Abstract
Background. Prospective studies of serum albumin concentration measurement as a low-cost predictor of human immunodeficiency virus (HIV) disease progression are needed for individuals initiating antiretroviral therapy (ART) in resource-limited settings.
Methods. Serum albumin concentration was measured at ART initiation for 2145 adults in Tanzania who were enrolled in a trial examining the effect of multivitamins on HIV disease progression. Participants were prospectively followed for mortality, morbidity, and anthropometric outcomes at monthly visits (median follow-up duration, 21.2 months). Proportional hazard models were used to analyze mortality, morbidity, and nutritional outcomes, while generalized estimating equations were used to analyze CD4+ T-cell counts.
Results. Individuals with hypoalbuminemia (defined as a serum albumin concentration of <35 g/L) at ART initiation had a hazard of death that was 4.52 times (95% confidence interval, 3.37–6.07; P < .001) that of individuals with serum albumin concentrations of ≥35 g/L, after multivariate adjustment. Hypoalbuminemia was also independently associated with the incidence of pulmonary tuberculosis (P < .001), severe anemia (P < .001), wasting (P = .002), and >10% weight loss (P = .012). Secondary analyses suggested that serum albumin concentrations of <38 g/L were associated with increased mortality and incident pulmonary tuberculosis. There was no association between serum albumin concentration and changes in CD4+ T-cell counts (P = .121).
Conclusions. Serum albumin concentrations can identify adults initiating ART who are at high risk for mortality and selected morbidities. Future research is needed to identify and manage conditions that reduce the serum albumin concentration.
Keywords: albumin, HIV, tuberculosis, nutrition, mortality
Clinical management of patients with human immunodeficiency virus (HIV) infection often relies on markers of disease progression, primarily CD4+ T-cell count and HIV load, to guide treatment plans [1]. Both CD4+ T-cell count and HIV load are routinely monitored in developed countries. However, in many resource-limited settings, routine viral load assessment is not in the national guidelines because of the high cost of testing and other logistical difficulties [2]. Accordingly, low-cost markers of HIV disease severity may improve HIV treatment in resource-limited settings.
Serum albumin is a plasma protein produced by the liver that has a role in many physiologic processes, including vasodilation, endothelial cell apoptosis, and antioxidant reactions [3, 4]. Individuals with conditions such as malnutrition, chronic inflammation, enteropathy, or liver disease can have reduced serum albumin concentrations [4]. As a result, the serum albumin concentration can be considered an indicator of overall health, and studies have shown that a low serum albumin concentration is a strong predictor of mortality for various acute and chronic illnesses [5–8].
Several studies of HIV-infected individuals have determined that hypoalbuminemia (defined as a serum albumin concentration of <35 g/L) is associated with more rapid progression to AIDS and all-cause mortality in developed countries [9–12]. Similar results were found in the few studies conducted in resource-limited settings, where monitoring the serum albumin concentrations may have a greater impact on clinical management and treatment of HIV infection. However, there is some indication that the association between serum albumin concentration and HIV progression may be modified by CD4+ T-cell counts [12–16]. A study of Kenyan HIV-infected women determined that hypoalbuminemia was significantly associated with mortality among those with CD4+ T-cell counts of ≥50 cells/µL (hazard ratio [HR], 5.7; 95% confidence interval [CI], 1.4–9.8) but not among women with CD4+ T-cell counts of <50 cells/µL (HR, 1.0; 95% CI, .4–2.5) [13]. No studies have investigated the association of serum albumin concentration with morbidity outcomes.
Here, we present the largest prospective study of serum albumin concentration and HIV disease progression to date to assess the association of serum albumin concentration with mortality and to detect effect modification by CD4+ T-cell count and other commonly assessed markers of disease severity. We also present the first prospective study examining the relationship between the serum albumin concentration and incident diagnosis of comorbidities. In secondary analyses, we examine whether the generally used 35 g/L cutoff to define hypoalbuminemia captures the relationship of serum albumin concentration with mortality and morbidity outcomes among HIV-infected individuals.
METHODS
Study Population
This prospective cohort study consists of HIV-infected adult men and women initiating ART enrolled in a trial of vitamins B-complex, C, and E at high levels versus standard levels of the recommended dietary allowance conducted in the HIV care and treatment clinics (CTC) in Dar es Salaam, Tanzania during 2006–2009 (Clinicaltrials.gov NCT00383669) [17]. Individuals were eligible for the study if they were aged ≥18 years, infected with HIV, initiated ART at enrollment, and intended to stay in Dar es Salaam city for at least 2 years. The primary outcome of the trial was HIV disease progression or death, and there was no difference between treatment arms [17]. At the time of the study, the Tanzanian national treatment guidelines recommended that HIV-infected patients with World Health Organization (WHO) HIV disease stage IV, a CD4+ T-cell count of <200 cells/μL, or WHO HIV stage III disease and a CD4+ T-cell count of <350 cells/μL initiate highly active ART [18]. Women who were pregnant or lactating were excluded from the study. First-line drug combinations included stavudine (d4T), lamivudine (3TC), nevirapine (NVP), zidovudine (AZT), and efavirenz (EFV). AZT was substituted for d4T for individuals who had peripheral neuropathy or could not tolerate d4T. EFV was substituted for NVP in patients who could not tolerate NVP. Cotrimoxazole prophylaxis was provided when CD4+ T-cell counts were <200 cells/μL, and treatment for all opportunistic infection was provided according to the national and WHO guidelines.
Serum Albumin and Baseline Covariate Assessment
A total of 3418 individuals consented and were enrolled into the parent trial. At enrollment, a structured interview was completed to collect information on demographic characteristics. Study physicians also performed a complete medical examination and assessed HIV disease stage in accordance with WHO guidelines [18]. Blood specimens were collected at baseline, and serum albumin concentrations were quantified using the Cobas Integra 400 Plus analyzer (Roche Diagnostics, Basel, Switzerland). Samples from 2145 individuals were available within 2 weeks of ART initiation. Absolute CD4+ T-cell count (FACS Calibur system, Becton Dickinson, San Jose, CA) and complete blood count (AcT5 Diff AL analyzer, Beckman Coulter, Miami, FL) were also determined at baseline. Height and weight measurements were obtained by trained research nurses, using standardized procedures.
Outcome Assessment and Definitions
Study participants were followed at monthly clinic visits, and individuals who missed a clinic visit were followed up by means of telephone calls and/or home visits, during which relatives or neighbors were questioned about the vital status of the participant. Physicians performed a clinical examination, assessed the WHO HIV disease stage, and diagnosed and treated any emerging illness [18]. Pneumonia, Kaposi sarcoma, and oral thrush were diagnosed on the basis of symptoms reported by patients and signs assessed by study physicians. Chronic diarrhea was defined as report of diarrheal symptoms for ≥14 days. Pulmonary tuberculosis was diagnosed according to Tanzanian National Tuberculosis and Leprosy Programme guidelines. Participants with symptoms of pulmonary tuberculosis provided 3 sputum samples: a spot sputum specimen at the first clinic visit during which symptoms were noted; an early morning specimen on the next day, before a second clinic visit; and a third sputum specimen at the second clinic visit. Participants received a diagnosis of pulmonary tuberculosis if at least 1 of the 3 sputum smears had acid-fast bacilli detected by Ziehl-Neelsen staining techniques or if chest radiography showed radiological features consistent with tuberculosis in the absence of a positive sputum smear result [19]. Presumptive diagnosis of extrapulmonary (EP) tuberculosis was based on known specific or constitutional features (eg, fever, weight loss, and night sweats) and organ-specific signs assessed by the study physician (eg, stony dullness on percussion and pleural effusion on aspiration for pleural tuberculosis). Height and weight were determined by clinic study nurses at each clinic visit. Wasting was defined as a body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) of <18.5 [20]. Weight loss of >10% from baseline was also selected as an outcome, on the basis of the definition of HIV-related wasting [21]. Absolute CD4+ T-cell count and a complete blood count were determined at clinic visits every 4 months. Anemia was defined as a hemoglobin concentration of <130 g/L for men and <120 g/L for women, whereas severe anemia was defined as a hemoglobin concentration of <85 g/L for both sexes [22].
Statistical Methods
Baseline hypoalbuminemia was defined as a serum albumin concentration of <35 g/L, which is the generally used clinical definition that was also used in previous studies of HIV-infected individuals [23]. We examined risk factors for baseline hypoalbuminemia with log-binomial regression models to obtain risk ratio estimates [24, 25]. In a few instances, the models did not converge, and log-Poisson models, which provide consistent but not fully efficient estimates of the relative risk and its confidence intervals (CIs), were used [26]. Variables included in this analysis were sex and baseline age (30, 30–39, 40–49, and ≥50 years), BMI (<16.0 [severely underweight], 16.0–18.4 [underweight], 18.5–25.0 [normal], and >25.0 [overweight]), WHO HIV disease stage (I/II, III, and IV), CD4+ T-cell count (<50, 50–99, 100–199, and ≥200 cells/μL), alanine transaminase (ALT) concentration (≤40 and >40 IU/L), and hemoglobin concentration (<8.5, 8.5–11, and ≥11 g/dL). Effect modification was assessed with the likelihood ratio test, using variables included in the risk factor analyses listed above.
Proportional hazard models were used to investigate the relationship between hypoalbuminemia at baseline and death, the incidence of WHO HIV stage IV disease or death, and the first occurrence of morbidity and nutritional outcomes, including pneumonia, oral thrush, pulmonary tuberculosis, chronic diarrhea, Kaposi sarcoma, EP tuberculosis, severe anemia, anemia, wasting, and >10% weight loss [27]. We excluded individuals who received a diagnosis of the event of interest at baseline. Individuals without events were censored at the date of last follow-up. The results of these analyses are reported as adjusted hazard ratios (HRs) and 95% CIs. In secondary analyses, we conducted continuous analyses of baseline serum albumin concentration to examine whether the generally used definition of hypoalbuminemia captured mortality and morbidity relationships for HIV-infected individuals, since the cutoff may differ by disease. The possible nonlinear relationship between serum albumin concentration and death and morbidity outcomes was examined nonparametrically with restricted cubic splines [28, 29]. Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We also present categorical analyses, using cutoffs suggested by continuous analyses.
Associations between serum albumin concentration at ART initiation and CD4+ T-cell counts were analyzed with generalized estimating equations (GEEs). Change in CD4+ T-cell count between consecutive visits was treated as a longitudinal continuous outcome, and baseline serum albumin concentration time since ART initiation, and baseline covariates (excluding baseline CD4+ T-cell count) were treated as explanatory variables. We used an m-dependent working correlation matrix (m = 1), which assumes the correlation coefficients of adjacent observations are nonzero and equal. Robust estimators of the variances, which are consistent estimators even if the working correlation matrix is misspecified, were used to construct CIs. The potential nonlinear relationship of the change in T-cell counts between consecutive visits over time was examined nonparametrically with restricted cubic splines for time since ART initiation [28, 29]. If a nonlinear relationship was found, we added the selected cubic spline terms to the model as covariates. To assess the association of baseline serum albumin concentration and change in CD4+ T- cell count between consecutive visits over time, we included the interactions between serum albumin concentration and time since ART initiation and its splines. The robust score test was used to determine whether the categorical serum albumin concentration groups differed in CD4+ T-cell count trajectory.
Confounders included in proportional hazard and GEE models were selected on the basis of our risk factor analysis and a review of the literature and consisted of sex and baseline age, BMI, WHO HIV disease stage (except for incidence of WHO stage IV disease or death), CD4+ T-cell count, hemoglobin concentration, and ALT concentration. Baseline CD4+ T cell count was not adjusted for in CD4+ T-cell analyses because this would have yielded biased estimates. Effect modification by all covariates listed above, randomized multivitamin regimen, and ART regimen were considered for all analyses. To assess whether effect modification was significant, we used the likelihood ratio test, for proportional hazard models, and the robust score test, for GEE analyses. Missing data for covariates were retained in the analysis, using the missing indicator method for variables [30]. All P values were 2 sided, and a P value of < .05 was considered statistically significant. Statistical analyses were performed using SAS v 9.2 (SAS Institute, Cary, NC, USA).
Ethics Statement
Written informed consent was obtained from all participants included in the parent trial. The trial protocol was approved by the institutional review boards of the Harvard School of Public Health, Muhimbili University of Health and Allied Sciences, the Tanzania Food and Drug Authority, and National Institute of Medical Research.
RESULTS
A total of 2172 individuals enrolled in the parent trial had their serum albumin concentration measured at ART initiation. There were no significant differences between individuals with serum albumin measurements at baseline and the trial population (n = 3418) in age, sex, or baseline CD4+ T-cell count, WHO HIV disease stage, hemoglobin concentration, and ALT concentration. Baseline characteristics of the study cohort are presented in Table 1, by serum albumin concentration. A total of 858 individuals (39.5%) had hypoalbuminemia at baseline. A multivariate cross-sectional analysis determined that baseline hypoalbuminemia was significantly associated with older age (P = .033), decreasing BMI (P < .001), more severe WHO HIV disease stage (P < .001), decreasing hemoglobin level (P < .001), and an ALT level of >40 IU/L (P = .003) at the baseline study visit (Table 1).
Table 1.
Baseline Characteristics of 2145 Study Participants at Antiretroviral Therapy (ART) Initiation, by Serum Albumin Concentration, and Univariate and Multivariate Risk Factor Analyses for Hypoalbuminemia
| Characteristic | No. (%) of Subjects, by Serum Albumin Concentration |
Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|---|---|
| ≥35 g/L (n = 1216) | <35 g/L (n = 929) | RR (95% CI) | P | RR (95% CI) | P | |
| Sex | ||||||
| Female (ref) | 831 (68.3) | 653 (70.3) | 1.0 | 1.0 | ||
| Male | 385 (31.7) | 276 (29.7) | .95 (.85–1.06) | .336 | 1.03 (1.28–1.77) | .491 |
| Age, y | ||||||
| <30 (ref) | 198 (16.3) | 122 (13.1) | 1.0 | .229a | 1.0 | .033a |
| 30–40 | 581 (47.8) | 466 (50.2) | 1.17 (1.00–1.36) | 1.13 (.98–1.30) | ||
| 40–50 | 326 (26.8) | 248 (26.7) | 1.13 (.96–1.34) | 1.17 (1.00–1.36) | ||
| >50 | 111 (9.1) | 93 (10.0) | 1.20 (.97–1.47) | 1.23 (1.02–1.49) | ||
| BMI | ||||||
| <16.0 | 28 (2.3) | 105 (11.4) | 2.00 (1.79–2.23) | <.001a | 1.56 (1.38–1.77) | <.001a |
| 16.0–18.5 | 179 (14.9) | 250 (27.0) | 1.47 (1.33–1.64) | 1.30 (1.17–1.44) | ||
| 18.5–25 | 741 (61.5) | 484 (52.4) | 1.0 | 1.0 | ||
| >25.0 | 257 (21.3) | 85 (9.2) | .79 (.38–1.64) | .78 (.64–.94) | ||
| WHO HIV disease stage | ||||||
| I/II | 360 (26.3) | 120 (14.0) | 1.0 | <.001a | 1.0 | <.001a |
| III | 654 (47.8) | 573 (67.0) | 1.87 (1.58–2.21) | 1.51 (1.28–1.77) | ||
| IV | 67 (7.0) | 162 (18.9) | 2.51 (2.10–3.01) | 1.72 (1.44–2.06) | ||
| CD4+ T-cell count, cells/μL | ||||||
| <50 | 227 (19.2) | 208 (23.5) | 1.05 (.91–1.22) | .117a | .96 (.84–1.11) | .762a |
| 50–100 | 215 (18.1) | 198 (22.4) | 1.07 (.92–1.23) | .99 (.87–1.14) | ||
| 100–200 | 507 (42.8) | 286 (32.3) | .80 (.70–.92) | .91 (.80–1.04) | ||
| >200 | 236 (19.9) | 193 (21.8) | 1.0 | 1.0 | ||
| Hemoglobin level, g/dL | ||||||
| <8.5 | 120 (10.5) | 308 (34.3) | 2.95 (2.56–3.40) | <.001a | 2.47 (2.13–2.88) | <.001a |
| 8.5–11 | 484 (42.2) | 416 (46.3) | 1.90 (1.64–2.20) | 1.77 (1.53–2.06) | ||
| ≥11 | 543 (47.3) | 175 (19.5) | 1.0 | 1.0 | ||
| ALT level, IU/L | ||||||
| ≤40 | 1102 (90.6) | 800 (86.1) | 1.0 | 1.0 | ||
| >40 | 114 (9.4) | 129 (13.9) | 1.27 (1.11–1.45) | <.001 | 1.20 (1.06–1.36) | .003 |
| ART regimenb | ||||||
| d4T, 3TC, NVP | 695 (57.7) | 575 (61.8) | … | … | ||
| d4T, 3TC, EFV | 96 (7.9) | 107 (11.5) | … | … | ||
| AZT, 3TC, NVP | 113 (9.3) | 60 (6.5) | … | … | ||
| AZT, 3TC, EFV | 312 (25.7) | 187 (20.1) | … | … | ||
Hypoalbuminemia was defined as a serum albumin concentration of <35 g/L.
Abbreviations: ALT, alanine transaminase; BMI, body mass index (calculated as the weight in kilograms divided by the height in meters squared); CI, confidence interval; HIV, human immunodeficiency virus; ref, reference group; RR, relative risk; WHO, World Health Organization.
a Test for trend.
b Not included in risk factor analyses because ART was prescribed after serum albumin concentration was assessed.
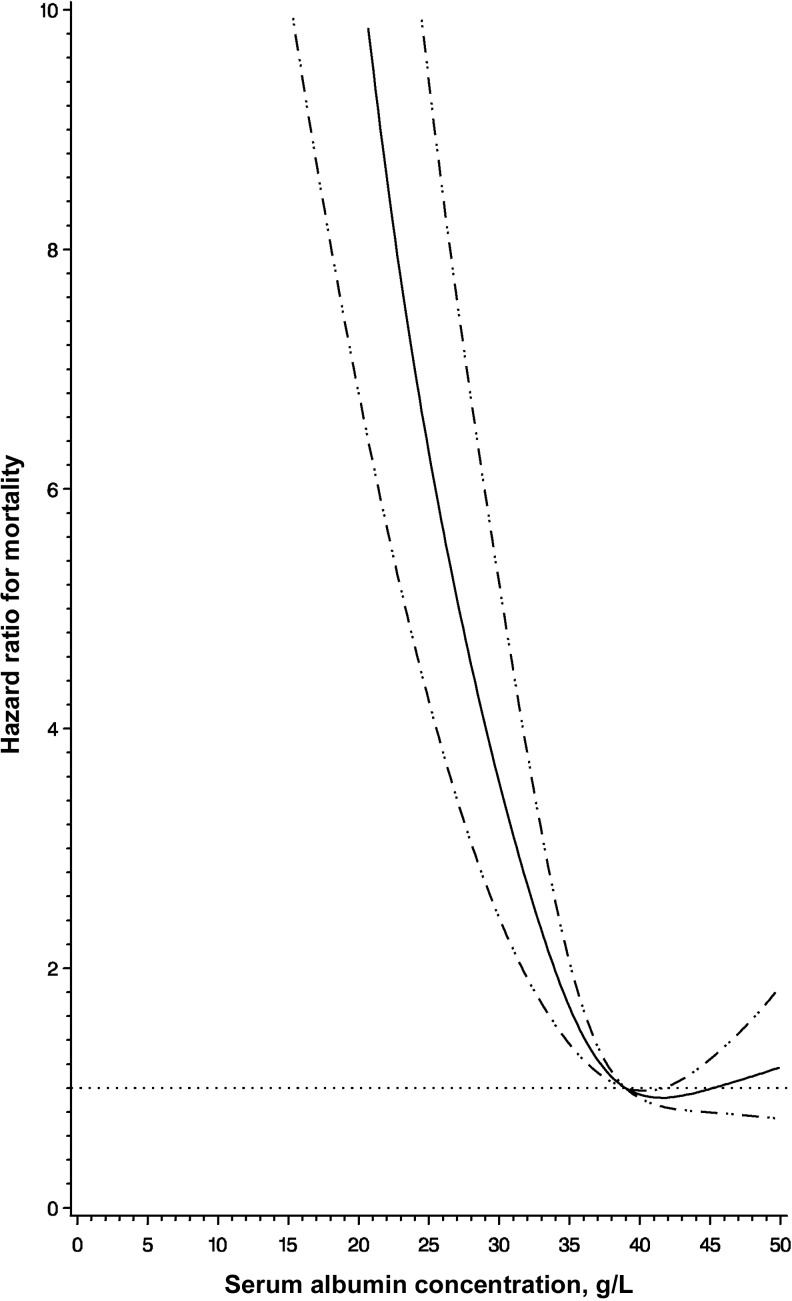
We then performed a prospective analysis of mortality, morbidity, and change in CD4+ T-cell count, by baseline serum albumin concentration. The median follow-up time of the cohort was 21.2 months, during which 238 deaths (mortality rate, 11.2%) were recorded. The cumulative incidence of mortality was 19.3% for individuals with hypoalbuminemia and 5.0% for those with a serum albumin concentration of ≥35 g/L. Individuals with hypoalbuminemia had a hazard of death that was 3.35 (95% CI, 2.42–4.63; P < .001) times that for individuals with a serum albumin concentration of ≥35 g/L, after adjustment for sex and baseline age, BMI, WHO HIV disease stage, CD4+ T-cell count, hemoglobin level, and ALT level. There was no detection of effect modification of the mortality association by baseline CD4+ T-cell count (P = .808). Hypoalbuminemia was significantly associated with increased mortality for individuals with baseline CD4+ T-cell counts of <50 cells/μL (HR, 3.39; 95% CI, 1.61–7.13; P =.001) and ≥50 cells/μL (HR, 3.21; 95% CI, 2.24–4.61; P < .001). Furthermore, no statistically significant effect modification by sex or baseline age, WHO disease stage, hemoglobin level, ALT level, randomized multivitamin regimen, and ART regimen was detected. Second, we analyzed serum albumin concentration continuously and found a significant nonlinear relationship with increasing risk of mortality for serum albumin concentrations <38 g/L (P = .002 for nonlinear relation; Figure 1). In a post hoc categorical analysis, individuals with a baseline serum albumin concentration of 35–38 g/L had a hazard of death that was 1.95 (95% CI, 1.17–3.24; P = .01) times that for individuals with a serum albumin concentration of ≥38 g/L, after multivariate adjustment.
Figure 1.
Restricted cubic spline analysis illustrating the shape of the adjusted relationship between continuous serum albumin concentration at antiretroviral therapy initiation and all-cause mortality. The solid line shows the estimated hazard ratio for serum albumin concentrations relative to the reference concentration of 38 g/L, with the horizontal dotted line designating a hazard ratio of 1.0. The 95% confidence intervals of the hazard ratio are represented by the dashed lines. Adjusted analyses controlled for sex and baseline age (30, 30–39, 40–49, and ≥50 years), body mass index (calculated as the weight in kilograms divided by the height in meters squared; <16.0, 16.0–18.4, 18.5–25.0, and >25.0), World Health Organization human immunodeficiency virus disease stage (I/II, III, and IV), CD4+ T-cell count (<50, 50–99, 100–199, and ≥200 cells/μL), hemoglobin level (<8.5, 8.5–11, and ≥11 g/dL), and alanine transaminase level (≤40 and >40 IU/L). P = .002 for nonlinear relation.
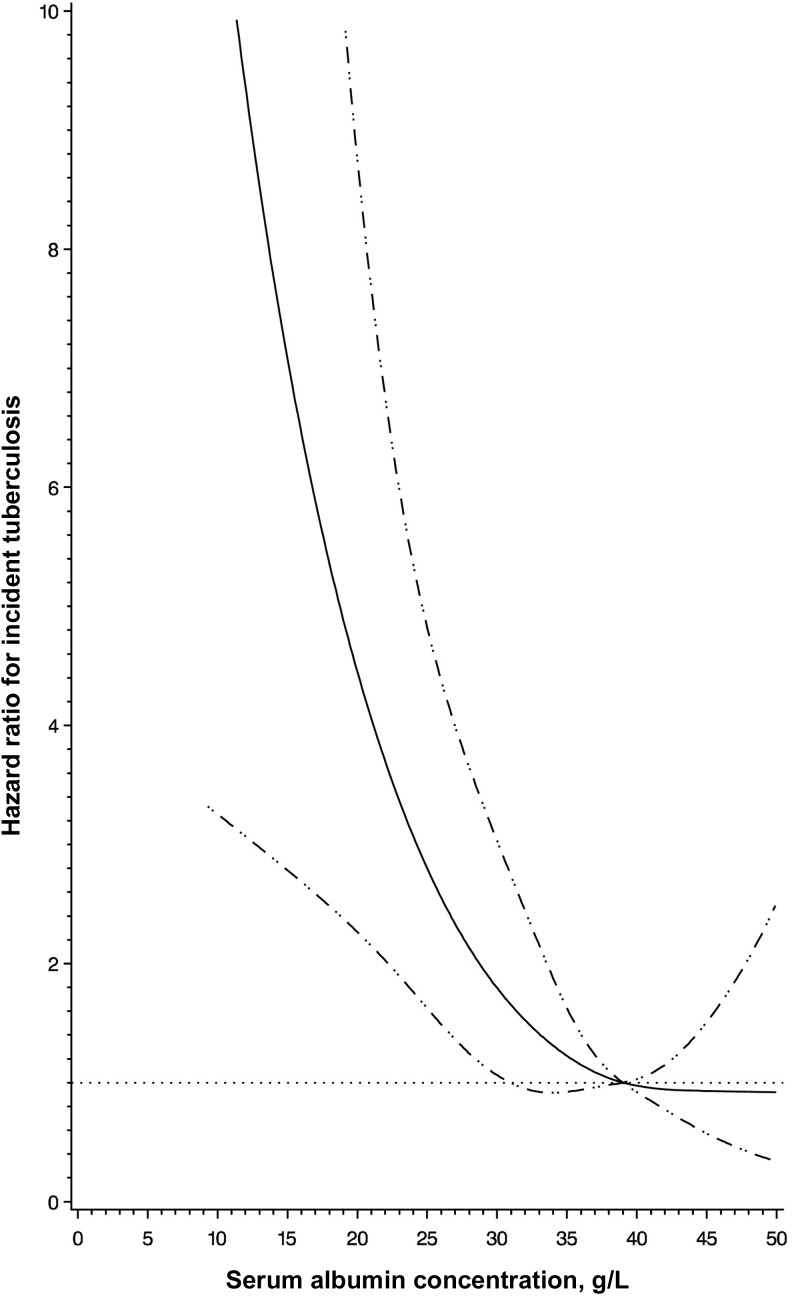
Study clinicians performed a clinical examination and diagnosed opportunistic infections and other comorbidities at monthly clinic visits. We present analyses of the composite incident WHO stage IV disease or death end point and comorbidities, by baseline presence of hypoalbuminemia, in Table 2. Hypoalbuminemia was significantly associated with incident WHO stage IV disease or death in univariate analysis (HR, 1.24; 95% CI, 1.08–1.43; P = .003), but there was no statistically significant association after multivariate adjustment (HR, 1.14; 95% CI, .97–1.33; P = .107). After multivariate adjustment, individuals with hypoalbuminemia had a significantly increased risk of incident pulmonary tuberculosis as compared to individuals with a serum albumin concentration of ≥35 g/L (HR, 1.80; 95% CI, 1.17–2.76; P = .007). When analyzing the relationship continuously, we found a significantly nonlinear relationship with an increasing hazard of pulmonary tuberculosis for serum albumin concentrations of <38 g/L (P = .03 for nonlinear relation; Figure 2). In a post hoc categorical analysis, individuals with a baseline serum albumin concentration 35–38 g/L had an increased but not statistically significant hazard of incident pulmonary tuberculosis as compared to individuals with a serum albumin concentration of ≥38 g/L, after multivariate adjustment (HR, 1.51; 95% CI, .72–3.15; P = .494). Serum albumin concentrations were not associated with the incidence of pneumonia, oral thrush, chronic diarrhea, and Kaposi sarcoma or with EP tuberculosis, after multivariate adjustment. There was also no indication of effect modification detected for any morbidity association by sex or baseline age, CD4+ T-cell count, WHO HIV disease stage, hemoglobin level, ALT level, randomized multivitamin regimen, and ART regimen.
Table 2.
Hazard Ratios (HRs) for All-Cause Mortality and Incident Morbidities Among Individuals With Versus Those Without Hypoalbuminemia at Baseline
| Outcome (No. of Events) | Crude HR (95% CI) | P | Adjusted HRa (95% CI) | P |
|---|---|---|---|---|
| Death (242) | 4.52 (3.37–6.07) | <.001 | 3.35 (2.42–4.63) | <.001 |
| WHO stage IV or death (785) | 1.24 (1.08–1.43) | .003 | 1.14 (.97–1.33) | .107 |
| Pneumonia (812) | 1.15 (1.00–1.32) | .051 | .96 (.83–1.12) | .633 |
| Oral thrush (238) | 1.07 (.83–1.39) | .602 | .83 (.62–1.11) | .209 |
| Pulmonary tuberculosis (107) | 2.35 (1.59–3.47) | <.001 | 1.80 (1.17–2.76) | .007 |
| Chronic diarrhea (87) | 1.15 (.75–1.76) | .526 | 1.06 (.66–1.71) | .810 |
| Kaposi sarcoma (49) | 1.21 (.68–2.13) | .520 | 1.07 (.57–2.03) | .828 |
| EP tuberculosis (35) | 2.11 (1.15–3.89) | .017 | 1.77 (.89–3.52) | .102 |
Hypoalbuminemia was defined as a serum albumin concentration of <35 g/L. Individuals with outcome events at baseline were excluded from analyses.
Abbreviations: CI, confidence interval; EP tuberculosis, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; WHO, World Health Organization.
a Adjusted for sex and baseline age (30, 30–39, 40–49, and ≥50 years), body mass index (calculated as the weight in kilograms divided by the height in meters squared; <16.0, 16.0–18.4, 18.5–25.0, and >25.0), WHO HIV disease stage (I/II, III, and IV, except for the outcome of WHO stage IV or death), CD4+ T-cell count (<50, 50–99, 100–199, and ≥200 cells/μL), hemoglobin level (<8.5, 8.5–11, and ≥11 g/dL), and alanine transaminase level (≤40 and >40 IU/L).
b WHO stage IV HIV disease.
Figure 2.
Restricted cubic spline analysis illustrating the shape of the adjusted relationship between continuous serum albumin concentration at antiretroviral therapy initiation and incident pulmonary tuberculosis. The solid line shows the estimated hazard ratio for serum albumin concentrations relative to the reference concentration of 38 g/L, with the horizontal dotted line designating a hazard ratio of 1.0. The 95% confidence intervals of the hazard ratio are represented by the dashed lines. Adjusted analyses controlled for sex and baseline age (30, 30–39, 40–49, and ≥50 years), body mass index (calculated as the weight in kilograms divided by the height in meters squared; <16.0, 16.0–18.4, 18.5–25.0, and >25.0), World Health Organization human immunodeficiency virus disease stage (I/II, III, and IV), CD4+ T-cell count (<50, 50–99, 100–199, and ≥200 cells/μL), hemoglobin level (<8.5, 8.5–11, and ≥11 g/dL), and alanine transaminase level (≤40 and >40 IU/L). P = .030 for nonlinear relation.
Hemoglobin levels were assessed every 4 months after the baseline ART initiation visit. During follow-up, 179 individuals (11.0%) experienced incident severe anemia. For individuals with baseline hypoalbuminemia, the hazard of incident severe anemia was 2.28 (95% CI, 1.67–3.10; P < .001) times that for individuals with a serum albumin concentration of ≥35 g/L, after multivariate adjustment (Table 3). Individuals with hypoalbuminemia also had an elevated but not statistically significant hazard of incident anemia (HR, 1.42; 95% CI, .96–2.12; P = .081). There was no indication of effect modification of anemia associations by sex, ART regimen, randomized multivitamin regimen, or any other variable.
Table 3.
Hazard Ratios (HR) for Incident Anemia and Anthropometric Outcomes Among Individuals With Versus Those Without Hypoalbuminemia at Baseline
| Outcome (No. of Events) | Crude HR (95% CI) | P | Adjusted HRa (95% CI) | P |
|---|---|---|---|---|
| Severe anemiab (179) | 2.61 (1.94–3.50) | <.001 | 2.28 (1.67–3.10) | <.001 |
| Anemiac (153) | 1.78 (1.23–2.58) | .002 | 1.42 (.96–2.12) | .081 |
| Wastingd (175) | 2.03 (1.51–2.74) | <.001 | 1.69 (1.22–2.35) | .002 |
| >10% weight losse (278) | 1.44 (1.13–1.83) | .003 | 1.40 (1.08–1.82) | .012 |
Hypoalbuminemia was defined as a serum albumin concentration of <35 g/L. Individuals with outcome events at baseline were excluded from analyses.
Abbreviations: AZT, zidovudine; BMI, body mass index (calculated as the weight in kilograms divided by the height in meters squared); CI, confidence interval; d4T, stavudine; EFV, efavirenz; HIV, human immunodeficiency virus; NVP, nevirapine; WHO World Health Organization; 3TC, lamivudine.
a Adjusted for sex and baseline age (30, 30–39, 40–49, and ≥50 years), WHO HIV disease stage (I/II, III, and IV), CD4+ T-cell count (<50, 50–99, 100–199, and ≥200 cells/μL), and alanine transaminase level (≤40 and >40 IU/L), for all outcomes; baseline BMI (<16.0, 16.0–18.4, 18.5–25.0, and >25.0), for anemia only; baseline hemoglobin level (<8.5, 8.5–11, and ≥11 g/dL), for wasting only; and baseline height, for wasting and >10% weight loss only.
b Defined as a hemoglobin level of <85 g/L for men and women.
c Defined as a hemoglobin level of <120 g/L for women and <130 g/L for men.
d Defined as a BMI of <18.5 kg/m2.
e Defined as a >10% decrease from baseline.
A total of 175 individuals (11.0%) experienced incident wasting during follow-up (Table 3). After multivariate adjustment, individuals with baseline hypoalbuminemia had an increased hazard of wasting as compared to individuals with a serum albumin concentration of ≥35 g/L (HR, 2.03; 95% CI, 1.51–2.74; P < .001; Table 3). Individuals with baseline hypoalbuminemia also had an increased hazard of >10% weight loss (HR, 1.40; 95% CI, 1.08–1.82; P = .012). There was no indication of effect modification by ART regimen, randomized multivitamin regimen, or any other factor for anthropometric analyses.
Absolute CD4+ T-cell count was assessed every 4 months. The change in CD4+ T-cell counts after ART initiation was nonlinear with individuals experiencing rapid increases in CD4+ T-cell counts during the first 6 months of ART with more moderate increases thereafter. After multivariate adjustment, there was no difference in the trajectory of change in CD4+ T-cell count between consecutive visits for individuals with hypoalbuminemia (P = .121) as compared to those with a serum albumin concentration of ≥35 g/L.
DISCUSSION
In this study, we found that hypoalbuminemia at ART initiation was independently associated with increased mortality, pulmonary tuberculosis, wasting, and >10% weight loss. We found no independent associations between serum albumin concentration and change in CD4+ T-cell count after ART initiation, incidence of WHO HIV stage IV disease or death, pneumonia, oral thrush, chronic diarrhea, Kaposi sarcoma, and EP tuberculosis. In secondary continuous analyses, the hazard of mortality and tuberculosis events appeared to be increased for serum albumin concentrations of <38 g/L.
This study confirms the findings from previous cohort studies that showed that hypoalbuminemia was associated with increased mortality among HIV-infected individuals [9–12]. Previous studies have suggested that hypoalbuminemia may not predict mortality at low CD4+ T-cell counts, because of a plateau effect in individuals with advanced HIV disease [10, 13]. In our study, which is the largest conducted to date, we found no statistical evidence of effect modification of the mortality association by CD4+ T-cell count. Hypoalbuminemia was significantly associated with increased mortality among individuals with a baseline CD4+ T-cell count of <50 cells/μL and appeared to be of the same magnitude as that among individuals with a baseline CD4+ T-cell count of ≥50 cells/μL. Furthermore, some of these previous studies did not adjust for BMI, which is likely to capture malnutrition and increase statistical power [11, 12, 14]. We felt it necessary to adjust for BMI, since BMI is routinely and easily measured in resource-limited settings and, consequently, because the serum albumin measurement would have limited clinical usefulness if its predictability was not independent of BMI.
We found that baseline hypoalbuminemia was significantly associated with increased risk of incident pulmonary tuberculosis and also had a borderline significant relationship with EP tuberculosis, which may be a result of low power or nondifferential misclassification. Individuals infected with Mycobacterium tuberculosis are characterized immunologically by acute phase responses, which are systemic inflammatory reactions that can lead to reductions in serum albumin concentrations [31, 32]. Worodria et al found that C-reactive protein (CRP), an acute phase response protein, which was not measured in our study, was significantly associated with incident tuberculosis-associated immune reconstitution inflammatory syndrome [33]. Accordingly, a possible explanation for our findings is that latent M. tuberculosis infection or M. tuberculosis infections that become clinically active after initiating ART will reduce serum albumin concentration.
In secondary analyses, we examined the relationship of serum albumin concentration with mortality and morbidity outcomes continuously. The results of these analyses suggest that individuals initiating ART with a serum albumin concentration of <38 g/L have an increased risk of mortality and pulmonary tuberculosis. A serum albumin concentration of <38 g/L has also been used as a diagnostic criterion by the International Society of Renal Nutrition and Metabolism for protein energy wasting in acute and chronic kidney disease [34]. Consequently, individuals with serum albumin concentrations of 35–38 g/L at ART initiation should also be identified as having an increased risk of adverse HIV treatment outcomes and managed appropriately.
Baseline hypoalbuminemia was also associated with incident severe anemia. These findings may be due to malnutrition and chronic inflammation, which decrease the serum albumin concentration and also suppress erythropoiesis [35, 36]. Studies among individuals undergoing hemodialysis and patients with acute coronary syndrome have also documented that the serum albumin concentration is a strong predictor of anemia cross-sectionally, as well as prospectively [37, 38]. Additionally, hypoalbuminemia was associated with incident wasting and weight loss. Serum albumin concentration is commonly considered a laboratory indicator for malnutrition; however, the association is not clear for HIV-infected individuals, particularly those with higher CD4+ T-cell counts [38, 39].
In this study, we found that serum albumin concentration was strongly associated with death and weight loss but not with the composite end point of WHO stage IV disease or death and change in CD4+ T-cell count. A plausible explanation linking these findings is that the serum albumin concentration is not associated with the incidence of morbidities that arise due to impaired CD4+ T-cell immune reconstitution, which would dilute the mortality association when WHO stage IV disease end points were included. There may also be increased nondifferential misclassification of morbidity outcomes as compared to the firm mortality end point, which will bias morbidity results to the null. Furthermore, the strong association of serum albumin concentration with weight loss may indicate that the serum albumin concentration is acting as an independent marker of increased severity of opportunistic infections. A limitation of this study is that we did not have data on the severity of opportunistic infections, and as a result future studies are needed to investigate this potential mechanism.
This study has several other important limitations. First, we were unable to adjust for HIV load, which may result in residual confounding. Nevertheless, HIV load is often not available in resource-limited settings. Second, we only had a single measurement of the serum albumin concentration, at ART initiation, and it is unclear from this study whether long-term concentrations or changes in serum albumin concentration after ART initiation are superior morbidity and mortality predictors.
Serum albumin concentration is a strong independent predictor of mortality, pulmonary tuberculosis, severe anemia, wasting, and weight loss among HIV-infected individuals initiating ART. Serum albumin concentration may be a useful and low-cost marker of disease severity in resource-limited settings with access to clinical chemistry equipment. Future research should focus on identification and management of conditions that reduce serum albumin concentration level, to improve the treatment and clinical management of individuals initiating ART.
Notes
Acknowledgments. We thank the study participants and field teams, including physicians, nurses, supervisors, laboratory, and the administrative staff, who made the study possible; Muhimbili National Hospital, Muhimbili University of Health and Allied Sciences, city and municipal medical offices of health, and the Ministry of Health and Social Welfare, for their institutional support and guidance; and Dr Edward Giovannucci, for support during the preparation of this manuscript.
Financial support. This work was supported by the National Institute of Child Health and Human Development (grant R01 HD32257) and the National Institute of Allergy and Infectious Diseases (award T32AI007358 to C. R. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4 lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mekonnen Y, Dukers NH, Sanders E, et al. Simple markers for initiating antiretroviral therapy among HIV-infected Ethiopians. AIDS. 2003;17:815–9. doi: 10.1097/00002030-200304110-00006. [DOI] [PubMed] [Google Scholar]
- 3.Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997;52:761–70. doi: 10.1038/ki.1997.393. [DOI] [PubMed] [Google Scholar]
- 4.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 5.Chan JC, Tsui EL, Wong VC Hospital Authority SARS Collaborative Group. Prognostication in severe acute respiratory syndrome: a retrospective time-course analysis of 1312 laboratory-confirmed patients in Hong Kong. Respirology. 2007;12:531–42. doi: 10.1111/j.1440-1843.2007.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2:1434–6. doi: 10.1016/s0140-6736(89)92042-4. [DOI] [PubMed] [Google Scholar]
- 7.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265–9. doi: 10.1054/clnu.2001.0438. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int, 2005;68:766–72. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 9.Sabin CA, Griffioen A, Yee TT, et al. Markers of HIV-1 disease progression in individuals with haemophilia coinfected with hepatitis C virus: A longitudinal study. Lancet. 2002;360:1546–51. doi: 10.1016/S0140-6736(02)11519-4. [DOI] [PubMed] [Google Scholar]
- 10.Feldman JG, Gange SJ, Bacchetti P, et al. Serum albumin is a powerful predictor of survival among HIV-1-infected women. J Acquir Immune Defic Syndr. 2003;33:66–73. doi: 10.1097/00126334-200305010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Shah S, Smith CJ, Lampe F, et al. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Med. 2007;8:38–45. doi: 10.1111/j.1468-1293.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]
- 13.Dao CN, Peters PJ, Kiarie JN, et al. Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses. 2011;27:1149–55. doi: 10.1089/AID.2010.0345. [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, Baeten JM, Richardson BA, et al. A decrease in albumin in early HIV type 1 infection predicts subsequent disease progression. AIDS Res Hum Retroviruses. 2007;23:1197–200. doi: 10.1089/aid.2007.0065. [DOI] [PubMed] [Google Scholar]
- 15.Baeten JM, McClelland RS, Wener MH, et al. Relationship between markers of HIV-1 disease progression and serum beta-carotene concentrations in Kenyan women. Int J STD AIDS. 2007;18:202–6. doi: 10.1258/095646207780132541. [DOI] [PubMed] [Google Scholar]
- 16.Olawumi HO, Olatunji PO. The value of serum albumin in pretreatment assessment and monitoring of therapy in HIV/AIDS patients. HIV Med. 2006;7:351–5. doi: 10.1111/j.1468-1293.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 17.Isanaka S, Mugusi M, Hawkins C, et al. Effect of high-dose multivitamin supplementation at the initiation of HAART on HIV disease progression and mortality in Tanzania: a randomized controlled trial. JAMA. 2012;308:1535–44. doi: 10.1001/jama.2012.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. Geneva: World Health Organization; 2004. [Google Scholar]
- 19.Enarson DA, Rieder HL, Arnadottir T, Trébucq A. Management of tuberculosis. A guide for low income countries. 5th ed. Paris, France: International Union Against Tuberculosis and Lung Disease; 2000. [Google Scholar]
- 20.World Health Organization Expert Committee. Physical status: the use and interpretation of anthropometry. 1995 WHO Technical Report Series 854. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 21.Polsky B, Kotler D, Steinhart C. HIV-associated wasting in the HAART era: Guidelines for assessment, diagnosis, and treatment. AIDS Patient Care STDs. 2001;15:411–23. doi: 10.1089/108729101316914412. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Nutritional anaemias. Report of a WHO Scientific Group. WHO Techn Rep Ser. 1968;405:9–10. [PubMed] [Google Scholar]
- 23.Dati F, Schumann G, Thomas L, et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470) International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. Eur J Clin Chem Clin Biochem. 1996;34:517–20. [PubMed] [Google Scholar]
- 24.Wacholder S. Binomial regression in GLIM: Estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–84. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 26.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 27.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 30.Miettinen O. Theoretical epidemiology. New York: John Wiley & Sons; 1985. [Google Scholar]
- 31.Robson SC, White NW, Aronson I, Woollgar R, Goodman H, Jacobs P. Acute phase response and the hypercoagulable state in pulmonary tuberculosis. Br J Haematol. 1996;93:943–9. doi: 10.1046/j.1365-2141.1996.d01-1722.x. [DOI] [PubMed] [Google Scholar]
- 32.Evans CA, Jellis J, Hughes SP, Remick DG, Friedland JS. Tumor necrosis factor alpha, interleukin 6 and interleukin 8 secretion and the acute phase response in patients with bacterial and tuberculous osteomyelitis. J Infect Dis. 1998;177:1582–7. doi: 10.1086/515313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, et al. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol. 2011;2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 35.Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32:S118–25. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Davis JL, Smith L. Serum albumin is strongly associated with erythropoietin sensitivity in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:98–104. doi: 10.2215/CJN.03330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bindra K, Berry C, Rogers J, et al. Abnormal haemoglobin levels in acute coronary syndromes. QJM. 2006;99:851–62. doi: 10.1093/qjmed/hcl117. [DOI] [PubMed] [Google Scholar]
- 38.Covinsky KE, Covinsky MH, Palmer RM, Sehgal AR. Serum albumin concentration and clinical assessments of nutritional status in hospitalized older people: different sides of different coins? J Am Geriatr Soc. 2002;50:631–7. doi: 10.1046/j.1532-5415.2002.50156.x. [DOI] [PubMed] [Google Scholar]
- 39.Dusingize JC, Hoover DR, Shi Q, et al. Association of serum albumin with markers of nutritional status among HIV-infected and uninfected Rwandan women. PLoS One. 2012;7:e35079. doi: 10.1371/journal.pone.0035079. [DOI] [PMC free article] [PubMed] [Google Scholar]