Abstract
Neurocognitive impairments are prevalent in persons seeking treatment for alcohol use disorders (AUDs). These impairments and their physical, social, psychological and occupational consequences vary in severity across persons, much like those resulting from traumatic brain injury; however, due to their slower course of onset, alcohol-related cognitive impairments are often overlooked both within and outside of the treatment setting. Evidence suggests that cognitive impairments can impede treatment goals through their effects on treatment processes. Although some recovery of alcohol-related cognitive impairments often occurs after cessation of drinking (time-dependent recovery), the rate and extent of recovery is variable across cognitive domains and individuals. Following a long hiatus in scientific interest, a new generation of research aims to facilitate treatment process and improve AUD treatment outcomes by directly promoting cognitive recovery (experience-dependent recovery). This review updates knowledge about the nature and course of cognitive and brain impairments associated with AUD, including cognitive effects of adolescent AUD. We summarize current evidence for indirect and moderating relationships of cognitive impairment to treatment outcome, and discuss how advances in conceptual frameworks of brain-behavior relationships are fueling the development of novel AUD interventions that include techniques for cognitive remediation. Emerging evidence suggests that such interventions can be effective in promoting cognitive recovery in persons with AUD and other substance use disorders, and potentially increasing the efficacy of AUD treatments. Finally, translational approaches based on cognitive science, neurophysiology, and neuroscience research are considered as promising future directions for effective treatment development that includes cognitive rehabilitation.
Keywords: Alcoholism, Cognitive impairment, Brain damage, Cognitive recovery, Cognitive enhancement, Cognitive training
Introduction
In 1987, a prescient overview of research on alcohol-associated impairment of the central nervous system and its potential significance to the efficacy of treatment for alcoholism appeared in Parsons, Butters and Nathan’s edited volume, Neuropsychology of Alcoholism: Implications for diagnosis and treatment (Parsons et al. 1987). Studies using computed tomography scans and electroencephalography had documented impairment to brain structure and function in chronic, heavy drinkers. In parallel, neuropsychological testing approaches documented that many persons with alcohol use disorders (AUD) experienced mild to severe impairment of abstract reasoning, visual-spatial skills, memory, and other information processes. There was evidence that cognitive deficits recovered over time following cessation, or substantially reduced, alcohol drinking, and that recovery of function could be facilitated if relevant tasks were practiced early in the recovery process. International experts in the field raised provocative research questions about the relation of cognitive impairment and recovery to the process and outcome of addiction treatment. Yet, enthusiasm was tempered by the modest and inconsistent relations between cognitive impairment and treatment efficacy that had been documented at that time.
Fifteen years later, the literature on neurocognitive impairment associated with AUD and implications for treatment was revisited (Bates et al. 2002a). Development of new brain imaging technologies and behavioral testing approaches had led to substantial progress in understanding the nature and course of alcohol-related neurocognitive impairments. Applications of this new knowledge to develop more effective treatments, however, had lagged behind. There were likely several reasons for the lack of progress in this area. One was the lack of conceptual models of indirect and moderating roles of neurocognitive impairment to represent the interrelated operation of cognitive factors with multiple other person and environmental factors that in concert contribute to treatment outcomes. In addition, few researchers had applied treatment innovations derived from basic cognitive science, and from other brain injury literatures, to the question of cognitive rehabilitation in AUD.
Fortunately, over the past 10 years, this trend has reversed. There has been a surge of clinical and research interest in the relation of cognitive impairment to addiction treatment outcome, and in developing new ways to intervene in non-adaptive neurocognitive processes that may interfere with recovery. Training of working memory and other executive functions shows potential promise to generalize to additional neurocognitive dispositions (e.g., Bickel et al. 2011) and potentially enhance positive substance use outcomes (e.g., Rupp et al. 2012). Both relatively brief and very time intensive cognitive remediation approaches are being explored in persons with a range of alcohol use behaviors spanning hazardous drinking to alcohol dependence, and a range of cognitive problems from cognitive bias to serious brain damage (e.g., Alfonso et al. 2011; Houben et al. 2011; Wilson et al. 2012). Finally, new conceptual models for intervention strategies that build on the bidirectional feedback streams between the brain and body are starting to emerge (Wiers et al. 2011b; Bates and Buckman in press). These novel interventions, described at the article’s end, use relatively simple muscle movements, rhythmical breathing exercises, aerobic exercise, meditation, or combined mental and physical interventions to train (or retrain) neurophysiological systems that support cognition, affect regulation, motivation, and resilience to stress, all of which are compromised by chronic heavy alcohol use.
Cognitive Impairment in AUD
A sizeable proportion of persons (estimated to be ~ 50% – 70%) diagnosed with AUD display some degree of neurocognitive deficit relative to healthy controls (Fein et al. 1990; Martin et al. 1986). Although many alcohol dependent adults fortunately demonstrate only subtle or transient cognitive disruptions (Bates and Convit 1999; Rourke and Loberg 1996), a sizable minority of these individuals display impairments as clinically severe as those seen in persons with traumatic brain injury (Bates 1997; Donovan et al. 2001). Chronic, heavy alcohol use appears to selectively affect cognitive abilities associated with controlled and effortful processing of novel information, and selective and divided attention, while sparing general intelligence, over-learned knowledge, and automatic information processes (summarized in Table 1, for review, see Bates et al. 2008; Oscar-Berman and Marinkovic 2007; Lyu and Lee 2012; Le Berre et al. 2010). Executive functions such as working memory and response inhibition, and the frontal lobe structures typically thought to subserve them, appear to be particularly vulnerable to alcohol-related impairment (Ratti et al. 2002; Oscar-Berman et al. 2004; Zinn et al. 2004; Noel et al. 2007; Lawrence et al. 2009; Loeber et al. 2009; Kopera et al. 2012; Ambrose et al. 2001; Pitel et al. 2007b). Fluid cognitive abilities, such as concept formation, abstraction, problem solving, and visual-spatial abilities are also often affected (Nixon 1995; Parsons 1998; Parsons and Farr 1981; Rourke and Loberg 1996; Weinstein and Shaffer 1993; Rupp et al. 2006).
Table 1.
Examples of neurocognitive functions and abilities often found to be vulnerable or resistant to impairment in persons with alcohol dependence.
| Ability | Vulnerable | Resistant |
|---|---|---|
| EXECUTIVE FUNCTIONS | ||
| Working memory | X | |
| Mental flexibility | X | |
| Self-monitoring | X | |
| Response inhibition | X | |
| FLUID ABILITIES | ||
| Concept formation | X | |
| Planning ability | X | |
| Abstraction | X | |
| Visuospatial skills | X | |
| Problem solving | X | |
| LEARNING & MEMORY | ||
| New Learning | X | |
| Autobiographical memory | X | |
| Prospective memory | X | |
| Episodic memory | X | |
| Implicit/automatic memory processes | X | |
| Procedural memory | X | |
| PSYCHOMOTOR SKILLS | ||
| Proprioception | X | |
| Gait stability | X | |
| CRYSTALLIZED ABILITIES | ||
| General intelligence | X | |
| Vocabulary | X | |
| Over-learned motor skills | X | |
| Over-learned general knowledge | X | |
| AUTOMATIC INFORMATION PROCESSES | X | |
| INFORMATION PROCESSING SPEED | X |
Episodic and autobiographical memory are often disrupted by chronic heavy alcohol use (Beatty et al. 1995; Le Berre et al. 2010; Weingardt et al. 1996; D'Argembeau et al. 2006). Occasionally, memory impairment may be severe, particularly in the context of thiamine deficiency as seen in those with Korsakoff’s syndrome, and has been compared to that observed in dementia (Mukamal et al. 2003; Xu et al. 2009; reviewed in Bates et al. 2008). Much of the existing literature on alcohol-related memory deficits utilizes tests of semantic and visual processing that assess retrospective memory at the level of encoding, storage, and retrieval of novel information. Recently, interest in prospective memory, or the memory needed to plan and carry out future actions (Ellis 1996), such as remembering to do the laundry or keep an appointment, has emerged in the addictions area. Prospective memory involves memory formation and retrieval as well as executive functions, planning and decision-making (Fish et al. 2010). Prospective memory functioning appears to be impaired by both chronic and acute alcohol use (Heffernan et al. 2002; Leitz et al. 2009; Montgomery et al. 2011; Griffiths et al. 2012) and may contribute to the memory problems encountered in everyday living both during and after addiction treatment.
Executive functioning and memory impairments have garnered particular attention as these domains of cognition are so conceptually tied to treatment outcome and maintenance of abstinence. Nonetheless, there is considerable heterogeneity in the likelihood of specific impairment and its severity in the AUD population, and individual studies do not consistently find the pattern of deficits noted above. Most information about impairment is from treatment samples and deficits may be less frequent or less severe in non treatment seeking populations (Fein and Greenstein 2012). In addition to methodological factors such as sample size, sample selection criteria, and time lapsed since last drink, alcohol-related cognitive deficits (and their recovery) are affected by psychological, social, and biological factors, and perhaps the interaction between these factors. Age, education, gender, other drug use, co-occurring mental disorders, childhood behavior disorders, and family history of alcoholism, for example, have all been implicated as risk factors for alcohol-induced cognitive impairments (Bates et al. 2002a; Oscar-Berman et al. 1997). This heterogeneity is consistent with evidence that cognitive ability levels in general are shaped by biological and genetic factors as well as by social and cultural influences (Finkel et al. 1995; Hedden et al. 2002). Further, some individuals with AUD will have cognitive impairments that pre-date their unhealthy alcohol use behaviors.
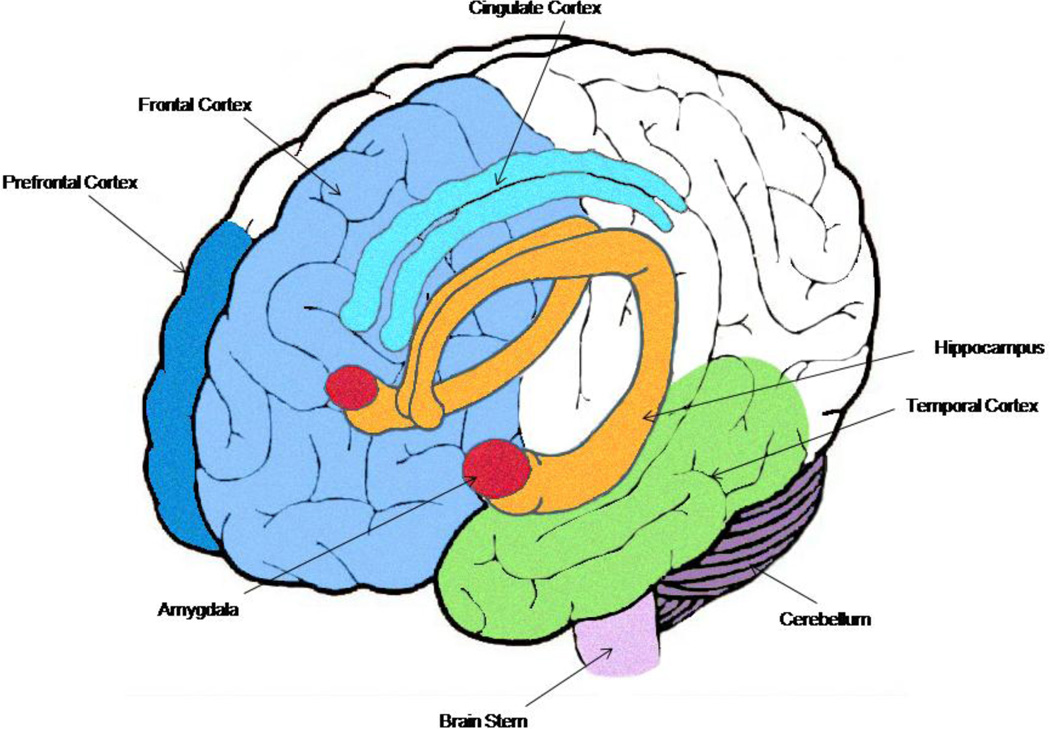
Strong support for neurostructural and neurofunctional changes associated with chronic heavy alcohol consumption parallels these observations (for full review, see Oscar-Berman and Marinkovic 2007). In addition to global atrophy in the brains of chronic heavy alcohol users (Oscar-Berman and Marinkovic 2007), there is compelling evidence that the frontal lobes (Pfefferbaum et al. 2009; Pfefferbaum et al. 1997; Fortier et al. 2011; Harris et al. 2008; Sorg et al. 2012; Cardenas et al. 2007), limbic system (Wrase et al. 2008; Makris et al. 2008; Anderson et al. 2012) and cerebellum (Fitzpatrick et al. 2012; Baker et al. 1999; Sullivan et al. 2000b), as well as the connections between them (Sullivan 2003; Rogers et al. 2012; Desmond et al. 2003), are particularly vulnerable (Figure 1). Structurally, this vulnerability is observed as region-specific volume reductions (e.g., Pfefferbaum et al. 1997; Fortier et al. 2011; Wrase et al. 2008; Makris et al. 2008; Sullivan et al. 2000a). Level of alcohol consumption (Cardenas et al. 2005; Pfefferbaum et al. 1998) and years of heavy drinking (Bjork et al. 2003) appear to importantly contribute to the severity of these neurostructural changes.
Fig. 1. Brain structure changes associated with chronic heavy alcohol use.
Early studies on the brain of alcohol dependent individuals consistently demonstrated decreased brain volume and ventricular and sulcal enlargement, although changes did not appear uniform across all brain structures. Current knowledge based on a variety of neuroimaging techniques suggests that limbic structures (amygdala = red, hippocampus = orange, temporal lobe gyri = green, cingulate cortex = turquoise), frontal cortical structures (prefrontal cortex and frontal cortex in shades of blue), and the cerebellum (dark purple) are especially vulnerable to chronic use. The extensive bidirectional connectivity between these structures also appears prone to disruption. Brain abnormalities may be fully or partially stabilized or reversed with extended abstinence. Current research is specifying region-specific recovery trajectories and risk for persistent versus reversible abnormalities.
Functionally, alcohol-related vulnerability is apparent as white matter abnormalities and changes in BOLD signal that vary by brain structure. For example, fractional anisotropy diffusion tensor magnetic resonance imaging shows compromised frontal lobe white matter integrity in persons with AUD in comparison to normal controls (Harris et al. 2008). Cardenas et al. (2007) further showed that chronic heavy alcohol users display a significantly greater loss of tissue in the frontal foci compared to light drinkers using deformation tensor morphometry imaging. Sullivan (2003) postulated that disruptions in the frontocerebellar circuitry underlie some of the commonly reported alcohol-related neuropsychological deficits in problem solving, visuospatial reasoning, and proprioception. Recent functional MRI studies report reduced frontocerebellar connectivity during a simple finger-tapping task (Rogers et al. 2012) and increased frontocerebellar activity during a working memory task (Desmond et al. 2003) in abstinent alcoholics compared to controls. Interestingly, Herting and colleagues (2011) found that alcohol-naive adolescents with a family history of alcoholism showed reduced frontocerebellar connectivity in comparison to negative family history controls.
The link between cognitive and neurobiological deficits in relation to alcohol use behaviors is well illustrated in a growing body of literature examining adolescents who drink heavily. Alcohol consumption increases sharply throughout adolescence, with upwards of 40% of 12th graders reporting drinking in the past 30 days (Johnston et al. 2012). Alcohol use disorders in adulthood often initiate in adolescence, with peak use at 17–18 years of age (Wagner and Anthony 2002). Executive skills, such as planning, problem-solving, memory, and inhibitory control, develop considerably both at the behavioral and neurobiological level during adolescence (Conklin et al. 2007; Luna et al. 2010). Processes like cortical thinning and neural pruning contribute to the natural brain maturation process during this crucial growth period (Clark and Tapert 2008; Durston et al. 2001).
Initial studies found that adolescents with AUD showed subtle alcohol-related cognitive deficits (Brown et al. 2000; Tapert and Brown 1999). These deficits extend to verbal memory, self-monitoring, psychomotor skills, sustained attention (Ferrett et al. 2010; Squeglia et al. 2009), and self-reported prospective memory (Heffernan and Bartholomew 2006; Heffernan et al. 2010). A longitudinal study over eight years showed that the use of substances, including alcohol, in adolescents predicts poor performance on visuospatial functioning, attention, memory, and learning abilities later in life (Tapert et al. 2002). Using latent class growth analysis, Anderson et al.(2010) identified six different alcohol use trajectories among adolescents undergoing treatment; continued and resurged chronic heavy alcohol use was associated with poorer occupational and family functioning over the next ten years. These maladaptive trajectories were also linked to poorer visuospatial processing, verbal and working memory, and attention (Hanson et al. 2011).
Heavy alcohol use in adolescents also affects various brain areas, including the prefrontal, frontal, and parietal cortices, hippocampus, thalamus, and the cerebellum (De Bellis et al. 2005; Nagel et al. 2005; Medina et al. 2008; Squeglia et al. 2012). Furthermore, gender differences in cognitive functioning have been detected in adolescents with AUD. In a spatial working memory task, girls with AUD showed greater overall brain activation patterns than their male counterparts, possibly to compensate for reduced functioning in the frontal and cingulate regions (Caldwell et al. 2005). Medina et al. (2008) found that prefrontal cortex volume differed between boys and girls with AUD. Overall, prefrontal cortex and white matter volumes were increased in boys and decreased in girls in comparison to their same-gender, non-AUD controls. A similar study found that adolescent alcohol and marijuana users showed deficits in white matter integrity in various cortical and subcortical tracts over an 18-month period. In particular, adolescents with increased alcohol use from baseline to follow-up had worse white matter integrity (Bava et al. 2012) and degradation positively correlated with increased quantity of alcohol consumption. Adolescent substance users have been found to exhibit not only white matter disorganization, but a corresponding increase in executive function difficulties (Clark et al. 2012).
Cognitive Recovery in AUD
Many studies, old and new, provide cross-sectional snapshots of potential cognitive recovery that unfolds after cessation of drinking in the absence of targeted cognitive remediation. This type of recovery, what Goldman (1990) termed time-dependent recovery, has been most commonly examined using between-group comparisons of an alcohol dependent sample to a control sample. Limited longitudinal designs have also compared the same alcohol dependent sample tested twice: at some point very early in treatment after detoxification, and again later in treatment or soon after the end of treatment. These studies have suggested that whereas some cognitive recovery, such as verbal learning, is observable early after detoxification, recovery in executive and memory domains is slower, occurring over one year and longer (Sullivan et al. 2000; Goldman 1990; Parsons 1998; Bates et al. 2002a; Bates et al. 2005; Manning et al. 2008; Bates et al. 2004; Dingwall et al. 2011; Rosenbloom et al. 2004; Rosenbloom et al. 2007). When considered collectively, as in a recent meta-analysis (Stavro et al. 2012) and review (Fernandez-Serrano et al. 2011), these studies paint a picture of substantial cognitive recovery in the short term (one month), with more modest increases across mid-term (up to one year), and long-term in multiple cognitive domains.
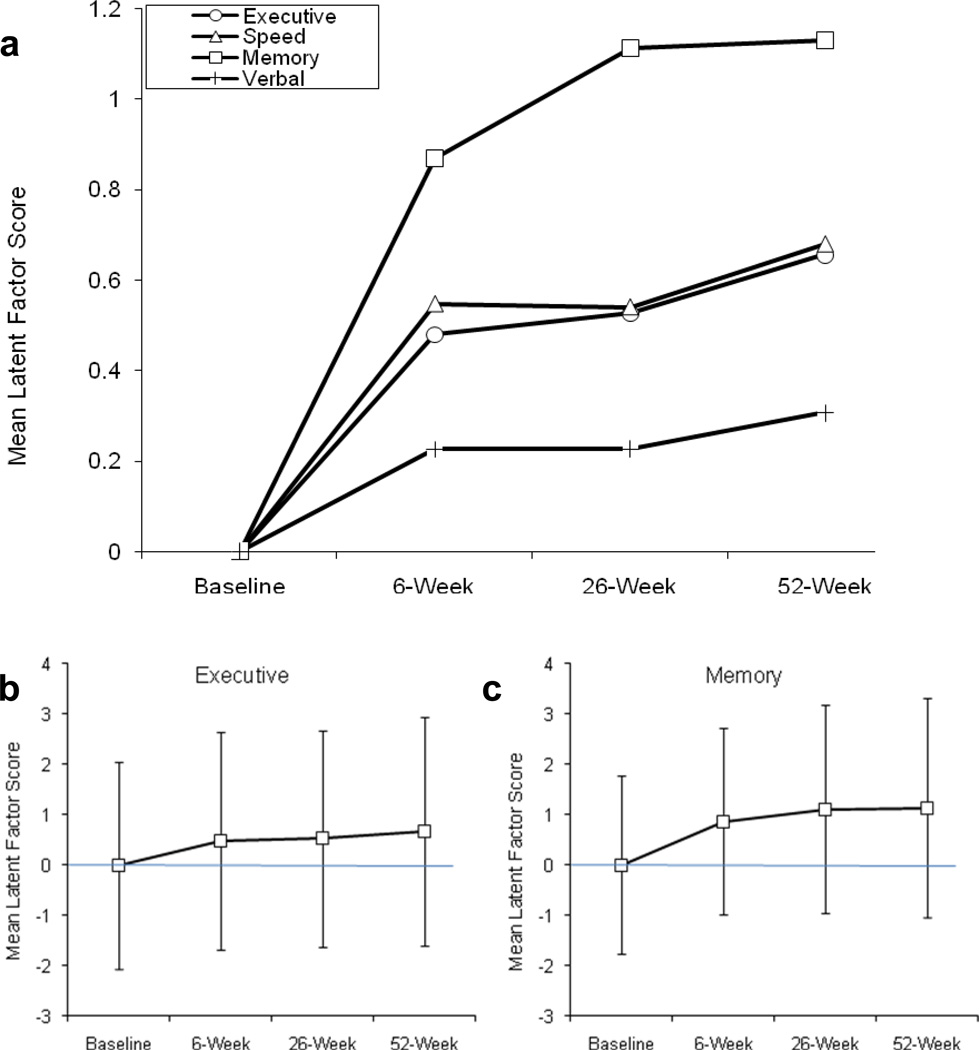
A few studies have used a longitudinal design with multiple assessment time points to directly track within-person changes in cognition over time. For example, Bartels and colleagues (2007) found considerable recovery of learning and memory performance in AUD patients with severe memory impairments who were assessed five times during 24 months of verified abstinence. In Figure 2 we present recovery data from a study that administered 15 neuropsychological tests to 196 men and women in treatment for AUD and other drug use disorders at four points in time across the first year of recovery (entry, 6 wks, 6 mons, and 12 mon). Results from the third and fourth assessments add to previously published data (Bates et al. 2004; 2005), We used a latent variable approach to parse the contribution of practice effects and measurement error from cognitive recovery (e.g., Bollen 1989; Friedman et al. 2008). Four latent ability constructs were replicated across time: executive functioning, memory, verbal ability, and complex information processing speed. Practice effects were identified as changes in the intercepts of individual test scores whereas recovery of function was identified as a significant change in the mean of the latent construct. Significant improvement in all four latent cognitive domains was observed, and each domain demonstrated a somewhat unique trajectory and extent of recovery.
Fig. 2. Improvement in cognitive functioning across the first year post-treatment entry in substance dependent individuals (n=197).
Panel (a) shows that the steepest increments in recovery in all domains occurred between treatment entry and the 6-week follow-up. Mean performance in the memory domain continued to significantly improve between the 6- and 26-week follow-ups. These improvements occurred after acute withdrawal and in the absence of intervention aimed at cognitive remediation. They did not reflect practice effects due to repeated exposure to the test materials (data not shown). Panels (b) and (c) show standard deviation error bars for executive and memory latent means. Note the considerable variability in latent ability across individuals at each follow up: individuals variously exhibited low or high stable levels of functioning across time, showed small, moderate or large increases in abilities, and even in some cases showed decreases in ability over time. Within-person improvement across 1 year following treatment may thus reflect improvements noted in cross-sectional studies of groups studied at different time points following cessation of drinking, or vary dramatically from this pattern.
Overall, these studies provide a starting point for understanding “natural” recovery processes following cessation of drinking. Yet, their ability to address the dynamics of the recovery process is limited because of differing sample characteristics, inconsistencies in temporal assessment time points in longitudinal designs, lack of knowledge about the cognitive deficits that predate alcohol use, and other considerations which hamper clinical application. Moreover, these studies and meta-studies capture recovery at the level of the group average rather than the individual. Note for example in Figure 2b and 2c, that while recovery is observable at the level of the mean, large individual differences in abilities exist and not all participants may demonstrate the same trajectory of recovery.
Although the AUD literature has been challenged by the same assessment and design issues that apply to the study of cognitive change over time in other clinical populations (Keith et al. 2002), the consistent observation that alcohol-related cognitive decline can resolve with time and abstinence suggests that, even after long-term heavy alcohol use, the brain is capable of recovery. Early neuroimaging studies suggested that structural brain abnormalities associated with chronic alcohol use can be, to some extent, stabilized or reversed with extended abstinence (for review see, Buhler and Mann 2011). The recovery of white matter, in particular, as length of abstinence increased, was recently supported in a meta-analysis (Monnig et al. 2012). Recent literature continues to extend these findings to better understand the spatial and temporal aspects of structural and functional recovery (Schulte et al. 2012; Buhler and Mann 2011). For example, recovery of some brain tissue and function is observed during the first few weeks of abstinence (Gazdzinski et al. 2005; Gazdzinski et al. 2010; Agartz et al. 2003; Durazzo et al. 2006; Bendszus et al. 2001; Parks et al. 2002; Bartsch et al. 2007) and other recovery becomes more evident over the subsequent months (Gazdzinski et al. 2005; Wobrock et al. 2009; Agartz et al. 2003; Colrain et al. 2012; Parks et al. 2002). Neural recovery also varies across brain structure (Cardenas et al. 2007; Parks et al. 2002; Gazdzinski et al. 2008). This body of research continues to grow and, in parallel with advances in neuroimaging and other strategies for gauging neural health, will likely address many currently unanswered questions about the reversible versus persistent neural effects of chronic heavy alcohol use.
A key focus of much current neuropsychological research is the loss of cognitive and behavioral flexibility that is observed in relation to chronic alcohol drinking and how the loss of this flexibility mirrors the loss of neural plasticity. Integrating current knowledge of time-dependent recovery on the cognitive and neural levels is no small task. Temporal inconsistencies in how chronic, heavy alcohol use affect neuropsychological functioning and neurological impairments have been suggested (Dao-Castellana et al. 1998). Significant neurological alterations may occur well before detectable clinically impaired neuropsychological functioning. Patients with AUD sometimes display comparable levels of neuropsychological, including executive, functioning in the laboratory, but have differential neural activation patterns compared to controls (Pfefferbaum et al. 2001). Untangling how neurological changes participate in behavioral dysfunction is complicated by the possibility that new brain areas may be recruited or differential cognitive strategies may be employed during task performance to compensate for neural damage elsewhere (Dao-Castellana et al. 1998; Fama et al. 2004; Chanraud et al. 2013). Compensatory neural processing is a strong current direction in the AUD field. A compensatory pattern of resting state connectivity in long-term abstinent alcoholics compared to controls was observed in Camchong et al. (2012). In addition, Chanraud et al. observed recruitment of alternative fronto-cerebellar functional pathways in recovering alcoholics who performed a working memory task at the level of controls (Chanraud et al. 2011) and demonstrated that this activational pattern fulfilled operational criteria for functional compensation (Chanraud et al. 2013). This suggests a role for neuroadaptive processes in the maintenance of normal cognitive performance.
The Relationship of Cognitive Impairment to AUD Treatment Outcome
That cognitive impairment should interfere with successful AUD treatment outcome appears to be self-evident. It is easy to see how memory problems, limited ability to communicate and understand ideas, difficulty in efficiently weighing competing information, and other cognitive problems would be serious challenges to persons of all ages attempting to interrupt well-established drinking patterns, and to accomplish the major life changes that this attempt often involves. Yet, the results of decades of research continue to suggest that the ways in which cognitive problems interfere with addiction treatment outcome, and behavioral flexibility more generally, are not simple or direct. Rather, their obtuse influence appears to be pervasive, potentially casting a wide net over phenomena as diverse as the relative valuation of present and future events (Amlung et al. 2012), the ascendance of automatic regulatory mechanisms (Houben et al. 2011), and the pursuit of unconscious goals (Levine et al. 2011).
It is likely that many of our previous research questions were phrased too simply and our methods took too little account of neurocognitive and behavioral adaptation processes. For example, neurocognitive deficits in persons with AUD are sometimes not evident at the behavioral level (Fein et al. 2009). As noted earlier, neuroimaging studies suggest that persons with an AUD may rely on different brain regions and circuits than healthy controls to maintain behavioral performance in neuropsychological and cognitive tasks (e.g., Chanraud et al. 2013). Similarly, the effects of cognitive deficit on treatment outcome may be masked by impaired and unimpaired persons relying more heavily on different therapeutic change processes (e.g., Bates et al. 2006). As elaborated in the following sections, new research suggests that cognition may be most relevant to behavior change within a cognitive-emotional scaffold of affect, intention, physiology, and the social environment.
Much previous research on cognitive impairments in individuals with AUD examined the direct effect of impairment on drinking outcomes without considering other pathways of influence. Literatures regarding the psychosocial impact of brain injury of other etiologies suggest that the effects of cognitive impairment are often indirect (mediated by other factors) and/or may moderate the influence of other intrapersonal or contextual factors. For example, impairment may affect psychosocial outcomes through its ability to change the salience of environmental features as well as the person’s emotional and motivational responses (e.g., Prigatano 1987; Prigatano et al. 1996; Ylvisaker and Feeney 1998). These concomitants of brain damage and cognitive impairment are relevant to AUD treatment strategies in view of the fact that motivation to change behavior, perceptions of self efficacy, the ability to form therapeutic alliance, and the use of social support to reinforce treatment goals are believed to be key therapeutic processes in many AUD treatments (Buckman et al. 2007; DiClemente 2007; Hartzler et al. 2011). Therefore, the use of a mediator–moderator variable distinction (Baron and Kenny 1986; MacKinnon et al. 2002) within the context of alternative conceptual models of brain–behavior relations may useful for understanding how cognitive impairments disrupt positive outcomes in individuals with AUD (reviewed in Bates et al. 2002a).
Mediation Models
One way in which cognitive impairment may impede positive addiction treatment outcome is by interrupting treatment processes which in turn mediate the influence of impairment on treatment outcomes (Bates et al. 2002a). Support for the notion that the influence of cognitive impairments on treatment outcome is indirect through treatment processes comes from multiple lines of research. First, early studies showed patients who were cognitively impaired remembered less treatment relevant information (Becker and Jaffe 1984; Sanchez-Craig and Walker 1982; Teichner et al. 2002; Godding et al. 1992) and learned fewer drink refusal skills (Smith and McCrady 1991) than unimpaired clients. In addition, some evidence suggested that cognitively-impaired individuals entering treatment for AUD may be viewed by treatment providers as being more inattentive and having lower motivation and greater denial compared to unimpaired clients (Goldman 1995), potentially compromising the treatment process itself. Impairment of executive functions, verbal memory, and mental speed were associated with “denial-related” treatment goals that were not achieved by alcohol dependent men and women who received inpatient treatment (Rinn et al. 2002). Moreover, poorer executive function and memory were associated with lower readiness to change drinking behaviour in a treatment sample (Le Berre et al. 2012), although there were also null findings (e.g., Bates et al. 2006). Even outside of treatment, cognitive dysfunction may impact motivation to change drinking behavior, although the relation of different cognitive functions to readiness to change may vary in treatment samples compared to heavy drinking, non-treatment seeking samples (Blume and Marlatt 2009; Blume et al. 2005). This further suggests that there may be multiple indirect pathways by which cognitive impairment can affect change processes in and outside of treatment, which in turn can reduce the likelihood of positive longer-term drinking outcomes.
Interestingly, there are surprisingly few studies that have addressed both aspects of mediation: namely, that cognitive impairment results in reduced learning, skill development, or motivation during treatment, and that this alteration in the treatment process results in poorer drinking outcomes. A recent study did provide support that cognitive impairment indirectly influences substance use outcomes by reducing the quality of coping skills acquired in CBT (Kiluk et al. 2011). Mediated relations also have been demonstrated, wherein impairment led to less treatment compliance and lower self-efficacy, but greater Alcoholics Anonymous Involvement (AAI), all of which, in turn, more proximally predicted drinking (Bates et al. 2006). Given that heightened treatment compliance, self-efficacy, and AAI are all consistent, positive predictors of AUD treatment outcome, it becomes clear that cognitive impairment can have countervailing effects on treatment processes that may cancel out in analyzing a direct effect of impairment on outcome. Thus while impairment appears to disrupt treatment processes such as compliance, coping skill training, and self efficacy, it may also increase behaviors, such as seeking social support for abstinence, that might be used to bolster treatment efficacy in impaired clients (Bates et al. 2006; Donovan et al. 2001).
Moderation Models
In addition to indirect or mediation models, cognitive deficits may serve to moderate the influence of treatment processes by interrupting or enhancing their influence on treatment outcomes. An example that has been replicated in multiple treatment samples is that cognitive impairment interacts with self-efficacy to resist drinking urges in affecting drinking behavior following treatment (Morgenstern and Bates 1999; Bates et al. 2006). The nature of the interaction was that impairment dissociated the positive relationship between self-efficacy and drinking outcomes that is typically found in unimpaired clients, such that increased self-efficacy was not as strong a prognostic indicator of positive outcomes in patients with clinically significant cognitive impairment. Cognitive impairment was also found to increase the influence of the social network on the drinking outcomes of persons receiving AUD treatment (Buckman et al. 2007; Buckman et al. 2008), although the nature of influence varied across samples. In the outpatient sample of Project MATCH, which presented with relatively less alcohol problem severity, a social network that supported sobriety was more predictive of positive drinking outcomes in cognitively impaired patients relative to unimpaired patients; conversely, in the relatively higher alcohol problem severity aftercare sample, a network that supported drinking was associated with more negative drinking outcomes in cognitively impaired versus unimpaired patients (Buckman et al. 2007). Moderation studies thus support the idea that the most salient mechanisms of behavior change may be different in persons with and without cognitive impairment. These results point to the substantial adaptability of humans, wherein different processes may be used to compensate for disruption caused by cognitive insult. It also appears likely that the nature of the disruption caused by cognitive impairment may be different depending on sample characteristics and the specific process factor examined.
A New Look at Direct Effects
Finally, as the field moves beyond classic neuropsychological assessments of impairment, newer cognitive behavioral tasks may uncover domains of deficits that are more directly related to behavioral treatment outcomes. For example, Carroll and colleagues (2011) recently found that performance on tests of IQ, motor speed, and attention did not directly affect outcome in a computer-assisted cognitive behavioural therapy (CBT), yet higher levels of risk taking on the Balloon Analogue Risk Task predicted lower treatment attendance and homework completion and poorer substance use outcomes. The predictive utility of performance on the risk taking task may suggest that certain inhibitory deficits, such as those also found in detoxified persons with AUD using the Hayling task (Noel et al. 2001), may be more directly associated with relapse, whereas other aspects of executive function are more likely to indirectly influence drinking outcomes through disruption of treatment processes. In the other drug literature, impaired affective decision making assessed using gambling tasks varied between opiate-dependent patients who maintained short term abstinence compared to those who relapsed (Passetti et al. 2008), pointing to the potential need to further examine the intersection of affect regulation and cognition in AUD. Additional support for the this link comes from neuroimaging data that shows that abstinent long-term alcoholics exhibit significantly less activation in the amygdala to emotionally-valenced facial expression stimuli compared to normal controls and impaired differentiation between negative and positive cues, at least under some conditions (Marinkovic et al. 2009; Gilman and Hommer 2008).
In summary, although there is a rudimentary understanding of several ways in which cognitive impairment can alter treatment engagement and process, this question is complicated by the range of sample characteristics, treatment modalities, and conceptual frameworks of treatment processes that have been studied. Perhaps more importantly, brain and cognitive status is not static following reduced alcohol use. Thus, the timing of treatment process assessments and cognitive recovery rates are conflated in longitudinal studies. Ultimately, there are unavoidable limitations in trying to link cognitive impairment and recovery to treatment process and outcome within treatments and research approaches that were not designed to directly affect those cognitive functions. A new generation of research aimed at therapeutically intervening to facilitate cognitive recovery, build cognitive strengths, or compensate for cognitive deficits may be more telling.
Facilitating recovery and relations to treatment outcomes
Evidence for improvements in cognitive functioning in many, but not all, individuals with alcohol-associated neurocognitive impairment suggests alternative trajectories of recovery as well as the well known individual differences in severity of impairment. We previously distinguished between time-limited impairment or deficits that appear to be direct or indirect consequences of heavy chronic alcohol use, which resolve with abstinence and improved health, versus persistent impairment that fails to resolve and, in fact, may predate alcohol consumption (Bates et al. 2002a). Treatment providers seldom have information regarding premorbid cognitive status to predict whether cognitive impairment is likely to be time-limited or persistent. Further, it is unknown in this population whether rehabilitation approaches designed to restore cognitive functioning or approaches that aim instead to facilitate compensatory strategies would be more effective in the face of impairments that may be mild or severe, time-limited or persistent impairment. Most of the AUD studies discussed below have focused on restorative rehabilitation. Compensatory training and aids also have been shown to improve cognition and memory in other populations with mild cognitive impairment (Fish et al. 2010) and thus merits further study in the AUD population.
Over 20 years ago, Goldman and colleagues published a series of studies suggesting that the practice of cognitive tasks enhances basic cognitive skills and facilitates performance on different cognitive measures among individuals recovering from AUD (e.g., Forsberg and Goldman 1987; Goldman and Goldman 1988; Roehrich and Goldman 1993; Stringer and Goldman 1988). Although their findings suggested that cognitive remediation (i.e., experience dependent recovery, Goldman 1990) could be a valuable component of treatment for AUD by helping patients to grasp treatment concepts more readily while simultaneously improving cognitive functioning in other areas, it was not until recently that empirical investigations began to follow up on these pioneering studies.
Several current approaches to cognitive rehabilitation in the addictions field have been framed within a number of dual process conceptual models of information processing that vary in respect to conceptual underpinnings, yet consistently distinguish between effortful executive control and automatic processing operations. This distinction is usually described as a conflict between processes that support conscious goals and intentions (that has variably been termed “reflective”, “executive”, “cool”, “frontal”, or “cortical”) and those processes that derive from attention capture by salient cues in the environment and impulsive or habitual modes of affective regulation (referred to as “impulsive”, “automatic”, “hot”, or “limbic”). With respect to control of alcohol use behaviors, the premise is that strengthening executive functions will increase an individual’s ability to override the impulsive use of alcohol. It is believed that alcohol use among dependent individuals is often completed automatically in response to environmental cues and affective states that are bound to drinking through associative learning processes, and thus the individual often is unaware of the internal and environmental triggers that instigate relapse and motivational failures.
Interventions to improve neurocognitive functioning
The importance of executive functions, and especially working memory, for maintaining and manipulating conscious, goal-relevant information (Houben et al. 2011) has inspired the application of several rehabilitation and training approaches to improve these functions. For example, seven weeks of Goal Management Training (GMT), a validated intervention for executive function impairment (Levine et al. 2011), combined with mindfulness-based mediation, which also has been associated with cognitive enhancement (Chiesa et al. 2011), produced improved working memory, response inhibition and decision making of outpatients with alcohol and drug problems compared to psychotherapy treatment as usual (Alfonso et al. 2011) (N=34). Bickel and colleagues (2011) (N=27) explored the functional relations between working memory and delay discounting in patients receiving treatment for stimulant use using a four-module computerized memory training program that was completed over a variable number of sessions (4 to 15) depending upon when patients’ performance reached an asymptote. Although an independent test of working memory did not improve significantly pre- to post-memory training, patients who received memory training with performance-contingent monetary rewards showed improved delay discounting performance compared to yoked controls. Control patients had completed a training condition wherein correct answers were cued and monetary reinforcement was not contingent on performance. Persons with alcohol and drug use disorders often have high levels of delay discounting reflecting their relatively greater devaluation of rewards as a function of length of time or delay until its receipt, compared to persons without disorders (Lejuez et al. 2010). Thus, this preliminary finding highlights the potential link between working memory capacity and improved future orientation and suggests one means by which behavioral flexibility to resist present-oriented urges to use alcohol and drugs for emotional modulation may be augmented. Additional research is needed, however, to determine if positive changes in delay discounting persist over time, and whether reductions in delay discounting translate into improved treatment outcomes.
A recent randomized controlled trial (N=41) compared intensive outpatient CBT focused treatment alone versus CBT supplemented with cognitive rehabilitation using a computer-assisted training program that comprised 62 exercises with alternate versions (Rupp et al. 2012). Training modules were selected to minimize overlap with cognitive outcome measures and included attention, executive function, and memory. Cognitive rehabilitation consisted of 12 individual sessions completed at the rate of three per week for four weeks. Some sessions focused on the individual’s impaired areas of functioning and some could be selected by the individual to enhance motivation. This intervention resulted in a significant, medium effect size increment in alertness, divided attention and working memory, delayed recall, visual-spatial construction, and a global measure of cognitive functioning, compared to the treatment-as-usual control. The cognitive rehabilitation group also showed greater, medium effect size decreases in psychological distress, psychological symptoms and craving from beginning to end of treatment compared to the control. Note that patients in this study were selected to have at least mild cognitive impairment and to be relatively free from co-occurring conditions. For patients with serious co-occurring psychiatric disorders, preliminary evidence suggests that five sessions of cognitive training over three weeks may also be effective in enhancing attention and cognitive flexibility compared to an attention placebo control during the sub-acute phase of detoxification (Goldstein et al. 2005).
Not all cognitive enhancement approaches have been successful with AUD populations. Griffiths and colleagues (2012) evaluated an imagery intervention to improve encoding in prospective memory, that is, the memory processes needed to carry out intended actions at an appropriate time in the future (Ellis 1996). The significance of prospective memory in brain injury, geriatric, and poly-substance use is gaining prominence (Groot et al. 2002; Zoellig et al. 2007; Rendell et al. 2007; Cuttler et al. 2012), and initial research suggests it is also impaired by both acute and chronic alcohol use (Heffernan et al. 2002; Leitz et al. 2009; Montgomery et al. 2011; Griffiths et al. 2012). In Griffiths et al. (2012) the alcohol dependent group, recruited from a residential service 7–10 days following medically assisted withdrawal, had prospective memory deficits compared to controls. The extent of deficit varied with disorder severity and weekly quantity of alcohol previously consumed. The imagery intervention improved prospective memory in the control group, but not the AUD group. The authors attributed the lack of benefit in the alcohol group to difficulties in forming or applying effective strategies. It also may be useful to examine rehabilitation of executive aspects of prospective memory such as cue monitoring (Fish et al. 2010), which are often impaired in AUD populations.
Only a few studies in recent years have evaluated whether interventions that improve neurocognitive functioning also improve alcohol outcomes in non-treatment seeking and treatment seeking populations. In one study, harmful problem drinkers, as defined by a standardized screening measure, were recruited via the internet to complete either 25 weekly sessions of three working memory training tasks or control tasks (Houben et al. 2011) (N=48). The difficulty level of the training tasks was adaptively adjusted to increase over time dependent on performance, whereas the control tasks remained at an easy level throughout the study. Working memory training significantly improved working memory and this improvement persisted at a 1-month follow-up. The active training group also showed an approximate 10 drink per week reduction in alcohol consumption by the end of training, with a trend for reduced drinking at follow-up. Mediation analyses suggested that there was an indirect effect of memory training through working memory capacity on drinking behavior, and the strength of this relationship depended on the strength of participants’ automatic impulses to consume alcohol. Working memory training affected greater reductions in alcohol drinking among those with strong automatic impulses to consume alcohol. If these results generalize to AUD treatment samples, it may be possible to develop a treatment component that builds resistance to urges to use alcohol by improving working memory.
At the opposite end of the severity continuum, preliminary results from an intensive psychosocial approach designed to accommodate severe levels of alcohol-related brain damage was reported from the UK (Wilson et al. 2012). Forty-one patients were referred from an acute general hospital inpatient setting to a five-phase community rehabilitation program: stabilization, assessment, therapeutic rehabilitation, adaptive rehabilitation, and social integration/relapse prevention. This is an intensive program, with the therapeutic rehabilitation phase based on the milieu-based approach summarized in Bates et al. (2002a) and augmented with cognitive enhancement training lasting up to 2 or more years. The final two phases focus on compensatory strategies for cognitive impairment, psychological and social support, and relapse prevention. The authors’ preliminary report showed an 85% reduction in the use of acute medical/surgical inpatient bed days per patient per year compared to the 5 years prior to referral. This approach to rehabilitation is suitable for individuals with severe alcohol-related brain damage including Korsakoff Syndrome, with the aim of rehabilitation and increasing independence of the individual to avoid costly and long-term institutionalizations. This inclusive program for severely impaired patients is clearly time and cost intensive, and would need substantial community or institutional support to operate. Nonetheless, it speaks to the possibility of augmenting cognitive and behavioral recovery among even the most severely impaired alcohol dependent persons through cognitively-mediated treatment strategies.
In summary, cognitive rehabilitation approaches seek to improve executive functions, memory and other cognitive abilities, and thereby enhance positive behavioral outcomes such as abstinence, reduced drinking, improved interpersonal relations, employment options and other life skills. Compensatory training has received less attention, except with severely impaired AUD patients (Wilson et al. 2012). However, given its success in other impaired groups, it requires further study in addiction populations with mild and severe impairments before conclusions can be drawn about its efficacy for time-limited and persistent impairment.
While this work is still in its infancy, the preliminary evidence described above supports the ability of cognitive interventions to enhance cognitive abilities, and points to the potential relationships of increased working memory and other cognitive skills to becoming more future oriented, experiencing reduced craving and psychological distress, and reducing alcohol drinking. Although replication of results in larger clinical trials is needed, this emerging literature, as well as advances in the cognitive rehabilitation field outside the area of addictions, support optimism that these and other interventions to enhance cognitive recovery, or to build new cognitive skills for the first time, will prove useful for increasing the efficacy of AUD treatments for persons who experience mild to severe cognitive impairment.
In order for this to best happen, future research needs to carefully consider choices regarding the use of control groups, measurement strategies, and treatment modalities (Kennedy and Turkstra 2006). In the area of addiction treatment, additional questions include how cognitive rehabilitation, compensation, or retraining approaches are best integrated into current AUD treatments for which there is already empirical support for efficacy, how improvements in specific cognitive domains are conceptualized mechanistically with respect to other hypothesized active elements of treatment, whether cognitive enhancement should be offered universally or only to those with some threshold level of cognitive impairment, and whether benefits of cognitive intervention are moderated by other person and environmental characteristics of the client. It is also of note that studies of cognitive impairment and AUD treatment have defined outcome success primarily in terms of changes in drinking behaviors, consistent with the primary outcome measures in AUD treatment research as a whole (Babor et al. 1994). The traumatic brain injury literature suggests that psychosocial functioning, health, and employment outcomes also are important indicators of improved quality of life following cognitive rehabilitation (e.g., Salazar et al. 2000; Sarajuuri et al. 2005). Early studies found neuropsychological impairment at AUD treatment entry was negatively related to employment status (Donovan et al. 1984; Donovan et al. 1985). Donovan and colleagues’ review (2005) further suggests that persons with AUD reported lower levels of quality of life compared to general population and other clinical groups. Thus, future AUD and cognitive rehabilitation research would benefit from expanding AUD treatment outcome definitions to include everyday functions as well as alcohol use outcomes.
Alternative Approaches to Neurocognitive Rehabilitation for AUD
Currently, many empirically-supported AUD treatments (e.g., cognitive behavioral therapy, motivational enhancement, 12-step facilitation and support groups) rely heavily on executive functions, explicit memory, and other effortful cognitive processes, such as impulse control, that are vulnerable to acute and chronic alcohol intake effects and thus may be most strongly impaired in the early days of abstinence and treatment. On the other hand, procedural and implicit memory systems and automatic information processes are less vulnerable to acute and chronic neurotoxic effects of alcohol (Fillmore et al. 1999; Tracy and Bates 1999; Ray and Bates 2006; Ray et al. 2012; Hayes et al. 2012). Unfortunately, automatic information processes, such as attention capture and implicit memory associations to alcohol cues, often serve to undermine effortful treatment goals (Robinson and Berridge 1993). Thus, interventions for intact automatic processes may be aimed at modifying counterproductive automatic information processing, rather than restoring their function, per se.
Behavioral flexibility requires input and action from both the body and the brain and is accomplished through continual bidirectional communication between them. Most cognitive rehabilitation strategies reviewed in the previous section sought to increase behavioral flexibility by directly building executive cognitive skills at the level of the brain. Several new approaches seek to enhance behavioral flexibility using interventions that focus on changing automatic cognitive processes (e.g., Wiers et al. 2011a) and indirectly affecting neurocognition via the neurophysiological feedback loops that regulate the flow of communication between the body and brain (Bates and Buckman in press). Here we describe several promising examples of methods for increasing behavioral flexibility and neurocognitive functioning based on manipulating the afferent stream of information from the body to the brain. These include including retraining automatic action tendencies, heart rate variability biofeedback, meditation, aerobic exercise, and combined mental and physical training. These rehabilitation approaches make fewer demands on effortful executive functions to support behavioral flexibility and thus may be particularly useful with AUD populations, even those who demonstrate impairment of effortful cognition.
Retraining Automatic Action Tendencies
Within the conceptual framework of Strack and Deutsch’s (2004) dual system model of reflective and impulsive information processing, Wiers and colleagues (2010) (N=42) applied a method for retraining automatic action tendencies to approach alcohol in hazardous drinkers, and then examined this method in a 4-session (200 trials per session during 15 min), randomized controlled trial of 214 persons diagnosed with alcohol dependence prior to their inpatient treatment (Wiers et al. 2011a). Persons with notable neurocognitive impairment were excluded. A training version of the Approach Avoidance Test (AAT) was used wherein participants either primarily pushed (avoidance) or pulled (approach) a joy stick in response to pictures of alcohol or non-alcohol beverages depending on the picture format. The clinical trial involved additional control and sham training conditions. The pulling and pushing was accompanied by a zooming feature that made the picture increase in size consistent with approach, or decrease in size consistent with avoidance, respectively. In both studies, changes in reaction times indicated that that training was effective in altering automatic action tendencies towards alcohol; those trained to avoid alcohol became faster in pushing alcohol pictures away. This training transferred to untrained pictures and an implicit word task. In the first study, heavy drinkers who were successfully trained in the avoid alcohol condition subsequently drank less in a taste test drinking session than did heavy drinkers in the approach alcohol condition (Wiers et al. 2010). In the clinical trial, participants trained to avoid alcohol showed improved drinking outcomes 1 year later compared to those in other conditions, although changes in approach bias reaction times did not statistically mediate clinical outcomes (Wiers et al. 2011a).
While this method, termed cognitive-bias modification (Wiers et al. 2011a), appears successful in interrupting automatic approach tendencies toward alcohol stimuli, important questions remain about its mechanism(s) of action. In particular, the role of arm movement and visual feedback (zooming) in affecting alcohol-avoid versus alcohol-approach associations merits further study because it raises intriguing questions about the use of peripheral functions to modify cognitive and affective information processing. For example, substantial psychological literatures support the premise that arm muscle flexion is associated with approach action tendencies that bring positive or desired stimuli closer to the self, whereas muscle extension is associated with avoidance action tendencies which push fearful or undesired stimuli away from the self (Strack and Deutsch 2004). Thus, the bi-directionality of neuromuscular communication provides a feedback loop wherein repeated pairing of a stimulus with an arm muscle extension may, with practice, interrupt a previously established automatic approach action response to that stimulus. Physiologically, this parallels what is known about muscle-brain communication wherein the brain coordinates the activity of all muscle fibers within and across muscle groups via cholinergic neuromuscular junctions (Ruff 2011) and muscle spindles relay information about the state of the muscle to the brain (Windhorst 2007). Further research on the neural circuitry and processes that integrate neuromuscular communication with cognition and affect may provide additional avenues for treatment development to increase behavioral flexibility by uncoupling established automatic cognitive and behavioral processes that support unhealthy alcohol use despite conscious intentions to change drinking behavior.
Heart rate variability (HRV) biofeedback
Another approach to influencing the brain via a peripheral feedback system is to intervene using a non-invasive biofeedback approach referred to as HRV biofeedback. This technique enhances neurocardiac signaling, improving communication between cardiovascular and neural control systems. Ineffective neurocardiac signaling increases stress reactivity as well as enhances attention capture by appetitive cues and may increase their salience by prolonging visceral reactivity (Bates and Buckman in press). HRV biofeedback involves learning to breath at a rate that synchronizes the cardiac oscillations being driven by respiratory sinus arrhythmia (i.e., the phenomena of heart acceleration with inhalation and deceleration with exhalation) with the cardiac oscillations driven by the baroreflex (i.e., the mechanism that links heart acceleration/deceleration to corresponding decreases/increases in blood pressure). When breathing rate is slowed to approximately 6 times per minute (compared to the natural breathing rate in the range of 12–20 breaths per minute), a dramatic increase in cardiac oscillations at 0.1 Hz occurs. This resonance response is thought to be therapeutic because it trains the feedback loop between the heart and brain, increasing baroreflex sensitivity and vagal modulation of neurocardiac response (Lehrer et al. 2003). That is, it activates afferent paths to the brain that set into motion a cascade of neural events that feedback information about bodily state to the central autonomic network (Benarroch 1997), a brain network that relays information between the frontal lobes, amygdala, and brainstem, as well as other structures. As such, it provides a window into the bidirectional information streams between the body and the brain that support adaptive responding and integrated mind-body responses to daily, as well as extreme, cognitive and emotional challenges.
HRV biofeedback improves symptoms of asthma (Lehrer et al. 2004), major depression (Karavidas et al. 2007), fibromyalgia (Hassett et al. 2007), neurosis (Chernigovskaia et al. 1990), post traumatic stress disorder (Zucker et al. 2009), and hypertension (McCraty et al. 2003). Individual differences in HRV are related to performance on executive function tasks and to brain activity in the prefrontal cortex (Thayer et al. 2009). Acute improvement in reaction time and consistency in cognitive performance (Prinsloo et al. 2011), and reductions in performance anxiety (Wells et al. 2012) have been reported even after a brief session of HRV biofeedback. It is now being explored as an intervention to interrupt maladaptive alcohol use behaviors and as an adjunct to cognitive behavior therapy in women seeking treatment for an AUD.
Mental and Physical (MAP) Training
In addition to the use of mental, neuromuscular, and neurocardiac training to affect cognitive change, it is now well established from the animal and human literatures that aerobic physical training also can improve cognitive functioning. One particularly intriguing application to cognitive remediation in AUD is what has been termed mental and physical (MAP) training that is thought to enhance cognition by directly supporting neurogenesis in the hippocampus (Curlik and Shors 2013). Data from animal studies indicate that physical (aerobic) exercise increased the production of new neurons in the brain (van Praag et al. 1999). However, most of these cells die unless the animal engages in some type of effortful learning, or mental training (Curlik and Shors 2013). The learning process itself must be achievable and the task should be challenging and new. Some of the important characteristics of the mental training are the need for sustained effort or concentration, many trials of practice, and difficulty in obtaining mastery. A key strength of this intervention strategy is that it provides a strong neuroscientific basis for one mechanistic route through which combined physical and mental training may enhance cognitive functioning in humans.
To model these training procedures in humans, Shors and colleagues are currently conducting a pilot study that combines aerobic exercise (physical training) with breathing meditation (mental training) two times per week for 8 weeks in emerging adults who are depressed and/or engage in hazardous drinking. This approach has the advantage of employing two intervention strategies that have individually received support for both cognitive enhancement and substance use treatment outcomes and are thus of unique interest to cognitive remediation in persons with AUD. More specifically, physical exercise enhances executive functions and effortful information processes in unimpaired children and adults as well as elderly persons with cognitive impairments ranging from mild to severe (see meta-analyses by Colcombe and Kramer 2003; Heyn et al. 2004; Sibley and Etnier 2003). The animal literature shows that exercise increases neurogenesis, which can facilitate cognitive flexibility (Burghardt et al. 2012; Shors et al. 2012). Exercise programs show promise for facilitating recovery in AUD populations (Brown et al. 2009). Similarly, several existing treatments for alcohol and other drug use disorders include meditative practices, and a recent review (Chiesa and Serretti 2010) suggested that mindfulness meditation is associated with activation of the prefrontal and anterior cingulate cortices and, over time, may enhance activity in brain areas that support attention. Their review of the effects of mindfulness meditation on cognition concluded that existing evidence supports the likelihood that this intervention is associated with improvements in selective and sustained attention, working memory and other executive functions (Chiesa et al. 2011), although more well-designed research with standardized meditation programs is needed to fully understand meditation effects on cognitive enhancement. Preliminary studies of mindfulness mediation as an intervention for substance use disorder suggest efficacy, but more research is needed in this area also to standardize administration and identify active elements of this intervention (Zgierska et al. 2009). Beyond the potential for cognitive enhancement, these interventions may promote stress resilience and increased emotional modulation, important relapse triggers in AUD.
In addition to neurogenesis and rescue of new neurons as one active element of MAP training, it is generally established that aerobic exercise increases baroreflex sensitivity and vagal modulation of neurocardiac response (Sandercock et al. 2005). In addition, slow breathing frequencies used in various types of meditation are at approximately 0.1 Hz (6 respiratory cycles/min) (Cysarz and Bussing 2005; Peng et al. 1999), a rate that causes the greatest modulation of heart rate by respiration (Keyl et al. 2000) achieved in HRV biofeedback. Thus, improved neurocardiac signaling may be another mechanism of action in the positive cognitive effects of aerobic exercise and meditation as well.
Conclusions
The majority of persons entering AUD treatment suffer from mild to severe cognitive impairments. Findings from a large number of studies have been sufficiently consistent across different neurocognitive assessment approaches, methods to control practice effects, and sample characteristics to conclude that many of them will show at least partial recovery from alcohol-related cognitive impairment over time when they stop or greatly reduce drinking alcohol. There may be untapped clinical value in treatment providers being able to give patients information about areas of cognitive impairment, the kind of life difficulties it is contributing to, the odds of meaningful gains in cognitive ability if they stop drinking, and how improved cognition would have downstream effects on improved emotional regulation and interpersonal relations. For example, questions concerning long-term employment options could be more adequately addressed. Nonetheless, precise knowledge about the threshold of neurocognitive impairments needed before complex behavioral patterns and outcomes are substantively affected remains limited, and understanding the role of cognitive recovery in AUD treatment process and drinking outcome has not proved straightforward. The addiction recovery literature is limited by the same considerations that apply more broadly to the questions of quantifying neuropsychological deficits and recovery across a broad range of disorders and environmental perturbations ranging from neurotoxic insult and traumatic brain injury to cardiopulmonary bypass surgery (Keith et al. 2002). More studies employing multiple time point assessments are needed to accurately capture the length of abstinence necessary to initiate recovery and tease apart the many influential malleable and static factors that impact recovery trajectories, which thus far appear to include age, education, gender, childhood behavior disorders, family history of alcoholism, duration of use and abstinence, medical complications from drinking, prior medically-supervised detoxifications, depression, social support, polydrug use, smoking and genotype (Moriyama et al. 2006; Bates et al. 2002b; Pitel et al. 2007a; Bates et al. 2004; Kalman et al. 2010; Bates et al. 2005; Levy et al. 2012; Buckman et al. 2008; Fein et al. 2006; Fein and McGillivray 2007; Rosenbloom et al. 2004; Loeber et al. 2010; Duka et al. 2003; Oscar-Berman et al. 2004; Gazdzinski et al. 2008; Mon et al. 2009; Durazzo et al. 2006; Mon et al. 2012). Studies that characterize trajectories of intra-individual changes in cognitive impairment over time are needed to move the field beyond understanding recovery at the level of the group, and instead capture change at the level of the individual. In doing so, cognitive impairment at treatment entry and prospects for recovery can be more directly used for clinical decision making (Chelune 2002).
Research conducted within treatments not designed to affect cognitive improvements suggests that cognitive impairment is importantly involved in mediated and moderated relations with treatment processes and other personal and environmental factors that affect outcomes. Yet, few studies over the past 30 years have addressed the question of whether methods to enhance the rate or nature of cognitive recovery also improve AUD treatment efficacy. In addition to the “naturally occurring” improvements in cognition that occur with abstinence, findings from recent studies suggest that cognitive rehabilitation and enhancement methods are promising strategies for facilitating neurocognitive recovery (or perhaps building new cognitive skills for the first time) and, in some cases, enhancing positive drinking outcomes. Studies designed to actively intervene in neurocognitive problems are better positioned to answer questions about how neurocognitive difficulties and improvement affect treatment process and outcome due to direct experimental manipulation of the construct of interest. While encouraging, findings in this area are preliminary and require replication. Progress will be hampered if expense and logistic difficulties involved in studying within-person changes in neurocognition, in adequately powered samples over sufficient periods of time, prevent larger clinical trials. Time-extended trials are needed to answer questions such as whether cognitive enhancement or rehabilitation interventions should be offered unilaterally, or only to those with a certain level of cognitive impairment; how long positive neurocognitive changes last, and what type of booster sessions might be needed to sustain or build on gains; and whether there are interactions with the primary treatment modality. Questions of the ecological validity of cognitive rehabilitation and enhancement techniques also need to be answered.
Current neuroscience, neurophysiology, and behavioral research suggests there may be value to AUD treatment development in exploring a broad range of approaches to neurocognitive rehabilitation that includes, for example, training in working memory and other executive and memory functions that are often impaired in AUD populations, as well as approaches that seek to alter cognitive biases that drive unconsciously-mediated behaviors that are believed to increase the likelihood of relapse. Further, as mechanisms of mind-body integration and afferent streams of influence on the brain become better understood, there is the potential for new, non-pharmacological behavioral interventions that affect neurocognition by manipulating peripheral systems. If such interventions are effective, web and smart phone applications could make these interventions available to large numbers of persons. Finally, continuing progress in understanding the neuroscientific underpinnings of broad health promoting interventions such as exercise and meditation may lead to novel combinations of mental and physical training that support brain health, and improved cognition and affective regulation in persons with AUD as well as those with co-occurring affective disorders.
Acknowledgments
This review was supported in part by the National Institute of Alcohol Abuse and Alcoholism through HHSN275201000003C, R01 AA015248, ARRA Administrative Supplement to R01 AA015248, K02 AA00325, and K01 AA017473.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, et al. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol Alcohol. 2003;38(1):71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-Garcia A. Combined goal management training and mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug and Alcohol Dependence. 2011;117(1):78–81. doi: 10.1016/j.drugalcdep.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcoholism, Clinical and Experimental Research. 2001;25(2):185–191. [PubMed] [Google Scholar]
- Amlung M, Sweet LH, Acker J, Brown CL, Mackillop J. Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict Biol. 2012 doi: 10.1111/adb.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Ramo DE, Cummins KM, Brown SA. Alcohol and drug involvement after adolescent treatment and functioning during emerging adulthood. Drug and Alcohol Dependence. 2010;107(2–3):171–181. doi: 10.1016/j.drugalcdep.2009.10.005. doi: http://dx.doi.org/10.1016/j.drugalcdep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ML, Nokia MS, Govindaraju KP, Shors TJ. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neuroscience. 2012;224:202–209. doi: 10.1016/j.neuroscience.2012.08.018. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Longabaugh R, Zweben A, Fuller RK, Stout RL, Anton RF, et al. Issues in the definition and measurement of drinking outcomes in alcoholism treatment research. Journal of Studies on Alcohol Supplement. 1994;12:101–111. doi: 10.15288/jsas.1994.s12.101. [DOI] [PubMed] [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 1999;91(2):429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartels C, Kunert HJ, Stawicki S, Kroner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol and Alcoholism. 2007;42(2):92–102. doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130(Pt 1):36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Bates ME. Stability of neuropsychological assessments early in alcoholism treatment. Journal of Studies on Alcohol. 1997;58:617–621. doi: 10.15288/jsa.1997.58.617. [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie EW, Fals-Stewart W, Voelbel G, Buckman JF. Risk factors and neuropsychological recovery in clients with alcohol use disorders who were exposed to different treatments. J Consult Clin Psychol. 2004;72(6):1073–1080. doi: 10.1037/0022-006X.72.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Experimental and Clinical Psychopharmacology. 2002a;10(3):193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Alcohol-related dementia. In: Publishing C, editor. Other Dementias. Delray Beach: FL: Geldmacher, D; 2008. pp. 123–145. [Google Scholar]
- Bates ME, Buckman JF. Integrating body and brain systems in addiction neuroscience. In: Miller P, editor. Encyclopedia of Addictive Behaviors. Elsevier; (in press). [Google Scholar]
- Bates ME, Convit A. Neuropsychology and neuroimaging of alcohol and illicit drug abuse. In: Calev A, editor. Assessment of Neuropsychological Functions in Psychiatric Disorders. Washington, DC: American Psychiatric Press; 1999. pp. 373–445. [Google Scholar]
- Bates ME, Labouvie EW, Voelbel GT. Individual differences in latent neuropsychological abilities at addictions treatment entry. Psychology of Addictive Behaviors. 2002b;16(1):35–46. doi: 10.1037//0893-164x.16.1.35. [DOI] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of Addictive Behaviors. 2006;20:241–253. doi: 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcoholism, Clinical and Experimental Research. 2005;29(3):367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug and Alcohol Dependence. 1995;37(3):247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Becker JT, Jaffe JH. Impaired memory for treatment-relevant information in inpatient men alcoholics. Journal of Studies on Alcohol. 1984;45(4):339–343. doi: 10.15288/jsa.1984.45.339. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network. In: Low PA, editor. Clinical Autonomic Disorders. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997. pp. 17–23. [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. AJNR Am J Neuroradiol. 2001;22(10):1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. The American Journal of Psychiatry. 2003;160(11):2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: what we know and what we need to know. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2009;37(2):117–125. doi: 10.1007/s12160-009-9093-8. [doi]. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Memory, executive cognitive function, and readiness to change drinking behavior. Addictive Behaviors. 2005;30(2):301–314. doi: 10.1016/j.addbeh.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33(2):220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism-Clinical and Experimental Research. 2000;24(2):164–171. [Article; Proceedings Paper]. [PubMed] [Google Scholar]
- Buckman JF, Bates ME, Cisler RA. Social networks and their influence on drinking behaviors: differences related to cognitive impairment in clients receiving alcoholism treatment. Journal of Studies on Alcohol and Drugs. 2007;68(5):738–747. doi: 10.15288/jsad.2007.68.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, Bates ME, Morgenstern J. Social support and cognitive impairment in clients receiving treatment for alcohol- and drug-use disorders: a replication study. Journal of Studies on Alcohol and Drugs. 2008;69(5):738–746. doi: 10.15288/jsad.2008.69.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the Human Brain: A Systematic Review of Different Neuroimaging Methods. Alcoholism-Clinical and Experimental Research. 2011;35(10):1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [Review]. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22(9):1795–1808. doi: 10.1002/hipo.22013. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40(3):194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-State Synchrony in Long-Term Abstinent Alcoholics. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138(2):115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, et al. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse. 2011;46(1):23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex. 2013;23(1):97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral Cortex. 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ. Making neuropsychological outcomes research consumer friendly: a commentary on Keith et al. (2002) Neuropsychology. 2002;16(3):422–425. doi: 10.1037//0894-4105.16.3.422. [DOI] [PubMed] [Google Scholar]
- Chernigovskaia NV, Vaschillo EG, Petrash VV, Rusanovskiĭ VV. Voluntary regulation of the heart contraction rate as a method for correcting the functional status of neurosis patients. Fiziol Cheloveka. 1990;16(2):58–64. [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [Review]. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med. 2010;40(8):1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction. 2012;107(1):206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Tapert SF. Introduction to alcohol and adolescent brain development. Alcohol Clin Exp Res. 2008;32(3):373–374. doi: 10.1111/j.1530-0277.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Padilla ML, Baker FC. Partial recovery of alcohol dependence-related deficits in sleep evoked potentials following 12 months of abstinence. Front Neurol. 2012;3:13. doi: 10.3389/fneur.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Curlik DM, 2nd, Shors TJ. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64(1):506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, McLaughlin RJ, Graf P. Mechanisms Underlying the Link between Cannabis Use and Prospective Memory. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysarz D, Bussing A. Cardiorespiratory synchronization during Zen meditation. European Journal of Applied Physiology. 2005;95(1):88–95. doi: 10.1007/s00421-005-1379-3. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Van Der Linden M, Verbanck P, Noel X. Autobiographical memory in non-amnesic alcohol-dependent patients. Psychol Med. 2006;36(12):1707–1715. doi: 10.1017/S0033291706008798. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, et al. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychological Medicine. 1998;28(5):1039–1048. doi: 10.1017/s0033291798006849. [Article]. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism, Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19(4):1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- DiClemente CC. Mechanisms, determinants and processes of change in the modification of drinking behavior. Alcohol Clin Exp Res. 2007;31(10 Suppl):13s–20s. doi: 10.1111/j.1530-0277.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- Dingwall KM, Maruff P, Cairney S. Similar profile of cognitive impairment and recovery for Aboriginal Australians in treatment for episodic or chronic alcohol use. Addiction. 2011;106(8):1419–1426. doi: 10.1111/j.1360-0443.2011.03434.x. [DOI] [PubMed] [Google Scholar]
- Donovan D, Mattson ME, Cisler RA, Longabaugh R, Zweben A. Quality of Life as an Outcome Measure in Alcoholism Treatment Research. Journal of Studies on Alcohol. 2005;66(Suppl15):119–139. doi: 10.15288/jsas.2005.s15.119. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Kivlahan DR, Kadden RM, Hill D. Cognitive impairment as a patient-treatment matching hypothesis. In: Longabaugh R, Wirtz PW, editors. Project MATCH Hypotheses: Results and Causal Chain Analyses. Vol. 8. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2001. pp. 62–81. Project MATCH Monograph Series. [Google Scholar]
- Donovan DM, Kivlahan DR, Walker RD. Clinical limitations of neuropsychological testing in predicting treatment outcome among alcoholics. Alcoholism: Clinical and Experimental Research. 1984;8(5):470–475. doi: 10.1111/j.1530-0277.1984.tb05704.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Kivlahan DR, Walker RD, Umlauf R. Derivation and validation of neuropsychological clusters among men alcoholics. Journal of Studies on Alcohol. 1985;46(3):205–211. doi: 10.15288/jsa.1985.46.205. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27(10):1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30(3):539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Ellis J. Prospective memory or the realisation of delayed intentions: A conceptual framework for research. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Hillsdale, NJ: Erlbaum; 1996. pp. 1–22. [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28(11):1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western Journal of Medicine. 1990;152(5):531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D. Gait and Balance Deficits in Chronic Alcoholics: No Improvement from 10 Weeks Through 1 Year Abstinence. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, McGillivray S. Cognitive performance in long-term abstinent elderly alcoholics. Alcohol Clin Exp Res. 2007;31(11):1788–1799. doi: 10.1111/j.1530-0277.2007.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Di Sclafani V, Barakos J, Harper C. Increased white matter signal hyperintensities in long-term abstinent alcoholics compared with nonalcoholic controls. Alcohol Clin Exp Res. 2009;33(1):70–78. doi: 10.1111/j.1530-0277.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism, Clinical and Experimental Research. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ferrett HL, Carey PD, Thomas KG, Tapert SF, Fein G. Neuropsychological performance of South African treatment-naive adolescents with alcohol dependence. Drug Alcohol Depend. 2010;110(1–2):8–14. doi: 10.1016/j.drugalcdep.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M, Gavrilescu D. Alcohol effects on intentional behavior: dissociating controlled and automatic influences. Experimental and Clinical Psychopharmacology. 1999;7(4):372–378. doi: 10.1037//1064-1297.7.4.372. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, McGue M, McClearn GE. Heritability of cognitive abilities in adult twins: comparison of Minnesota and Swedish data. Behav Genet. 1995;25(5):421–431. doi: 10.1007/BF02253371. [DOI] [PubMed] [Google Scholar]
- Fish J, Wilson BA, Manly T. The assessment and rehabilitation of prospective memory problems in people with neurological disorders: a review. Neuropsychol Rehabil. 2010;20(2):161–179. doi: 10.1080/09602010903126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LE, Jackson M, Crowe SF. Characterization of Cerebellar Ataxia in Chronic Alcoholics Using the International Cooperative Ataxia Rating Scale (ICARS) Alcoholism-Clinical and Experimental Research. 2012;36(11):1942–1951. doi: 10.1111/j.1530-0277.2012.01821.x. [Article]. [DOI] [PubMed] [Google Scholar]
- Forsberg LK, Goldman MS. Experience-dependent recovery of cognitive deficits in alcoholics: Extended transfer of training. Journal of Abnormal Psychology. 1987;96(4):345–353. doi: 10.1037//0021-843x.96.4.345. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, et al. Reduced Cortical Thickness in Abstinent Alcoholics and Association with Alcoholic Behavior. Alcoholism-Clinical and Experimental Research. 2011;35(12):2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137(2):201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug and Alcohol Dependence. 2005;78(3):263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133(Pt 4):1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162(2):133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13(3–4):423–434. doi: 10.1111/j.1369-1600.2008.00111.x. doi:ADB111 [pii] [DOI] [PubMed] [Google Scholar]
- Godding PR, Fitterling JM, Schmitz JM, Seville JL, Parisi SA. Discriminative utility of a brief cognitive status assessment with alcoholics and the impact of cognitive status on acquisition of treatment-relevant information. Psychology of Addictive Behaviors. 1992;6(1):34–40. [Google Scholar]
- Goldman MS. Experience-dependent neuropsychological recovery and the treatment of chronic alcoholism. Neuropsychology Review. 1990;1(1):75–101. doi: 10.1007/BF01108859. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Recovery of cognitive functioning in alcoholics - the relationship to treatment. Alcohol Health and Research World. 1995;19(2):148–154. [PMC free article] [PubMed] [Google Scholar]
- Goldman RS, Goldman MS. EXPERIENCE-DEPENDENT COGNITIVE RECOVERY IN ALCOHOLICS - A TASK COMPONENT STRATEGY. Journal of Studies on Alcohol. 1988;49(2):142–148. doi: 10.15288/jsa.1988.49.142. [Article]. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Haas GL, Shemansky WJ, Barnett B, Salmon-Cox S. Rehabilitation during alcohol detoxication in comorbid neuropsychiatric patients. J Rehabil Res Dev. 2005;42(2):225–234. doi: 10.1682/jrrd.2004.03.0040. [DOI] [PubMed] [Google Scholar]
- Griffiths A, Hill R, Morgan C, Rendell PG, Karimi K, Wanagaratne S, et al. Prospective memory and future event simulation in individuals with alcohol dependence. Addiction. 2012;107(10):1809–1816. doi: 10.1111/j.1360-0443.2012.03941.x. [DOI] [PubMed] [Google Scholar]
- Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8(5):645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in Neuropsychological Functioning Over 10 Years Following Adolescent Substance Abuse Treatment. Psychology of Addictive Behaviors. 2011;25(1):127–142. doi: 10.1037/a0022350. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32(6):1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. doi:ACER661 [pii] [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Witkiewitz K, Villarroel N, Donovan D. Self-efficacy change as a mediator of associations between therapeutic bond and one-year outcomes in treatments for alcohol dependence. Psychol Addict Behav. 2011;25(2):269–278. doi: 10.1037/a0022869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett AL, Radvanski DC, Vaschillo E, Vaschillo B, Sigal LH, Karavidas MK, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32(1):1–10. doi: 10.1007/s10484-006-9028-0. [doi]. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Fortier CB, Levine A, Milberg WP, McGlinchey R. Implicit memory in Korsakoff's syndrome: a review of procedural learning and priming studies. Neuropsychol Rev. 2012;22(2):132–153. doi: 10.1007/s11065-012-9204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji LJ, Jing Q, Jiao S. Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychology. 2002;16(1):65–73. doi: 10.1037//0894-4105.16.1.65. [DOI] [PubMed] [Google Scholar]
- Heffernan T, Clark R, Bartholomew J, Ling J, Stephens S. Does binge drinking in teenagers affect their everyday prospective memory? Drug and Alcohol Dependence. 2010;109(1–3):73–78. doi: 10.1016/j.drugalcdep.2009.12.013. [Article]. [DOI] [PubMed] [Google Scholar]
- Heffernan TM, Bartholomew J. Does excessive alcohol use in teenagers affect their everyday prospective memory? Journal of Adolescent Health. 2006;39(1):138–140. doi: 10.1016/j.jadohealth.2005.10.010. [Article]. [DOI] [PubMed] [Google Scholar]
- Heffernan TM, Moss M, Ling J. Subjective ratings of prospective memory deficits in chronic heavy alcohol users. Alcohol and Alcoholism. 2002;37(3):269–271. doi: 10.1093/alcalc/37.3.269. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54(4):2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a Grip on Drinking Behavior: Training Working Memory to Reduce Alcohol Abuse. Psychol Sci. 2011;22(7):968–975. doi: 10.1177/0956797611412392. [Article]. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. MONITORING THE FUTURE. NATIONAL SURVEY RESULTS ON DRUG USE, 1975–2011. Volume II. College Students and Adults Ages 19–50. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30(1):12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. Preliminary Results of an Open Label Study of Heart Rate Variability Biofeedback for the Treatment of Major Depression. Appl Psychophysiol Biofeedback. 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Keith JR, Puente AE, Malcolmson KL, Tartt S, Coleman AE, Marks HF., Jr Assessing postoperative cognitive change after cardiopulmonary bypass surgery. Neuropsychology. 2002;16(3):411–421. [PubMed] [Google Scholar]
- Kennedy MR, Turkstra L. Group intervention studies in the cognitive rehabilitation of individuals with traumatic brain injury: challenges faced by researchers. Neuropsychol Rev. 2006;16(4):151–159. doi: 10.1007/s11065-006-9012-8. [DOI] [PubMed] [Google Scholar]
- Keyl C, Dambacher M, Schneider A, Passino C, Wegenhorst U, Bernardi L. Cardiocirculatory coupling during sinusoidal baroreceptor stimulation and fixed-frequency breathing. Clin Sci (Lond) 2000;99(2):113–124. [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Carroll KM. Relationship of cognitive function and the acquisition of coping skills in computer assisted treatment for substance use disorders. Drug Alcohol Depend. 2011;114(2–3):169–176. doi: 10.1016/j.drugalcdep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, et al. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46(7):665–671. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104(6):1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, Pitel AL, Allain P, Eustache F, et al. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res. 2010;34(11):1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. doi:ACER1277 [pii] [doi]. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, Pinon K, Allain P, Pitel AL, et al. Cognitive barriers to readiness to change in alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(9):1542–1549. doi: 10.1111/j.1530-0277.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65(5):796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, et al. Biofeedback treatment for asthma. Chest. 2004;126(2):352–361. doi: 10.1378/chest.126.2.352. [doi] 126/2/352 [pii]. [DOI] [PubMed] [Google Scholar]
- Leitz JR, Morgan CJA, Bisby JA, Rendell PG, Curran HV. Global impairment of prospective memory following acute alcohol. Psychopharmacology. 2009;205(3):379–387. doi: 10.1007/s00213-009-1546-z. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res. 2010;34(8):1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Schweizer TA, O'Connor C, Turner G, Gillingham S, Stuss DT, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5 doi: 10.3389/fnhum.2011.00009. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, Manove E, Weiss RD. Recovery of cognitive functioning in patients with co-occurring bipolar disorder and alcohol dependence during early remission from an acute mood episode. Ann Clin Psychiatry. 2012;24(2):143–154. [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44(4):372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, et al. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol. 2010;45(6):541–547. doi: 10.1093/alcalc/agq065. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Lee SH. Gender Differences in the Link Between Excessive Drinking and Domain-Specific Cognitive Functioning Among Older Adults. Journal of Aging and Health. 2012;24(8):1380–1398. doi: 10.1177/0898264312459346. [Article]. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. doi:S0006-3223(08)00110-8 [pii] [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning V, Wanigaratne S, Best D, Hill RG, Reed LJ, Ball D, et al. Changes in neuropsychological functioning during alcohol detoxification. Eur Addict Res. 2008;14(4):226–233. doi: 10.1159/000156479. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33(11):1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. doi:ACER1026 [pii] [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Adinoff B, Weingartner H, Mukherjee AB, Eckardt MJ. Alcoholic organic brain disease: nosology and pathophysiologic mechanisms. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1986;10(2):147–164. doi: 10.1016/0278-5846(86)90069-2. [DOI] [PubMed] [Google Scholar]
- McCraty R, Atkinson M, Tomasino D. Impact of a workplace stress reduction program on blood pressure and emotional health in hypertensive employees. Journal of Complementary and Alternative Medicine. 2003;9:355–369. doi: 10.1089/107555303765551589. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism-Clinical and Experimental Research. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ. Brain-derived neurotrophic factor genotype is associated with brain gray and white matter tissue volumes recovery in abstinent alcohol-dependent individuals. Genes Brain Behav. 2012 doi: 10.1111/j.1601-183X.2012.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res. 2009;33(8):1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: a meta-analysis. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery C, Ashmore KV, Jansari A. The effects of a modest dose of alcohol on executive functioning and prospective memory. Human Psychopharmacology-Clinical and Experimental. 2011;26(3):208–215. doi: 10.1002/hup.1194. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Bates ME. Effects of executive function impairment on change processes and substance use outcomes in 12-step treatment. Journal of Studies on Alcohol. 1999;60(6):846–855. doi: 10.15288/jsa.1999.60.846. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Muramatsu T, Kato M, Mimura M, Kashima H. Family history of alcoholism and cognitive recovery in subacute withdrawal. Psychiatry Clin Neurosci. 2006;60(1):85–89. doi: 10.1111/j.1440-1819.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289(11):1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ. Assessing cognitive impairment. Alcohol Health and Research World. 1995;19:97–103. [PMC free article] [PubMed] [Google Scholar]
- Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21(6):778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, et al. Supervisory attentional system in nonamnesic alcoholic men. Arch Gen Psychiatry. 2001;58(12):1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28(4):667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: The neurological effects of alcohol. Alcohol Health and Research World. 1997;21(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2002;26(9):1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcoholism: Clinical and Experimental Research. 1998;22(4):954–961. [PubMed] [Google Scholar]
- Parsons OA, Butters N, Nathan PE. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. New York: Guilford Press; 1987. [Google Scholar]
- Parsons OA, Farr SP. The neuropsychology of alcohol and drug use. In: Filskov SB, Boll TJ, editors. Handbook of Clinical Neuropsychology. New York: Wiley; 1981. pp. 320–365. [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94(1–3):82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus JE, Liu Y, Khalsa G, Douglas PS, Benson H, et al. Exaggerated heart rate oscillations during two meditation techniques. Int J Cardiol. 1999;70(2):101–107. doi: 10.1016/s0167-5273(99)00066-2. doi:S0167-5273(99)00066-2 [pii]. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(1 Pt 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65(8):680–690. doi: 10.1016/j.biopsych.2008.10.039. doi:S0006-3223(08)01385-1 [pii] [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21(3):521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55(10):905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, et al. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcoholism, Clinical and Experimental Research. 2007a;31(7):1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, et al. Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcoholism, Clinical and Experimental Research. 2007b;31(2):238–248. doi: 10.1111/j.1530-0277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Personality and psychosocial consequences after brain injury. In: Meier MJ, Benton AL, Diller L, editors. Neuropsychol Rehabil. New York: Guilford Press; 1987. pp. 355–378. [Google Scholar]
- Prigatano GP, Glisky E, Konoff P. Cognitive rehabilitation after traumatic brain injury. In: Corrigan PW, Yudofsky SC, editors. Cognitive Rehabilitation for Neuropsychiatric Disorders. Washington, DC: American Psychiatric Press; 1996. p. 459. [Google Scholar]
- Prinsloo GE, Rauch HGL, Lambert MI, Muench F, Noakes TD, Derman WE. The effect of short duration heart rate variability (HRV) biofeedback on cognitive performance during laboratory induced cognitive stress. Applied Cognitive Psychology. 2011;25:792–801. [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are impaired? Acta Neurol Scand. 2002;105(4):276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Bates ME. Acute alcohol effects on repetition priming and word recognition memory with equivalent memory cues. Brain & Cognition. 2006;60(2):118–127. doi: 10.1016/j.bandc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Ray S, Mun EY, Buckman JF, Udo T, Bates ME. Memory for emotional picture cues during acute alcohol intoxication. J Stud Alcohol Drugs. 2012;73(5):718–725. doi: 10.15288/jsad.2012.73.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Gray TJ, Henry JD, Tolan A. Prospective memory impairment in "ecstasy" (MDMA) users. Psychopharmacology. 2007;194(4):497–504. doi: 10.1007/s00213-007-0859-z. [DOI] [PubMed] [Google Scholar]
- Rinn W, Desai N, Rosenblatt H, Gastfriend DR. Addiction denial and cognitive dysfunction: A preliminary investigation. Journal of Neuropsychiatry & Clinical Neurosciences. 2002;14(1):52–57. doi: 10.1176/jnp.14.1.52. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research: Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roehrich L, Goldman MS. Experience-dependent neuropsychological recovery and the treatment of alcoholism. J Consult Clin Psychol. 1993;61(5):812–821. doi: 10.1037//0022-006x.61.5.812. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol Clin Exp Res. 2012;36(2):294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18(3):589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O'Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with change in brain structure. Psychiatry Res. 2007;155(2):91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Loberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. New York: Oxford University Press; 1996. pp. 423–485. [Google Scholar]
- Ruff RL. Endplate contributions to the safety factor for neuromuscular transmission. Muscle Nerve. 2011;44(6):854–861. doi: 10.1002/mus.22177. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Drexler A, Hausmann A, Hinterhuber H, Kurz M. Executive function and memory in relation to olfactory deficits in alcohol-dependent patients. Alcoholism-Clinical and Experimental Research. 2006;30(8):1355–1362. doi: 10.1111/j.1530-0277.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs. 2012;73(4):625–634. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: A randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. Journal of the American Medical Association. 2000;283(23):3075–3081. doi: 10.1001/jama.283.23.3075. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Walker K. Teaching coping skills to chronic alcoholics in a coeducational halfway house: I. Assessment of programme effects. British Journal of Addiction. 1982;77(1):35–50. doi: 10.1111/j.1360-0443.1982.tb03248.x. [DOI] [PubMed] [Google Scholar]
- Sandercock GRH, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: Inferences from meta-analysis. Medicine and Science in Sports and Exercise. 2005;37(3):433–439. doi: 10.1249/01.mss.0000155388.39002.9d. [DOI] [PubMed] [Google Scholar]
- Sarajuuri JM, Kaipio ML, Koskinen SK, Niemela MR, Servo AR, Vilkki JS. Outcome of a comprehensive neurorehabilitation program for patients with traumatic brain injury. Arch Phys Med Rehabil. 2005;86(12):2296–2302. doi: 10.1016/j.apmr.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Schulte T, Oberlin BG, Kareken DA, Marinkovic K, Muller-Oehring EM, Meyerhoff DJ, et al. How acute and chronic alcohol consumption affects brain networks: insights from multimodal neuroimaging. Alcohol Clin Exp Res. 2012;36(12):2017–2027. doi: 10.1111/j.1530-0277.2012.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM, II, Nokia MS. Use it or lose it: How neurogenesis keeps the brain fit for learning. Behavioural Brain Research. 2012;227(2):450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- Smith DE, McCrady BS. Cognitive impairment among alcoholics: Impact on drink refusal skill acquisition and treatment outcome. Addictive Behaviors. 1991;16(5):265–274. doi: 10.1016/0306-4603(91)90019-e. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, et al. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry. 2012;71(3):262–268. doi: 10.1016/j.biopsych.2011.09.022. doi:S0006-3223(11)00922-X [pii] [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain Response to Working Memory Over Three Years of Adolescence: Influence of Initiating Heavy Drinking. Journal of Studies on Alcohol and Drugs. 2012;73(5):749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating Moderate to Heavy Alcohol Use Predicts Changes in Neuropsychological Functioning for Adolescent Girls and Boys. Psychology of Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8(3):220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- Stringer AY, Goldman MS. EXPERIENCE-DEPENDENT RECOVERY OF BLOCK DESIGN PERFORMANCE IN MALE-ALCOHOLICS - STRATEGY TRAINING VERSUS UNSTRUCTURED PRACTICE. Journal of Studies on Alcohol. 1988;49(5):406–411. doi: 10.15288/jsa.1988.49.406. [Article]. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism, Clinical and Experimental Research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000a;14(3):341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000;14(2):178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism-Clinical and Experimental Research. 2000b;24(5):611–621. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Roitzsch JC, Herron J, Thevos A. Substance abuse treatment outcomes for cognitively impaired and intact outpatients. Addictive Behaviors. 2002;27(5):751–763. doi: 10.1016/s0306-4603(01)00207-6. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [doi]. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Bates ME. The selective effects of alcohol on automatic and effortful memory processes. Neuropsychology. 1999;13(2):282–290. doi: 10.1037//0894-4105.13.2.282. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Weingardt KR, Stacy AW, Leigh BC. Automatic activation of alcohol concepts in response to positive outcomes of alcohol use. Alcoholism: Clinical and Experimental Research. 1996;20(1):25–30. doi: 10.1111/j.1530-0277.1996.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Weinstein CS, Shaffer HJ. Neurocognitive aspects of substance abuse treatment: A psychotherapist's primer. Psychotherapy. 1993;30:317–333. [Google Scholar]
- Wells R, Outhred T, Heathers JA, Quintana DS, Kemp AH. Matter over mind: a randomised-controlled trial of single-session biofeedback training on performance anxiety and heart rate variability in musicians. PLoS One. 2012;7(10):e46597. doi: 10.1371/journal.pone.0046597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients' approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011a;22(4):490–497. doi: 10.1177/0956797611400615. [Randomized Controlled Trial Research Support, Non-U S Gov't]. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining Automatic Action Tendencies Changes Alcoholic Patients' Approach Bias for Alcohol and Improves Treatment Outcome. Psychol Sci. 2011b;22(4):490–497. doi: 10.1177/0956797611400615. [Article]. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105(2):279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [Article]. [DOI] [PubMed] [Google Scholar]
- Wilson K, Halsey A, Macpherson H, Billington J, Hill S, Johnson G, et al. The psycho-social rehabilitation of patients with alcohol-related brain damage in the community. Alcohol Alcohol. 2012;47(3):304–311. doi: 10.1093/alcalc/agr167. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73(4–6):155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wolwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: a MRI study. Eur Arch Psychiatry Clin Neurosci. 2009;259(3):143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165(9):1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [Article]. [DOI] [PubMed] [Google Scholar]
- Xu G, Liu X, Yin Q, Zhu W, Zhang R, Fan X. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin Neurosci. 2009;63(1):43–49. doi: 10.1111/j.1440-1819.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- Ylvisaker M, Feeney TJ. Collaborative Brain Injury Intervention: Positive Everyday Routines. San Diego: Singular Publishing Group, Inc.; 1998. [Google Scholar]
- Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness Meditation for Substance Use Disorders: A Systematic Review. Substance Abuse. 2009;30(4):266–294. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcohol Clin Exp Res. 2004;28(9):1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]
- Zoellig J, West R, Martina M, Altgassen M, Lemke U, Kliegel M. Neural correlates of prospective memory across the lifespan. Neuropsychologia. 2007;45(14):3299–3314. doi: 10.1016/j.neuropsychologia.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: a pilot study. Appl Psychophysiol Biofeedback. 2009;34(2):135–143. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]