. For instance, for calcium, in the Swedish study by Larsson et al. [47] daily intake of 1445 mg or more is compared to an intake of less than 956 mg/day, whereas in the Japanese study by Ishihara et al. [51] daily intake of 661 mg or more is compared with an intake of less than 337 mg/day. Careful reading of the exposure categories (given in the figures) is therefore necessary when evaluating the findings. In addition, the specific confounding factors adjusted for at the analysis stage differ between studies.
Of the studies that were full-text reviewed, 76 were excluded as they did not reveal information at the level of subsite location. Given the high proportion of cohort studies failing to report subsite-specific estimates, the potential publication bias prohibited a formal meta-analysis [59]. A further rationale for this decision is the lack of uniformity in exposure categories within each risk factor.
In summary, the strength of the association between dietary components and cancer of the large bowel may partially depend on the anatomic location within the colorectum. The most consistent finding is the stronger association between alcohol and rectal cancer, compared with alcohol and proximal and distal colon cancer. Meat (red and processed) is possibly more strongly associated with risk of distal colon cancer and rectal cancer than the risk of proximal colon cancer. For fiber, milk, and calcium there seem to be only minor differences in relative risk across the subsites. However, caution is required as the number of papers presenting risk estimates by colorectal subsite is limited, particularly for milk and garlic. Also, most of the subsite-specific analyses report non-significant findings.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
The authors thank Lena Leder for her contribution to the data extraction and paper preparation.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; 2012. (IARC Cancer Base, no. 10). [Google Scholar]
- 2.Kee F, Wilson RH, Gilliland R, Sloan JM, Rowlands BJ, Moorehead RJ. Changing site distribution of colorectal cancer. British Medical Journal. 1992;305(6846):p. 158. doi: 10.1136/bmj.305.6846.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slater GI, Haber RH, Aufses AH., Jr. Changing distribution of carcinoma of the colon and rectum. Surgery, Gynecology & Obstetrics. 1984;158:216–218. [PubMed] [Google Scholar]
- 4.Toyoda Y, Nakayama T, Ito Y, Ioka A, Tsukuma H. Trends in colorectal cancer incidence by subsite in Osaka, Japan. Japanese Journal of Clinical Oncology. 2009;39(3):189–191. doi: 10.1093/jjco/hyn144. [DOI] [PubMed] [Google Scholar]
- 5.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Diseases of the Colon and Rectum. 2002;45(8):1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 6.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. Journal of Clinical Gastroenterology. 2007;41(2):173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 7.Beart RW, Melton LJ, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Diseases of the Colon & Rectum. 1983;26:393–398. doi: 10.1007/BF02553382. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, Randimbison L, La Vecchia C. Trends in subsite distribution of colorectal cancers and polyps from the Vaud Cancer Registry. Cancer. 1993;72(1):46–50. doi: 10.1002/1097-0142(19930701)72:1<46::aid-cncr2820720111>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Mitry E, Benhamiche AM, Couillault C, et al. Effect of age, period of diagnosis and birth cohort on large bowel cancer incidence in a well-defined French population, 1976–1995. European Journal of Cancer Prevention. 2002;11(6):529–534. doi: 10.1097/00008469-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. American Journal of Gastroenterology. 2008;103(5):1249–1256. doi: 10.1111/j.1572-0241.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 11.Takada H, Ohsawa T, Iwamoto S, et al. Changing site distribution of colorectal cancer in Japan. Diseases of the Colon & Rectum. 2002;45:1249–1254. doi: 10.1007/s10350-004-6400-0. [DOI] [PubMed] [Google Scholar]
- 12.Thörn M, Bergström R, Kressner U, Sparén P, Zack M, Ekbom A. Trends in colorectal cancer incidence in Sweden 1959–93 by gender, localization, time period, and birth cohort. Cancer Causes and Control. 1998;9(2):145–152. doi: 10.1023/a:1008826109697. [DOI] [PubMed] [Google Scholar]
- 13.Jass JR. Subsite distribution and incidence of colorectal cancer in New Zealand, 1974–1983. Diseases of the Colon & Rectum. 1991;34:56–59. doi: 10.1007/BF02050208. [DOI] [PubMed] [Google Scholar]
- 14.Scheiden R, Pescatore P, Wagener Y, Kieffer N, Capesius C. Colon cancer in Luxembourg: a national population-based data report, 1988–1998. BMC Cancer. 2005;5, article 52 doi: 10.1186/1471-2407-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962–2006: an interpretation of the temporal patterns by anatomic subsite. International Journal of Cancer. 2010;126(3):721–732. doi: 10.1002/ijc.24839. [DOI] [PubMed] [Google Scholar]
- 16.Li FY, Lai MD. Colorectal cancer, one entity or three. Journals of Zhejiang University-Science B. 2009;10:219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slattery ML, Curtin K, Wolff RK, et al. A comparison of colon and rectal somatic DNA alterations. Diseases of the Colon & Rectum. 2009;52(7):1304–1311. doi: 10.1007/DCR.0b013e3181a0e5df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Cancer Research Found and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC, USA: 2007. [Google Scholar]
- 19.World Cancer Research Found and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. Continuous Update Project, Colorectal Cancer Report 2010, Summary. Washington, DC, USA: 2011. [Google Scholar]
- 20.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutrition and Cancer. 2009;61(1):47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 21.Bergsma-Kadijk JA, van’t Veer P, Kampman E, Burema J. Calcium does not protect against colorectal neoplasia. Epidemiology. 1996;7(6):590–597. doi: 10.1097/00001648-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. International Journal of Cancer. 2006;119(11):2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 23.Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS ONE. 2011;6(6, article e20456) doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. Journal of the National Cancer Institute. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. The Journal of the American Medical Association. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- 26.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Annals of Internal Medicine. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 27.Klatsky AL, Armstrong MA, Friedman GD, Hiatt RA. The relations of alcoholic beverage use to colon and rectal cancer. American Journal of Epidemiology. 1988;128(5):1007–1015. doi: 10.1093/oxfordjournals.aje.a115045. [DOI] [PubMed] [Google Scholar]
- 28.Stemmermann GN, Nomura A, Chyou PH. The influence of dairy and nondairy calcium on subsite large-bowel cancer risk. Diseases of the Colon and Rectum. 1990;33(3):190–194. doi: 10.1007/BF02134177. [DOI] [PubMed] [Google Scholar]
- 29.Razzak AA, Oxentenko AS, Vierkant RA, et al. Alcohol intake and colorectal cancer risk by molecularly defined subtypes in a prospective study of older women. Cancer Prevention Research. 2011;4:2035–2043. doi: 10.1158/1940-6207.CAPR-11-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Research. 1994;54(9):2390–2397. [PubMed] [Google Scholar]
- 31.Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. The Journal of the American Medical Association. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 32.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. International Journal of Cancer. 2005;113(5):829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 33.Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. Journal of the National Cancer Institute. 2005;97(12):906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato Y, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, Tsuji I. Meat consumption and risk of colorectal cancer in Japan: the Miyagi cohort study. European Journal of Cancer Prevention. 2006;15(3):211–218. doi: 10.1097/01.cej.0000197455.87356.05. [DOI] [PubMed] [Google Scholar]
- 35.Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Research. 2010;70(6):2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin A, Joo J, Bak J, et al. Site-specific risk factors for colorectal cancer in a Korean population. PLoS One. 2011;6, article e23196 doi: 10.1371/journal.pone.0023196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa women’s health study. American Journal of Epidemiology. 1994;139(1):1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs CS, Giovannucci EL, Colditz GA, et al. Dietary fiber and the risk of colorectal cancer and adenoma in women. New England Journal of Medicine. 1999;340(3):169–176. doi: 10.1056/NEJM199901213400301. [DOI] [PubMed] [Google Scholar]
- 39.Larsson SC, Giovannucci E, Bergkvist L, Wolk A. Whole grain consumption and risk of colorectal cancer: a population-based cohort of 60 000 women. British Journal of Cancer. 2005;92(9):1803–1807. doi: 10.1038/sj.bjc.6602543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura AM, Hankin JH, Henderson BE, et al. Dietary fiber and colorectal cancer risk: the multiethnic cohort study. Cancer Causes and Control. 2007;18(7):753–764. doi: 10.1007/s10552-007-9018-4. [DOI] [PubMed] [Google Scholar]
- 41.Schatzkin A, Mouw T, Park Y, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. American Journal of Clinical Nutrition. 2007;85(5):1353–1360. doi: 10.1093/ajcn/85.5.1353. [DOI] [PubMed] [Google Scholar]
- 42.Kabat GC, Shikany JM, Beresford SA, et al. Dietary carbohydrate, glycemic index, and glycemic load in relation to colorectal cancer risk in the Women’s Health Initiative. Cancer Causes and Control. 2008;19(10):1291–1298. doi: 10.1007/s10552-008-9200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egeberg R, Olsen A, Loft S, et al. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. British Journal of Cancer. 2010;103(5):730–734. doi: 10.1038/sj.bjc.6605806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy N, Norat T, Ferrari P, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC) PLoS One. 2012;7, article e39361 doi: 10.1371/journal.pone.0039361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCullough ML, Robertson AS, Rodriguez C, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States) Cancer Causes and Control. 2003;14(1):1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- 46.Larsson SC, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. American Journal of Clinical Nutrition. 2005;82(4):894–900. doi: 10.1093/ajcn/82.4.894. [DOI] [PubMed] [Google Scholar]
- 47.Larsson SC, Bergkvist L, Rutegård J, Giovannucci E, Wolk A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. American Journal of Clinical Nutrition. 2006;83(3):667–673. doi: 10.1093/ajcn.83.3.667. [DOI] [PubMed] [Google Scholar]
- 48.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutrition and Cancer. 2002;43(1):39–46. doi: 10.1207/S15327914NC431_4. [DOI] [PubMed] [Google Scholar]
- 49.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. Journal of the National Cancer Institute. 2002;94(6):437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 50.Flood A, Peters U, Chatterjee N, Lacey JV, Jr., Schairer C, Schatzkin A. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiology Biomarkers and Prevention. 2005;14(1):126–132. [PubMed] [Google Scholar]
- 51.Ishihara J, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Dietary calcium, vitamin D, and the risk of colorectal cancer. American Journal of Clinical Nutrition. 2008;88(6):1576–1583. doi: 10.3945/ajcn.2008.26195. [DOI] [PubMed] [Google Scholar]
- 52.Wu AH, Paganini-Hill A, Ross RK, Henderson BE. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. British Journal of Cancer. 1987;55(6):687–694. doi: 10.1038/bjc.1987.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine—low-folate diets, and risk of colon cancer in men. Journal of the National Cancer Institute. 1995;87(4):265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen A, Johansen C, Grønbæk M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut. 2003;52(6):861–867. doi: 10.1136/gut.52.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akhter M, Kuriyama S, Nakaya N, et al. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi Cohort Study. European Journal of Cancer. 2007;43(2):383–390. doi: 10.1016/j.ejca.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Ferrari P, Jenab M, Norat T, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC) International Journal of Cancer. 2007;121(9):2065–2072. doi: 10.1002/ijc.22966. [DOI] [PubMed] [Google Scholar]
- 57.Bongaerts BWC, van den Brandt PA, Goldbohm RA, de Goeij AFPM, Weijenberg MP. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. International Journal of Cancer. 2008;123(10):2411–2417. doi: 10.1002/ijc.23774. [DOI] [PubMed] [Google Scholar]
- 58.Park JY, Dahm CC, Keogh RH, et al. Alcohol intake and risk of colorectal cancer: results from the UK Dietary Cohort Consortium. British Journal of Cancer. 2010;103(5):747–756. doi: 10.1038/sj.bjc.6605802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egger M, Smith GD, Sterne JA. Uses and abuses of meta-analysis. Clinical Medicine. 2001;1:478–484. doi: 10.7861/clinmedicine.1-6-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin J, Zhang SM, Cook NR, Manson JE, Lee IM, Buring JE. Intakes of calcium and vitamin D and risk of colorectal cancer in women. American Journal of Epidemiology. 2005;161(8):755–764. doi: 10.1093/aje/kwi101. [DOI] [PubMed] [Google Scholar]
- 61.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. Journal of Nutrition. 2002;132(11):3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 62.Povey AC, Hall CN, Badawi AF, Cooper DP, O’Connor PJ. Elevated levels of the pro-carcinogenic adduct, O(6)-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut. 2000;47(3):362–365. doi: 10.1136/gut.47.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hague A, Manning AM, Hanlon KA, Huschtscha LI, Hart D, Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. International Journal of Cancer. 1993;55(3):498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 64.Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Current Opinion in Clinical Nutrition and Metabolic Care. 2004;7(5):563–567. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Wong JM, de Souza R, Kendall CWC, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. Journal of Clinical Gastroenterology. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nature Reviews Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 68.Pöschl G, Seitz HK. Alcohol and cancer. Alcohol and Alcoholism. 2004;39(3):155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 69.Simanowski UA, Stickel F, Maier H, Gärtner U, Seitz HK. Effect of alcohol on gastrointestinal cell regeneration as a possible mechanism in alcohol-associated carcinogenesis. Alcohol. 1995;12(2):111–115. doi: 10.1016/0741-8329(94)00091-3. [DOI] [PubMed] [Google Scholar]


