Abstract
The “nonclassic” role of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) has been recently widely recognized. In type 1 diabetes mellitus (T1D), it plays an immunomodulatory role through the vitamin D receptor (VDR) present on pancreatic and immune cells. Specific VDR allelic variants have been associated with T1D in many countries. Furthermore, vitamin D deficiency has been prevalent in T1D, and the seasonal and latitude variability in the incidence of T1D can be partly explained by the related variability in vitamin D level. In fact, retrospective studies of vitamin D supplementation during pregnancy or infancy showed a lower incidence of T1D. We will review the different mechanisms of the vitamin D protective effect against insulitis and present the available data on the role of vitamin D deficiency in the control, progression, and complications of T1D.
1. Introduction
Type 1 diabetes (T1DM) is an autoimmune disease occurring in the pancreatic islets [1]. It accounts for 90% of diabetes in children and adolescents [2]. Its incidence varies considerably worldwide, being highest in Finland and Sardinia [3], probably related to genetic, dietary, and environmental factors that might interfere with its pathogenesis [4]. The annual incidence has been increasing worldwide, possibly related to higher socioeconomic status and degree of urbanization [5]. Recently, there has been appealing evidence on the “nonclassic” role of vitamin D in many autoimmune diseases including rheumatoid arthritis, scleroderma, psoriasis, multiple sclerosis, and also T1DM [6, 7]. In fact, in addition to its skeletal effects and control of calcium hemostasis, 1,25-DihydroxyvitaminD3 (1,25(OH)2D3) showed potent antiproliferative and immunomodulatory properties [8].
In this paper, we will review the available data on the relationship between vitamin D and T1DM trying to elucidate the immunomodulatory mechanisms of vitamin D on pancreatic insulitis, seasonal and latitude effects, protective effects of supplements on T1DM incidence, complications and progression.
2. Immunomodulatory Effect of Vitamin D
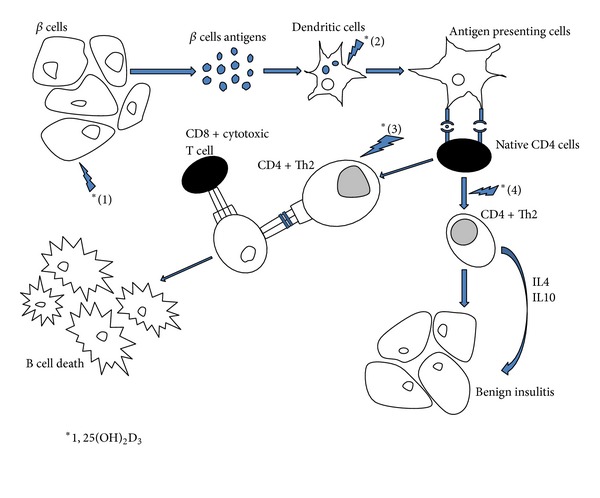
1,25(OH)2D3 plays an immunomodulatory role in the prevention of T1DM, through the vitamin D receptor (VDR) expressed in antigen presenting cells, activated T cells [9], and pancreatic islet β-cells [10]; this has been demonstrated in many trials done on nonobese diabetic mice (NOD)—a murine model of human IDDM, spontaneously developing diabetes mellitus (DM)—using 1,25(OH)2D3 or its analogue (1,25(OH)2D3, MC1288 (20-epi-1,25(OH)2D3), or KH1060 (1,25(OH)2-20-epi-22-oxa-24,26,27,-trishomovitamin D) [9]. Conversely, 1,25(OH)2D3-deficient mice were at higher risk of developing DM, with a more aggressive course when deficiency is present early in life [11, 12]. 1,25(OH)2D3, administered early on, protects against or reduces the severity of pancreatic insulitis via a dual action, on the pancreatic beta cells and on the immune cells [13]. Furthermore, administration of 1,25(OH)2D3 in combination with cyclosporine A, after the onset of the autoimmune attack, which is known as a prediabetic state, can prevent clinical diabetes [14].
At the level of the pancreatic islets, 1,25(OH)2D3 decreased in vivo and in vitro proinflammatory chemokine and cytokine expression (e.g., IL6), which are implicated in the pathogenesis of T1DM making β-cells less chemoattractive and less prone to inflammation; this results in decreased T cell recruitment and infiltration, increased regulatory cells, and arrest of the autoimmune process [15–17]. Furthermore, 1,25(OH)2D3 decreases MHC class I expression leading to reduced vulnerability of islet β-cells to cytotoxic T lymphocytes [18].
At the level of the immune system, 1,25(OH)2D3 inhibits the differentiation and maturation of dendritic cells and promotes their apoptosis [19], preventing their transformation into antigen presenting cells which is the first step in the initiation of an immune response [20]. It has been also demonstrated that 1,25(OH)2D3 restores the suppressor cells, decreases Th1 cytokine production—responsible for β-cell death—and shifts the immune response toward Th2 pathway, leading to benign insulitis [21–24]. The addition of 1,25(OH)2D3 inhibits the production of Il-6, a direct stimulator of Th17 cells [19], implicated in many autoimmune diseases, including T1D [20]. On the other hand, 1,25(OH)2D3 exerts antiapoptotic effects on the cytokine-induced pancreatic β-cells apoptosis. It induces and maintains high levels of A20 gene protein, which leads to decreased nitric oxide (NO) levels. In fact, NO induces directly beta cell dysfunction and death, and, indirectly, through the induction of Fas expression [25]; Fas is a transmembrane cell surface receptor and a member of the tumor necrosis factor (TNF) receptor family. It is stimulated by inflammatory cytokines secreted by islet-infiltrating mononuclear cells. It renders the β-cells in T1DM susceptible to Fas-Ligand-induced apoptosis mediated by tissue-infiltrating Fas-Ligand-positive T lymphocytes [26]. Decreasing NO levels leads to down regulation of all the aforementioned mechanisms and allows cytoprotective effects on islet cells. In addition, 1,25(OH)2D3 has been found to be able to counteract the cytokine-induced Fas expression in human pancreatic islets, both at the mRNA and protein levels, modulating the cascade of death signals and preventing cell apoptosis [27] (Figure 1).
Figure 1.
3. Vitamin D Polymorphism
Vitamin D and its analogues exert their actions through the nuclear VDR which is responsible for transducing the action of the active form of vitamin D, 1,25(OH)2D3 [28]. The VDR gene is located on chromosome 12q12-q14 in humans [29]. Polymorphisms within the VDR gene may be associated with altered gene expression or gene function [29], and many reports revealed their association with many physiologic and pathologic phenotypes, though inconsistently [30]. Five single nucleotide polymorphisms (SNP) in exon 2 (FokI), intron 8 (BsmI, Tru9I, ApaI), and exon 9 (TaqI) have been defined historically in VDR gene, by the associated restriction enzyme [31]. Association studies of VDR allelic variations and T1D done in many countries, including different populations (southern [32] and northern [33] India, Iran [34], Spain [35], Romania [36, 37], Turkey [38, 39], Hungary [40], Portugal [41], UK, US, Norway [42], Japan [42, 43], Finland [42, 44], Poland [45], Croatia [46, 47], Brazil [48], Uruguay [49], Germany [50–52], Greece [53], Bangladesh [54], Taiwan [55], Chile [56], and Italy [57]) yielded conflicting results; some showed significant association while others failed to reach statistical significance, as shown in Table 1. These different results may be related to differences in ethnic background of the populations studied, interactions with other genetic or environmental factors involved in the pathogenesis of T1DM [34], and possibly differences in ultraviolet radiation exposure [58]. In fact, VDR polymorphisms, with the potential exception of the FokI allele variant which has a differential effect on the immune system [59], may not have any functional effect, so, the VDR itself may not be the disease affecting locus but rather a marker locus in linkage disequilibrium with the real disease locus, and the discrepant findings may reflect variable strength of linkage disequilibrium in different populations [41].
Table 1.
VDR gene polymorphism and type 1 DM.
| Population | VDR polymorphism associated with IDDM |
|---|---|
| Bangladesh | FokI, BsmI, ApaI, TaqI |
| Brazil | No association |
| Chile | BsmI, ApaI, TaqI¹ |
| Croatia | Tru91, FokI² |
| Finland | No association |
| Germany | TakI, ApaI, BsmI¹, TruI³ |
| Greece | FokI, BsmI, ApaI, TaqI |
| Hungary | BsmI, ApaI, Tru914 |
| India Northern | FokI, TaqI |
| India Southern | BsmI |
| Italy | No association |
| Iran | TaqI |
| Japan |
BsmI, FokI |
| Norway | No association |
| Polish |
No association |
| Portugal | No association |
| Romania | No association |
| Spain | FokI |
| Taiwan | BsmI, ApaI |
| Turkey | FokI |
| United States | No association |
| United Kingdom | No association |
1Combined.
²Dalmatian population.
³In one study, in combination with TruI, VDR polymorphisms were protective against DM type I.
4Combined, only in girls.
The largest meta-analysis to date investigating the association between polymorphisms in VDR gene and T1DM risk found that BsmI polymorphism is associated with a significantly increased risk of T1DM, whereas the FokI, ApaI, and TaqI polymorphisms do not appear to have a significant association with overall T1DM risk. The BsmI variant B allele (BB or Bb) carriers might have a 30% increased risk of T1DM when compared with the bb homozygote carriers [60].
4. Prevalence of Low Vitamin D Level in Type I DM
Given the association between vitamin D and T1DM and the possible role that vitamin D deficiency might play in its pathogenesis, many observational studies have assessed the 25-hydroxyvitamin D (25-OH D) level in T1DM patients (Table 2) and found a significant higher prevalence of 25-OH D deficiency in T1DM patients compared to controls. In Switzerland, in a cross-sectional study, 60–84% of T1DM were 25-OH D deficient [61]. In Qatar, in a case control study, 90.6% of T1DM children versus 85.3% of nondiabetic children had vitamin D deficiency [62]. Similarly, in North India in a case-control study, 58% of T1DM and only 32% of controls had 25-OH D deficiency [63]. In Northeastern US, in a cross-sectional study, it has been found that 15% of T1D patients were 25-OH D deficient and 61% were insufficient, findings inversely associated with age [64]. All these studies showed significantly lower mean 25-OH D level in T1DM compared to controls [62, 63, 65–68]. In addition, in the Diabetes Incidence Study in Sweden (DISS), 25-OH D level was lower in diabetics compared to controls, not only at the onset of diabetes but also at 8-year followup [69].
Table 2.
Mean 25OH D level in T1DM in different countries.
| Country | Mean 25OH D level (nmol/L) |
|---|---|
| Australia | 78.7 |
| Egypt | 46.75 |
| Florida | 53 |
| Qatar | 39.8 |
| Sweden | 82.5 |
| Switzerland | 45.7 |
| USA (North Eastern) | 67 |
However, only one study, in Florida, a solar rich region in the United States, found no difference in 25-OH D levels in diabetics (recently or more than 5 months diagnosed) compared to their first degree relatives and controls [70].
A pilot study, comparing 25-OH D level in T1DM and type 2 diabetes mellitus (T2DM) showed a higher prevalence of deficiency in T2DM compared to T1DM, and more severe deficiency, independent of age, sex, BMI, and insulin treatment (mean adjusted 25-OH D level 18.1 ± 1.4 ng/mL in T2DM versus 22.9 ± 1.6 ng/mL in T1DM) [71].
5. Effect of Latitude on Vitamin D Level and T1DM Incidence
Dermal vitamin D synthesis is a major source of circulating 25-OH D and its metabolites [72]. Sun exposure, strongly related to latitude, predicts 25-OH D level. Many observational studies showed increased T1DM prevalence at northern latitudes where sun exposure is reduced [6].
In Australia, an ecologic analysis of immune-related disorders showed a positive association of T1DM prevalence with both increasing southern latitude of residence and decreasing regional annual ambient ultraviolet radiation (UVR), with an evident threefold increase in prevalence from the northernmost region to the southernmost region [73]. Similar results were found with increasing latitude in Sweden [74] and China [75]. In Norway, a nationwide prospective study showed higher rate of T1DM in southern county and lowest in northern county [76].
The EURODIAB collaborative, a large multicenter case-control study including 7 centers, Austria, Bucharest, Bulgaria, Latvia, Lithuania, Luxembourg, North Ireland representing most European countries and Israel, in a report based on 16 362 cases registered during the period 1989–1994 by 44 centers and covering a population of about 28 million children, found a high incidence rate in northern and north western Europe and low in central, southern, and eastern Europe with the exception of Sardinia which presented higher rates than neighboring countries [77], with reverse prevalence, being higher in southern areas [78]. In a worldwide study assessing the pattern of incidence of diabetes in 51 different countries, according to latitude and solar UVR, the incidence rates were higher at higher latitudes and lower ultraviolet B irradiance, adjusted for cloud cover, as inversely associated with incidence rates [79].
Note that interpretation of international correlations is particularly difficult because there are many confounding factors such as affluence and genetic variation. Within country analysis provides probably more precise information [80].
6. Seasonal Variability in the Incidence of T1DM
Variability in sun exposure during pregnancy or early developmental stages in infancy has been also suggested as an important environmental factor influencing T1DM onset, possibly related to changes in 25-OH D levels, with highest birth dates of diabetic patients in spring–summer months with an opposite pattern of disease onset peaking in autumn and winter [80]. Consistent results were found in Ukraine (highest variability in western Europe) [81], Sweden [82], Greece [83], Ireland (significant in boys only) [84], Slovenia [85], Germany [86], The Netherlands [87], Britain [88], New Zealand [89], and Sardinia [90]. However, a multicenter cohort study in Europe found no seasonal variations [91]. Similarly, no significant differences in parameters studied in diabetics and controls were detected in Denmark [92]. In a Lebanese T1D population, El Baba et al. showed seasonal variation in glucose control but failed to establish a significant correlation between seasonal changes in 25-OH D levels and HbA1c [93, 94]. In fact, ethnicity may be a confounding factor [95]. Furthermore, none of these studies have shown data about 25-OH D levels, given that they were retrospective. Also, given that viral infections—proven to be involved in the pathogenesis of T1DM—may have also seasonal variations, the evidence of vitamin D involvement in seasonal variations of T1DM needs to be demonstrated with more accurate data.
7. Vitamin D Supplementation and Risk of Developing T1DM
Many studies have assessed the effect of vitamin D supplementation during pregnancy, infancy, or early adulthood and the risk of developing T1DM later on in life (Table 3).
Table 3.
Vitamin D supplementation and risk of T1DM development.
| Author Study design (year) |
Country | Population | Vitamin D supplements | Duration | Results (relative risk of T1D with vitamin D supplements) |
|---|---|---|---|---|---|
| EURODIAB (no authors listed) Case control study (1999) [96] |
7 countries in Europe | 746 T1DM and 2188 controls | Vitamin D supplementation during infancy | 31 years | 0.67 (0.53, 0.86) |
| Stene et al. Case control study (2000) [98] |
Norway | 78 T1DM and 980 controls | Cod liver oil to pregnant women | 16 years | 0.36 (0.14–0.9) |
| Hyppönen et al. Birth control, prospective study (2001) [97] |
Finland | 81 T1DM and 10366 controls | Cod liver oil to children during the first year of life (2000IU daily) | 31 years | 0.22 (0.05–0.89) for regular or irregular vitamin D intake versus no supplements 0.12 (0.03–0.51) for regular vitamin D supplements versus no supplements |
| Fronczak et al. Cohort study (2003) [100] |
Colorado | 16 T1DM and 206 controls | Vitamin D supplementation in food, during the third trimester of pregnancy (250IU daily) | 4 years | 0.37 (0.17–0.78) |
| Stene and Joner Case control study (2003) [99] |
Norway | 545 T1DM and 1668 controls | Cod liver oil in the first year of life, at 7–12 months of age (10 mcg daily for at least 5 times per week) | 15 years | 0.74 (0.56–0.99) |
| Tenconi et al. Case control study (2007) [101] |
North Italy | 159 T1DM and 318 controls | Vitamin D supplementation during lactation | 29 years | 0.33 (0.14–0.81) |
| Brekke et al. Cohort retrospective and prospective study (2007) [102] |
Sweden | 8.7% at 1 year and 8.9% at 2.5 years had positive antibodies | Vitamin D supplementation during pregnancy (10 mcg daily) | 2.5 years | 0.71 (0.17–0.78) |
| Marjamäki et al. Birth cohort study (2010) [103] |
Finland | 165 patients with positive antibodies and 4297 control | Vitamin D supplements during pregnancy (mean supplements 5.1 mcg and 1.3 mcg in food, daily) | 4 years | No significant protective effect |
The EURODIAB focused on early exposures and risk of T1DM. Vitamin D intake during infancy was assessed by questionnaire or interview (recalled). It showed that vitamin D supplements (given for the prevention of rickets) have a protective effect, even after adjustment for various confounders [96].
Hyppönen et al., in his finish birth cohort study found that vitamin D supplementation of 200 IU daily (as cod liver oil), given to children, was associated with a lower incidence of T1DM during a follow-up period of 31 years [97].
In the Norway pilot study, Stene et al. demonstrated a protective effect of vitamin D supplements, only when given as cod liver oil to pregnant women and not when given in other forms of supplementation or when given to children, suggesting a protective effect in utero [98]. However, in his larger case control study, he found a protective effect of cod liver oil when given during the first year of life only and when given ≥5 times per week. No protective effect was detected if vitamin D was given during pregnancy, conflicting results with what have been shown previously by the same group for unknown reasons [99].
Similarly, in the DAISY (Diabetes Autoimmunity Study in the Young) study in Colorado, that recruited at birth and followed children at increased risk for T1DM, as determined by HLA-DR genotype or by family history of T1DM, there was a protective effect of vitamin D taken through food only and not as supplements [100]. More recently, Tenconi et al. demonstrated a protective effect of vitamin D given during lactation [101].
The ABIS (All Babies in Southeast Sweden) study is a large, prospective, population-based cohort study in Sweden that found vitamin D supplementation, given as drops 10 mcg daily, decreased significantly the incidence of glutamic acid decarboxylase autoantibodies or IA-2A in the offspring at 1 year, but not at 2.5 years [102].
Furthermore, the Diabetes Prediction and Prevention study (DIPP), which is a population-based birth cohort of infants at genetic risk of T1DM, showed no significant protective effects of vitamin D whether given with food or as supplements [103].
A meta-analysis of the results of observational studies suggests that the risk of T1DM is 29% reduced in those who were supplemented in childhood with vitamin D compared to those who were not [104]. There was some evidence of dose-response effect—higher supplementation resulting in better protection—and the timing of supplementation predicted a favorable response when given between 7 and 12 months, critical period for immunity to become competent [105].
To note that all these studies have several limitations including recall bias, the absence of 25-OH D level, and the absence of quantitative assessment of vitamin D intake; the dose of vitamin D given was not always mentioned. Randomized controlled trials with long periods of followup are needed to establish causality and to suggest the best formulation, dose, duration, and period of supplementation with vitamin D that would allow appropriate protection against T1DM [104].
8. Possible Explanation of Vitamin D Deficiency in Diabetes
One of the plausible mechanisms of vitamin D deficiency in diabetics is decreased binding proteins; this has been initially demonstrated in diabetic rats [106]. Later on, in humans, it has been found that the urinary loss of vitamin D binding protein (VDBP) is secondary to diminished function or availability of megalin or low-density lipoprotein-related protein 2 (LRP2), correlated with proteinuria. In fact, Megalin is a receptor to many ligands, including albumin, vitamin-binding protein, lipoproteins, hormones, enzymes, and drugs responsible for their reabsorption in the proximal tubule. It facilitates the generation of 1,25(OH)2D3 following the reabsorption of the VDBP—25OHD complex by via megalin endocytic receptor [107]. Furthermore, a study on pubertal T1DM patients showed altered vitamin D regulatory mechanisms with relative decrease in 1,25(OH)2D3 plasma concentration and increased 24,25-dihydroxyvitamin D levels in diabetics compared to their healthy counterparts [68]. Note that 25-OH D level upon presentation with diabetic ketoacidosis can be falsely lowered by acidosis and improves with its resolution without any supplementation [108].
9. Vitamin D Deficiency and Risk of Diabetic Complications
Vitamin D deficiency is associated with increased inflammatory markers in diabetics including CRP, monocyte toll-like receptor (TLR) 2, TLR4, and nuclear factor-κB (NFκB) expression; this might predict increased microvascular complications. However, no statistically significant difference was found in 25-OH D levels in diabetics with microvascular complications compared to those without [109]. On the other hand, another study showed that persistent microalbuminuria is associated with lower 25-OH D levels in T1DM compared to controls [110]. Cardiovascular diseases increased with low 25-OH D levels in the general population [111] but these results have not been specifically studied in diabetics.
25-OH D deficiency has been prevalent upon the initial presentation of T1DM patients who presented with DKA, making it a contributing factor. However, given that levels improved spontaneously after correction of acidosis, the direct contribution of 25-OH D deficiency in the acute presentation of DKA remains controversial [108].
10. Vitamin D Supplementation Effect on Progression and Control of Diabetes
Given that vitamin D deficiency increases the risk of diabetes development and supplementation showed protective effects, many studies looked at the protective effect of vitamin D on diabetes progression and control. One randomized controlled study aimed to assess the effect calcitriol (given as 0.25 mcg every other day) compared to nicotinamide, within 4 weeks of diabetes diagnosis, on the preservation of β-cell function; it showed no improvement in C-peptide and HbA1c levels but significantly lower insulin doses in the calcitriol-treated group [112]. Even when the dose of calcitriol was increased to 0.25 mcg daily and after a followup of 2 years, there was no protective effect of such supplementation on C-peptide levels [113]. Conversely, in LADA patients, when calcitriol (0.5 mcg daily) was added to insulin, it showed stabilization or improvement in fasting and 2 h after 75-g glucose load C-peptide level at 1 year, especially in those whose diabetes duration was less than 1 year [114]. Similarly, in a study in Saudi Arabia, vitamin D3 supplementation to T1DM patients who were deficient showed improvement in glucose control (with significantly lower HbA1c) when 25OH D level reached >75 nmol/L at 12 weeks [115].
11. Guidelines of Vitamin D Supplementation in Children
The American Academy of Pediatrics and the Canadian Pediatric Association recommended vitamin D supplementation of 400 IU daily, starting the first few days of life [116]. The Institute of Medicine (IOM) recommended that the adequate intake and RDA for children below 1 year of age is 400 IU/d and for all individuals of 1 year to 70 years should be 600 IU/d [117]. It seems prudent to ensure that all infants in the United States and other areas with comparable sunlight exposure receive enough vitamin D, especially in winter [118]. Whether these recommended doses are enough to allow extraskeletal benefits of vitamin D is still unknown.
Until now, no specific recommendations regarding vitamin D supplementation in patients with T1DM or at risk of developing autoimmune diabetes [119] but intakes between 5 mcg daily and the 25 mcg daily, tolerable upper intake level, may be desirable [118].
12. Conclusion
1,25(OH)2D3 immunomodulatory effects have shown significant protection against pancreatic insulitis in animal studies [13–18, 21, 22, 120, 121]. In humans, retrospective analysis and observational studies demonstrated high prevalence of 25-OH D deficiency in patients with T1DM [61–68] and suggested a contributory role in the pathogenesis of T1DM, specially with certain allelic variations of the VDR [32–57]. Conversely, vitamin D supplementation during pregnancy and early childhood decreased the risk of autoimmune diabetes [96–104] and perhaps, even after the onset of diabetes, it may improve glycemic control [114, 115]. Despite all these data, the best dose to be used and the target population in order to decrease the incidence of T1DM have not been yet defined. Abiding by the IOM and the American Academy of Pediatrics recommendations on vitamin D supplementations, at least, improves the 25OH D level.
References
- 1.Harrison LC, Honeyman MC, Morahan G, et al. Type 1 diabetes: lessons for other autoimmune diseases? Journal of Autoimmunity. 2008;31(3):306–310. doi: 10.1016/j.jaut.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Craig ME, Hattersley A, Donaghue K. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatric Diabetes. 2009;7(10, supplement 12):3–12. doi: 10.1111/j.1399-5448.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 3.Lammi N, Taskinen O, Moltchanova E, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia. 2007;50(7):1393–1400. doi: 10.1007/s00125-007-0690-4. [DOI] [PubMed] [Google Scholar]
- 4.Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Current Opinion in Endocrinology, Diabetes and Obesity. 2011;18(4):248–251. doi: 10.1097/MED.0b013e32834872ce. [DOI] [PubMed] [Google Scholar]
- 5.Moroni L, Bianchi I, Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmunity Reviews. 2012;11:A386–A392. doi: 10.1016/j.autrev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: a systematic review. Seminars in Arthritis and Rheumatism. 2011;40(6):512–531. doi: 10.1016/j.semarthrit.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutolo M, Pizzorni C, Sulli A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmunity Reviews. 2011;11:84–87. doi: 10.1016/j.autrev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine Reviews. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 9.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. Journal of Steroid Biochemistry and Molecular Biology. 2005;97(1-2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic β-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134(4):1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 11.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Archives of Biochemistry and Biophysics. 2003;417(1):77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 12.Giulietti A, Gysemans C, Stoffels K, et al. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47(3):451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes. 1992;41(11):1491–1495. doi: 10.2337/diab.41.11.1491. [DOI] [PubMed] [Google Scholar]
- 14.Casteels KM, Mathieu C, Waer M, et al. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology. 1995;136(3):866–872. doi: 10.1210/endo.136.3.7867594. [DOI] [PubMed] [Google Scholar]
- 15.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146(4):1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 16.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. Journal of Immunology. 2004;173(4):2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- 17.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 18.Riachy R, Vandewalle B, Belaich S, et al. Beneficial effect of 1,25 dihydroxyvitamin D3 on cytokine-treated human pancreatic islets. Journal of Endocrinology. 2001;169(1):161–168. doi: 10.1677/joe.0.1690161. [DOI] [PubMed] [Google Scholar]
- 19.Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58(6):1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Annals of the Rheumatic Diseases. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37(6):552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu C, Waer M, Casteels K, Laureys J, Bouillon R. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology. 1995;136(3):866–872. doi: 10.1210/endo.136.3.7867594. [DOI] [PubMed] [Google Scholar]
- 23.Overbergh L, Decallonne B, Waer M, et al. 1α,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543) Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 24.Khoo AL, Joosten I, Michels M. 1, 25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134(4):459–468. doi: 10.1111/j.1365-2567.2011.03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riachy R, Vandewalle B, Conte JK, et al. 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology. 2002;143(12):4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
- 26.Savinov AY, Tcherepanov A, Green EA. Contribution of Fas to diabetes development. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:628–632. doi: 10.1073/pnas.0237359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riachy R, Vandewalle B, Moerman E, et al. 1,25-dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11(2):151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 28.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine Reviews. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 29.Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPTM. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes and Metabolism. 2005;31(4, part 1):318–325. doi: 10.1016/s1262-3636(07)70200-8. [DOI] [PubMed] [Google Scholar]
- 31.Ramos-Lopez E, Jansen T, Ivaskevicius V, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Annals of the New York Academy of Sciences. 2006;1079:327–334. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 32.McDermott MF, Ramachandran A, Ogunkolade BW, et al. Allelic variation in the vitamin D receptor influences susceptibility to IDDM in Indian Asians. Diabetologia. 1997;40(8):971–975. doi: 10.1007/s001250050776. [DOI] [PubMed] [Google Scholar]
- 33.Israni N, Goswami R, Kumar A, Rani R. Interaction of Vitamin D receptor with HLA DRB1∗0301 in Type 1 diabetes patients from North India. PLoS ONE. 2009;4(12) doi: 10.1371/journal.pone.0008023.e8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammadnejad Z, Ghanbari M, Ganjali R, et al. Association between vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in Iranian population. Molecular Biology Reports. 2012;39(2):831–837. doi: 10.1007/s11033-011-0805-3. [DOI] [PubMed] [Google Scholar]
- 35.Martí G, Audí L, Esteban C, et al. Association of vitamin D receptor gene polymorphism with type 1 diabetes mellitus in two Spanish populations. Medicina Clinica. 2004;123(8):286–290. doi: 10.1016/s0025-7753(04)74494-2. [DOI] [PubMed] [Google Scholar]
- 36.Nejentsev S, Cooper JD, Godfrey L, et al. Analysis of the vitamin D receptor gene sequence variants in type 1 diabetes. Diabetes. 2004;53(10):2709–2712. doi: 10.2337/diabetes.53.10.2709. [DOI] [PubMed] [Google Scholar]
- 37.Guja C, Marshall S, Welsh K, et al. The study of CTLA-4 and vitamin D receptor polymorphisms in the Romanian type 1 diabetes population. Journal of Cellular and Molecular Medicine. 2002;6(1):75–81. doi: 10.1111/j.1582-4934.2002.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahin SB, Cetinkalp S, Erdogan M. Fas, Fas ligand, and vitamin D receptor fokI gene polymorphisms in patients with Type 1 diabetes mellitus in the aegean region of Turkey. Genet Test Mol Biomarkers. 2012;16(10):1179–1183. doi: 10.1089/gtmb.2012.0173. [DOI] [PubMed] [Google Scholar]
- 39.Gogas Yavuz D, Keskin L, Kıyıcı S, et al. Vitamin D receptor gene BsmI, FokI, ApaI, TaqI polymorphisms and bone mineral density in a group of Turkish type 1 diabetic patients. Acta Diabetologica. 2011;48(4):329–336. doi: 10.1007/s00592-011-0284-y. [DOI] [PubMed] [Google Scholar]
- 40.Györffy B, Vásárhelyi B, Krikovszky D, et al. Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. European Journal of Endocrinology. 2002;147(6):803–808. doi: 10.1530/eje.0.1470803. [DOI] [PubMed] [Google Scholar]
- 41.Lemosa M, Fagulhab A, Coutinhoc E. Lack of association of vitamin D receptor gene polymorphisms with susceptibility to type 1 diabetes mellitus in the Portuguese population. Human Immunology. 2008;69:134–138. doi: 10.1016/j.humimm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Shimada A, Kanazawa Y, Motohashi Y, et al. Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. Journal of Autoimmunity. 2008;30(4):207–211. doi: 10.1016/j.jaut.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Ban Y, Taniyama M, Yanagawa T, et al. Vitamin D receptor initiation codon polymorphism influences genetic susceptibility to type 1 diabetes mellitus in the Japanese population. BMC Medical Genetics. 2001;2, article 7 doi: 10.1186/1471-2350-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turpeinen H, Hermann R, Vaara S, et al. Vitamin D receptor polymorphisms: no association with type 1 diabetes in the Finnish population. European Journal of Endocrinology. 2003;149(6):591–596. doi: 10.1530/eje.0.1490591. [DOI] [PubMed] [Google Scholar]
- 45.Fichna M, Zurawek M, Januszkiewicz-Lewandowska D, Fichna P, Nowak J. PTPN22, PDCD1 and CYP27B1 polymorphisms and susceptibility to type 1 diabetes in Polish patients. International Journal of Immunogenetics. 2010;37(5):367–372. doi: 10.1111/j.1744-313X.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 46.Boraska V, Škrabić V, Zeggini E, et al. Family-based analysis of vitamin D receptor gene polymorphisms and type 1 diabetes in the population of South Croatia. Journal of Human Genetics. 2008;53(3):210–214. doi: 10.1007/s10038-007-0234-2. [DOI] [PubMed] [Google Scholar]
- 47.Zemunik T, Škrabić V, Boraska V, et al. Fokl polymorphism, vitamin D receptor, and interleukin-1 receptor haplotypes are associated with type 1 diabetes in the Dalmatian population. Journal of Molecular Diagnostics. 2005;7(5):600–604. doi: 10.1016/S1525-1578(10)60593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mory DB, Rocco ER, Miranda WL, Kasamatsu T, Crispim F, Dib SA. Prevalence of vitamin D receptor gene polymorphisms FokI and BsmI in Brazilian individuals with type 1 diabetes and their relation to β-cell autoimmunity and to remaining β-cell function. Human Immunology. 2009;70(6):447–451. doi: 10.1016/j.humimm.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Mimbacas A, Trujillo J, Gascue C, Javiel G, Cardoso H. Prevalence of vitamin D receptor gene polymorphism in a Uruguayan population and its relation to type 1 diabetes mellitus. Genetics and Molecular Research. 2007;6(3):534–542. [PubMed] [Google Scholar]
- 50.Fassbender WJ, Goertz B, Weismüller K, et al. VDR gene polymorphisms are overrepresented in German patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Hormone and Metabolic Research. 2002;34(6):330–337. doi: 10.1055/s-2002-33262. [DOI] [PubMed] [Google Scholar]
- 51.Pani MA, Knapp M, Donner H, et al. Vitamin D receptor allele combinations influence genetic susceptibility to 1 diabetes in Germans. Diabetes. 2000;49(3):504–507. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Lopez E, Jansen T, Ivaskevicius V, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Annals of the New York Academy of Sciences. 2006;1079:327–334. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 53.Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E. Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clinical Immunology. 2009;133(2):276–281. doi: 10.1016/j.clim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Ogunkolade BW, Boucher BJ, Prahl JM, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51(7):2294–2300. doi: 10.2337/diabetes.51.7.2294. [DOI] [PubMed] [Google Scholar]
- 55.Chang TJ, Lei HH, Yeh JI, et al. Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clinical Endocrinology. 2000;52(5):575–580. doi: 10.1046/j.1365-2265.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- 56.García D, Angel B, Carrasco E, Albala C, Santos JL, Pérez-Bravo F. VDR polymorphisms influence the immune response in type 1 diabetic children from Santiago, Chile. Diabetes Research and Clinical Practice. 2007;77(1):134–140. doi: 10.1016/j.diabres.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Bianco MG, Minicucci L, Calevo MG, Lorini R. Vitamin D receptor polymorphisms: are they really associated with type 1 diabetes? European Journal of Endocrinology. 2004;151(5):641–642. doi: 10.1530/eje.0.1510641. [DOI] [PubMed] [Google Scholar]
- 58.Ponsonby AL, Pezic A, Ellis J, et al. Variation in associations between allelic variants of the vitamin D receptor gene and onset of type 1 diabetes mellitus by ambient winter ultraviolet radiation levels: a meta-regression analysis. American Journal of Epidemiology. 2008;168(4):358–365. doi: 10.1093/aje/kwn142. [DOI] [PubMed] [Google Scholar]
- 59.Van Etten E, Verlinden L, Giulietti A, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. European Journal of Immunology. 2007;37(2):395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Li W, Liu J. Polymorphisms in the vitamin D receptor gene and type 1 diabetes mellitus risk: an update by meta-analysis. Molecular and Cellular Endocrinology. 2012;355:135–142. doi: 10.1016/j.mce.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Janner M, Ballinari P, Mullis PE, Flück CE. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Medical Weekly. 2010;140:p. w13091. doi: 10.4414/smw.2010.13091. [DOI] [PubMed] [Google Scholar]
- 62.Bener A, Alsaied A, Al-Ali M, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetologica. 2009;46(3):183–189. doi: 10.1007/s00592-008-0071-6. [DOI] [PubMed] [Google Scholar]
- 63.Borkar VV, Devidayal VS, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatric Diabetes. 2010;11(5):345–350. doi: 10.1111/j.1399-5448.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 64.Svoren BM, Volkening LK, Wood JR, Laffel LMB. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. Journal of Pediatrics. 2009;154(1):132–134. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pozzilli P, Manfrini S, Crinò A, et al. Low levels of 25-hydroxyvitamin D3 and 1, 25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Hormone and Metabolic Research. 2005;37(11):680–683. doi: 10.1055/s-2005-870578. [DOI] [PubMed] [Google Scholar]
- 66.Greer RM, Portelli SL, Hung BS. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatric Diabetes. 2012;14(1):31–41. doi: 10.1111/j.1399-5448.2012.00890.x. [DOI] [PubMed] [Google Scholar]
- 67.Hamed EA, Faddan NH, Elhafeez HA. Parathormone—25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr Diabete. 2011;12(6):536–546. doi: 10.1111/j.1399-5448.2010.00739.x. [DOI] [PubMed] [Google Scholar]
- 68.Rodland O, Markestad T, Aksnes L, Aarskog D. Plasma concentration of vitamin D metabolites during puberty of diabetic children. Diabetologia. 1985;28(9):663–666. doi: 10.1007/BF00291972. [DOI] [PubMed] [Google Scholar]
- 69.Littorin B, Blom P, Schölin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49(12):2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 70.Bierschenk L, Alexander J, Wasserfall C, Haller M, Schatz D, Atkinson M. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care. 2009;32(11):1977–1979. doi: 10.2337/dc09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Cesar DJ, Ploutz-Snyder R, Weinstock RS, Moses AM. Vitamin D deficiency is more common in type 2 than in type 1 diabetes [6] Diabetes Care. 2006;29(1):p. 174. doi: 10.2337/diacare.29.1.174. [DOI] [PubMed] [Google Scholar]
- 72.DeLuca HF. Overview of general physiologic features and functions of vitamin D. The American Journal of Clinical Nutrition. 2004;80(6, supplement):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 73.Staples JA, Ponsonby AL, Lim LLY, McMichael AJ. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: iatitude, regional ultraviolet radiation, and disease prevalence. Environmental Health Perspectives. 2003;111(4):518–523. doi: 10.1289/ehp.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nystrom L, Dahlquist G, Ostman J, et al. Risk of developing insulin-dependent diabetes mellitus (IDDM) before 35 years of age: indications of climatological determinants for age at onset. International Journal of Epidemiology. 1992;21(2):352–358. doi: 10.1093/ije/21.2.352. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z, Wang K, Tianlin LI, et al. Childhood diabetes in China: enormous variation by place and ethnic group. Diabetes Care. 1998;21(4):525–529. doi: 10.2337/diacare.21.4.525. [DOI] [PubMed] [Google Scholar]
- 76.Joner G, Stene LC, Søvik O. Nationwide, prospective registration of type 1 diabetes in children aged <15 years in Norway 1989–1998: no increase but significant regional variation in incidence. Diabetes Care. 2004;27(7):1618–1622. doi: 10.2337/diacare.27.7.1618. [DOI] [PubMed] [Google Scholar]
- 77.Variation and trends in incidence of childhood diabetes in Europe. The Lancet. 2000;355(9207):873–876. [PubMed] [Google Scholar]
- 78.Casu A, Pascutto C, Bernardinelli L, Songini M. Bayesian approach to study the temporal trend and the geographical variation in the risk of type 1 diabetes: the Sardinian Conscript type 1 diabetes registry. Pediatric Diabetes. 2004;5(1):32–38. doi: 10.1111/j.1399-543X.2004.00037.x. [DOI] [PubMed] [Google Scholar]
- 79.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391–1398. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 80.Merriman TR. Type 1 diabetes, the A1 milk hypothesis and vitamin D deficiency. Diabetes Research and Clinical Practice. 2009;83(2):149–156. doi: 10.1016/j.diabres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Vaiserman AM, Carstensen B, Voitenko VP, et al. Seasonality of birth in children and young adults (0-29 years) with type 1 diabetes in Ukraine. Diabetologia. 2007;50(1):32–35. doi: 10.1007/s00125-006-0456-4. [DOI] [PubMed] [Google Scholar]
- 82.Samuelsson U, Johansson C, Ludvigsson J. Month of birth and risk of developing insulin dependent diabetes in south east Sweden. Archives of Disease in Childhood. 1999;81(2):143–146. doi: 10.1136/adc.81.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalliora MI, Vazeou A, Delis D, Bozas E, Thymelli I, Bartsocas CS. Seasonal variation of type 1 diabetes mellitus diagnosis in Greek children. Hormones. 2011;10(1):67–71. doi: 10.14310/horm.2002.1294. [DOI] [PubMed] [Google Scholar]
- 84.Roche EF, Lewy H, Hoey HMCV, Laron Z. Differences between males and females in the seasonality of birth and month of clinical onset of disease in chilren with type 1 diabetes mellitus in Ireland. Journal of Pediatric Endocrinology and Metabolism. 2003;16(5):779–782. doi: 10.1515/jpem.2003.16.5.779. [DOI] [PubMed] [Google Scholar]
- 85.Ursic-Bratina N, Battelino T, Kržišnik C, Laron-Kenet T, Ashkenazi I, Laron Z. Seasonality of birth in children (0–14 years) with type 1 diabetes mellitus in Slovenia. Journal of Pediatric Endocrinology and Metabolism. 2001;14(1):47–52. doi: 10.1515/jpem.2001.14.1.47. [DOI] [PubMed] [Google Scholar]
- 86.Kordonouri O, Shuga N, Lewy H, Ashkenazi I, Laron Z. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in Berlin differs from the general population. European Journal of Pediatrics. 2002;161(5):291–292. doi: 10.1007/s00431-002-0941-9. [DOI] [PubMed] [Google Scholar]
- 87.Jongbloet PH, Groenewoud HM, Hirasing RA, Van Buuren S. Seasonality of birth in patients with childhood diabetes in The Netherlands. Diabetes Care. 1998;21(1):190–191. doi: 10.2337/diacare.21.1.190. [DOI] [PubMed] [Google Scholar]
- 88.Rothwell PM, Staines A, Smail P, Wadsworth E, McKinney P. Seasonality of birth of patients with childhood diabetes in Britain. British Medical Journal. 1996;312(7044):1456–1457. doi: 10.1136/bmj.312.7044.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willis JA, Scott RS, Darlow BA, Lewy H, Ashkenazi I, Laron Z. Seasonality of birth and onset of clinical disease in children and adolescents (0–19 years) with type 1 diabetes mellitus in Canterbury, New Zealand. Journal of Pediatric Endocrinology and Metabolism. 2002;15(5):645–647. doi: 10.1515/jpem.2002.15.5.645. [DOI] [PubMed] [Google Scholar]
- 90.Songini M, Casu A, Ashkenazi I, Laron Z. Seasonality of birth in children (0–14 years) and young adults (0–29 years) with type 1 diabetes mellitus in Sardinia differs from that in the general population. Journal of Pediatric Endocrinology and Metabolism. 2001;14(6):781–783. doi: 10.1515/jpem.2001.14.6.781. [DOI] [PubMed] [Google Scholar]
- 91.Rothwell PM, Gutnikov SA, McKinney PA, Schober E, Ionescu-Tirgoviste C, Neu A. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. British Medical Journal. 1999;319(7214):887–888. doi: 10.1136/bmj.319.7214.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bock T, Pedersen CR, Volund A, Pallesen CS, Buschard K. Perinatal determinants among children who later develop IDDM. Diabetes Care. 1994;17(10):1154–1157. doi: 10.2337/diacare.17.10.1154. [DOI] [PubMed] [Google Scholar]
- 93.El Baba K, Zantout MS, Arabi A, Azar ST. Seasonal variations of glucose control in Lebanese patients with type 1 diabetes. Biological Rhythm Research. 2010;41(2):91–97. [Google Scholar]
- 94.El Baba K, Zantout M, Akel R, Azar S. Seasonal variation in vitamin D and HbA(1c) levels in patients with type 1 diabetes mellitus in the Middle East. International Journal of General Medicine. 2011;4:635–638. doi: 10.2147/IJGM.S23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laron Z, Lewy H, Wilderman I, et al. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in homogenous and heterogeneous populations. Israel Medical Association Journal. 2005;7(6):381–384. [PubMed] [Google Scholar]
- 96.Vitamin D supplement in early childhood and risk for Type I (insulin- dependent) diabetes mellitus. Diabetologia. 1999;42(1):51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 97.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. The Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 98.Stene LC, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia. 2000;43(9):1093–1098. doi: 10.1007/s001250051499. [DOI] [PubMed] [Google Scholar]
- 99.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. American Journal of Clinical Nutrition. 2003;78(6):1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 100.Fronczak CM, Barón AE, Chase HP, et al. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–3242. doi: 10.2337/diacare.26.12.3237. [DOI] [PubMed] [Google Scholar]
- 101.Tenconi MT, Devoti G, Comelli M, et al. Major childhood infectious diseases and other determinants associated with type 1 diabetes: a case-control study. Acta Diabetologica. 2007;44(1):14–19. doi: 10.1007/s00592-007-0235-9. [DOI] [PubMed] [Google Scholar]
- 102.Brekke HK, Ludvigsson J. Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatric Diabetes. 2007;8(1):11–14. doi: 10.1111/j.1399-5448.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 103.Marjamäki L, Niinistö S, Kenward MG, et al. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010;53(8):1599–1607. doi: 10.1007/s00125-010-1734-8. [DOI] [PubMed] [Google Scholar]
- 104.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Archives of Disease in Childhood. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 105.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with Vitamin D reduce the risk or modify the course of autoimmune diseases? A systamatic review of the literature. Autoimmunity Reviews. 2012;12:127–136. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Nyomba BL, Verhaeghe J, Thomasset M, Lissens W, Bouillon R. Bone mineral homeostasis in spontaneously diabetic BB rats. I. Abnormal vitamin D metabolism and impaired active intestinal calcium absorption. Endocrinology. 1989;124(2):565–572. doi: 10.1210/endo-124-2-565. [DOI] [PubMed] [Google Scholar]
- 107.Thrailkill KM, Jo CH, Cockrell GE. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? The Journal of Clinical Endocrinology & Metabolism. 2011;96(1):142–149. doi: 10.1210/jc.2010-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huynh T, Greer RM, Nyunt O, et al. The association between ketoacidosis and 25(OH)-vitamin D3 levels at presentation in children with type 1 diabetes mellitus. Pediatric Diabetes. 2009;10(1):38–43. doi: 10.1111/j.1399-5448.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 109.Devaraj S, Yun JM, Duncan-Staley CR, Jialal I. Low vitamin d levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. American Journal of Clinical Pathology. 2011;135(3):429–433. doi: 10.1309/AJCPJGZQX42BIAXL. [DOI] [PubMed] [Google Scholar]
- 110.Verrotti A, Basciani F, Carle F, Morgese G, Chiarelli F. Calcium metabolism in adolescents and young adults with type 1 diabetes mellitus without and with persistent microalbuminuria. Journal of Endocrinological Investigation. 1999;22(3):198–202. doi: 10.1007/BF03343541. [DOI] [PubMed] [Google Scholar]
- 111.Judd S, Tangpricha V, Vitamin D. Deficiency and risk for cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitocco D, Crinò A, Di Stasio E, et al. The effects of calcitriol and nicotinamide on residual pancreatic β-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI) Diabetic Medicine. 2006;23(8):920–923. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 113.Bizzarri C, Pitocco D, Napoli N, et al. No protective effect of calcitriol on β-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33(9):1962–1963. doi: 10.2337/dc10-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li X, Liao L, Yan X, et al. Protective effects of 1-α-hydroxyvitamin D3 on residual β-cell function in patients with adult-onset latent autoimmune diabetes (LADA) Diabetes/Metabolism Research and Reviews. 2009;25(5):411–416. doi: 10.1002/dmrr.977. [DOI] [PubMed] [Google Scholar]
- 115.Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Annals of Saudi Medicine. 2010;30(6):454–508. doi: 10.4103/0256-4947.72265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 117.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harris S. Can vitamin D supplementation in infancy prevent type 1 diabetes? Nutrition Reviews. 2002;60(4):118–121. doi: 10.1301/00296640260085868. [DOI] [PubMed] [Google Scholar]
- 119.Holick M, Binkley N, Bischoff-Ferrari H, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 120.Penna G, Adorini L. 1α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. Journal of Immunology. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 121.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. Journal of Immunology. 2000;164(9):4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]


