Abstract
Background. Comprehensive analyses of host, viral, and immune factors associated with severe respiratory syncytial virus (RSV) infection in adults have not been performed.
Methods. Adults with RSV infection identified in both outpatient and inpatient settings were evaluated. Upper and lower respiratory tract virus load, duration of virus shedding, select mucosal chemokine and cytokine levels, humoral and mucosal immunoglobulin responses, and systemic T-cell responses were measured.
Results. A total of 111 RSV-infected adults (61 outpatients and 50 hospitalized patients) were evaluated. Hospitalized subjects shed virus in nasal secretions at higher titers and for longer durations than less ill outpatients, had greater mucosal interleukin 6 (IL-6) levels throughout infection, and had higher macrophage inflammatory protein 1α (MIP-1α) levels early in infection. Persons >64 years old and those with more severe disease had a higher frequency of activated T cells in the blood than younger, less ill subjects at infection. Multivariate analysis found that the presence of underlying medical conditions, female sex, increased mucosal IL-6 level, and longer duration of virus shedding were associated with severe disease. Older age and increased nasal MIP-1α levels were of borderline statistical significance.
Conclusions. Multiple factors, but not older age, are independently associated with severe RSV infection in adults. The presence of underlying medical conditions had the greatest influence on disease severity.
Keywords: respiratory syncytial virus, adults, pathogenesis, viral shedding, immune response
Respiratory syncytial virus (RSV), a single-stranded RNA virus of the family Paramyxoviridae, is the leading cause of severe respiratory illness in infants and young children [1, 2]. A hallmark of RSV infection is incomplete immunity, leading to reinfection throughout life [3–5]. Although reinfection is generally mild, certain adult populations can have severe illness with lower respiratory tract symptoms, resulting in hospitalization and death [3, 6–8]. Factors associated with severe disease in infants are well described and include underlying congenital cardiac or neurological conditions, bronchopulmonary dysplasia, prematurity, low RSV-specific serum antibody titers, and high virus load [2, 9–11]. Additionally, it has been considered that innate and adaptive T-cell responses, as well as cytokine responses, such as interleukin 6 (IL-6) level, strongly influence the pathogenesis of RSV disease in infants [12–14]. Although less well studied, analogous risk factors also appear to be operative in adults. Previously, we found that severe RSV disease in adults was associated with underlying cardiopulmonary disease, frailty, and low serum levels of RSV-specific antibody [15, 16]. In addition, high virus load in hospitalized persons has been associated with respiratory failure [15]. Thus, as in infants, disease severity in adults is likely multifactorial, as advancing age is associated with increased frequency of underlying medical conditions and global changes in immune function [17].
However, a comprehensive assessment of factors, including involvement of the lower airways, virus load, local and systemic cytokine responses, and T-lymphocyte activation during infection, has not been performed in adults. Therefore, we examined these factors in adults with a wide spectrum of disease severity.
METHODS
Patient Population and Illness Surveillance
The study spanned 3 winters, from 2005 through 2008, in Rochester, New York. Two cohorts of adults were recruited after they provided informed consent. The first included persons ≥21 years of age living independently in the community, some with underlying medical conditions. Subjects were enrolled in the late summer and early fall and were followed for development of RSV infection during subsequent winters. They remained under surveillance for 1–3 winters and were asked to call study personnel to report symptoms consistent with respiratory illness (ie, new or worse cough, increase in chronic sputum production, sore throat or nasal congestion, and worsening dyspnea, with or without fever) when they were evaluated as described below.
The second cohort included persons ≥21 years of age hospitalized at Rochester General Hospital during the winter with an admitting diagnosis consistent with an acute respiratory infection (ie, community-acquired pneumonia, acute exacerbation of chronic obstructive pulmonary disease, bronchitis, congestive heart failure, respiratory failure, or acute viral syndrome). Exclusion criteria included residence in a long-term care facility or presence of a significant immunocompromising condition (ie, receipt of chemotherapy or radiation therapy within 6 months of illness, human immunodeficiency virus infection, or receipt of immunosuppressive medications including >15 mg prednisone for >3 weeks).
At enrollment, demographic and medical history, baseline functional score, resting saturated oxygen (SaO2) level, and findings on a focused respiratory examination were recorded. The study was approved by the institutional review boards of the University of Rochester and Rochester General Hospital.
Illness Evaluation
For the prospective cohort, most illness and daily assessments were performed by a study nurse during home visits. For the hospitalized cohort, initial evaluations occurred in the hospital, and visits after discharge took place at home. Symptoms and signs of respiratory illness were recorded, and a nasal swab specimen was obtained by gently swabbing one nostril just below the inferior turbinate for 5 seconds. The nasal swab specimen was placed in 3 mL of distilled water, kept on ice, and processed immediately by reverse transcription polymerase chain reaction (RT-PCR) for RSV RNA. Sputum samples were diluted in an equal volume of distilled water and vortexed vigorously before processing as described elsewhere [18]. RSV-positive subjects were seen again as soon as feasible, generally within 48 hours, when a directed history was obtained, a physical examination was performed, and the following specimens were collected: repeat nasal swab specimen from 1 nostril and a sputum specimen for quantitative RT-PCR analysis, a nasal swab specimen from the opposite nostril for cytokine measurements, a respiratory condensate obtained during a 5-minute interval, 30 mL of heparinized blood for lymphocyte purification, and 10 mL of blood for serum IL-6 and RSV antibody determinations. Heparinized blood was kept at room temperature and transported to the laboratory for lymphocyte purification within 12 hours. Other samples were processed immediately and frozen at −80°C until assayed. Subjects were reevaluated daily if possible for clinical signs and symptoms of respiratory illness and collection of a nasal swab specimen and sputum specimen for RSV RT-PCR. Assessment visits and collection of nasal swab and sputum samples continued until 2 consecutive samples were RT-PCR negative for RSV. Nasal swab and blood samples were also obtained between days 12 and 16 and days 25 and 32 after symptom onset for cytokine measurements, peripheral blood mononuclear cell (PBMC) purification, and serum antibody determinations.
Diagnosis of RSV Infection, Identification of RSV Group, and Determination of RSV Titer
RSV infection, including virus group, was diagnosed using real-time RT-PCR performed on initial nasal swab and sputum specimens as described.[19] Positive and subsequent nasal swab and sputum specimens were assayed using a published quantitative RSV RT-PCR [20]. RSV titers were expressed as plaque-forming unit (PFU) equivalents per milliliter of sample. Peak nasal and sputum titers were defined as the highest titer on any day.
Antibody Measurement
Serum immunoglobulin G (IgG) titers to purified RSV F, Ga, and Gb proteins and neutralizing antibody titers to RSV groups A and B were determined as described elsewhere [16, 21]. Nasal immunoglobulin A (IgA) titers to RSV F, Ga, and Gb proteins was determined and corrected to a total protein concentration of 100 µg/mL, according to published methods [22].
Cytokine Measurement
High-sensitivity enzyme immunoassays (Biosource International, Camarillo, CA) were used for measuring IL-6, IL-8, and macrophage inflammatory protein 1α (MIP-1α) levels in nasal swab and serum specimens. Nasal cytokine values were corrected to a total protein concentration of 100 µg/mL.
Multicolor Flow Cytometry of T Cells
PBMCs were isolated within 12 hours of collection in sodium heparin sulfate tubes, divided into aliquots of 1 million cells, and frozen in liquid nitrogen, as described elsewhere [23]. Frozen cells were thawed and diluted in complete Roswell Park Memorial Institute medium containing 10% fetal calf serum and were stained with live/dead-PacOrange (Invitrogen, Carlsbad, CA) and incubated for 20 minutes with the following anti-human antibodies: CD3-PE-Cy5.5 (Invitrogen), CD4-PE-Cy5 (eBioscience, San Diego, CA), CD8-PE-TR (Invitrogen), HLA-DR-AlexaF700 (eBioscience), APC-CD56 (BD Biosciences, San Diego, CA), CD38-Pacific Blue (BD Biosciences) and for exclusion: CD19-PE-Cy7 (BD Bioscienes), CD16-PE-Cy7 (BD Biosciences), CD14-APC-Cy7 (BD Biosciences). Cells were then washed, and permeabilized with BD Cytoperm/Cytofix. Then, cells were incubated with anti-human Ki67-FITC (Invitrogen) and Bcl-2-PE (Invitrogen). Cells were collected on an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star, v 8.8.7). Total PBMCs were gated using forward scatter and side scatter channels. Antibodies to CD14, CD16, and CD19 were used to exclude nonspecific staining of B cells and monocytes.
RESULTS
We evaluated 111 RSV-infected subjects from the 2 cohorts during 3 consecutive winters; 61 were outpatients, and 50 were hospitalized patients. Subjects ranged in age from 24 to 95 years, 61% were female, and 84% were white. Group A and B RSV were identified in each of the 3 years, of which 40% were group A, 56% were group B, and 4% were untyped.
Analysis According to Illness Severity
In the absence of a validated severity scoring system for nonpneumonic respiratory tract infections, we defined severe disease as the disease requiring hospitalization and mild disease as disease managed on an outpatient basis. Hospitalized subjects were approximately 14 years older than outpatients and more likely to be smokers and to have underlying chronic obstructive pulmonary disease (COPD), diabetes, and cardiac disease and to use inhaled corticosteroids at baseline (Table 1). Hospitalized subjects more often had dyspnea, a lower SaO2 value, and rales and wheezing on lung examination; were ill longer prior to evaluation (5.5 vs 3.1 days; P < .0001); and had symptoms that persisted longer (25 vs 17 days; P < .0001).
Table 1.
Demographic and Clinical Characteristics of Respiratory Syncytial Virus–Infected Outpatients and Hospitalized Patients
| Characteristic | Outpatients (n = 61) | Hospitalized Patients (n = 50) | P |
|---|---|---|---|
| Age, y | <.0001 | ||
| Mean ± SD | 56.7 ± 17.1 | 70.5 ± 14.7 | |
| Range | 24.4–91.1 | 41.5–95.8 | |
| Male sex | 24 (33) | 20 (40) | NS |
| Never smoked | 30 (49) | 15 (30) | .05 |
| Home O2 therapy | 5 (8) | 10 (20) | .10 |
| Underlying condition | |||
| COPD | 10 (16) | 24 (48) | .0004 |
| CHF | 0 (0) | 13 (26) | .0001 |
| DM | 5 (8) | 16 (32) | .003 |
| CAD | 3 (5) | 16 (32) | .0002 |
| Medication | |||
| Oral corticosteroids | 2 (3) | 7 (14) | .076 |
| Inhaled corticosteroids | 11 (18) | 18 (36) | .05 |
| Symptom duration before diagnosis, d | 3.1 ± 1.8 | 5.5 ± 3.8 | <.0001 |
| Treatment with systemic GCS | 3 (5) | 33 (66) | <.0001 |
| Illness duration, d | 16.8 ± 5.8 | 25 ± 9.7 | <.0001 |
| Signs and symptoms | |||
| Temperature, °C | 36.8 ± 0.6 | 37.1 ± 1.0 | .01 |
| Respiratory rate, breaths/min | 15.6 ± 3.8 | 23.8 ± 7.0 | <.0001 |
| SaO2 on room air, % | 97.4 ± 2.2 | 92.1 ± 7.3 | <.0001 |
| Dyspnea | 20 (33) | 47 (94) | .0001 |
| Wheezing on examination | 7 (11) | 40 (80) | .0001 |
| Rales on examination | 3 (5) | 16 (32) | .0002 |
Data are no. (%) of patients or mean value ± SD. Outpatients had mild disease, whereas hospitalized patients had severe disease.
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GCS, glucocorticosteroids; NS, not significant; SaO2, oxygen saturation.
Group A and B viruses were equally distributed between patients with mild and those with severe illnesses (Table 2). There was a trend toward higher peak virus titers in nasal and lower airway secretions among hospitalized persons, but neither reached statistical significance. Because subjects were evaluated at variable times after symptom onset, nasal virus shedding patterns for inpatients and outpatients were developed by combining data from all subjects. This showed that shedding began 1–2 days after symptom onset, peaked by day 3, and began to decline by days 5–6 for both groups (Supplementary Figure 1). Overall, hospitalized persons had slightly higher titers on most days. Hospitalized persons shed virus in nasal secretions for 3 days longer than mildly ill outpatients (13.1 vs 9.8 days; P = .003), although the duration of lower respiratory tract shedding was similar. Hospitalized subjects with respiratory failure had significantly higher mean nasal virus titers (±SD) than hospitalized subjects not requiring ICU care (3.4 ± 1.2 vs 2.5 ± 1.6 log10 PFU/mL; P = .04).
Table 2.
Viral, Antibody, and Cytokine Measurements for Respiratory Syncytial Virus (RSV)–Infected Outpatients and Hospitalized Patients
| Variable | Outpatients (n = 61) | Hospitalized Patients (n = 50) | P |
|---|---|---|---|
| RSV group | |||
| A | 27 | 17 | NS |
| B | 32 | 32 | NS |
| Untyped | 3 | 1 | NS |
| Peak RSV titer, log10 PFU/mL | |||
| Nasal specimens | 2.3 ± 1.2 | 2.8 ± 1.3 | .09 |
| Sputum specimens | 2.5 ± 1.8 | 3.3 ± 1.8 | .06 |
| Duration of RSV shedding, d | |||
| Nasal specimens | 9.8 ± 4.8 | 13.1 ± 6.3 | .003 |
| Sputum specimens | 9.1 ± 4.0 | 10.1 ± 5.4 | NS |
| Acute-phasea serum IgG titer, log2 dilution | |||
| To RSV F | 15.4 ± 1.4 | 16.1 ± 1.6 | .018 |
| To RSV Ga | 14.2 ± 1.7 | 15.1 ± 1.8 | .005 |
| To RSV Gb | 14.0 ± 1.3 | 14.6 ± 1.8 | .04 |
| Acute-phasea serum neutralizing antibody titer, log2 | |||
| To RSV group A | 10.7 ± 1.5 | 11.5 ± 2.1 | .022 |
| To RSV group B | 11.0 ± 1.5 | 11.6 ± 2.1 | .076 |
| Acute-phasea nasal IgA titer, log2 | |||
| To RSV F | 3.2 ± 0.9 | 2.7 ± 1.4 | .045 |
| To RSV Ga | 3.9 ± 1.2 | 3.2 ± 1.3 | .003 |
| To RSV Gb | 3.9 ± 1.3 | 2.8 ± 1.3 | <.0001 |
| Serum IL-6 level, pg/mL | |||
| Acute phasea | 5.9 ± 12.5 | 9.2 ± 21.2 | NS |
| Day 28 | 5.9 ± 16.8 | 7.4 ± 15.5 | NS |
| Nasal IL-6 level, pg/mL | |||
| Acute phasea | 3.6 ± 3.4 | 7.6 ± 7.8 | <.001 |
| Day 10–12 | 2.3 ± 3.4 | 5.1 ± 5.3 | .001 |
| Day 28 | 1.7 ± 1.8 | 5.7 ± 4.5 | <.001 |
| Nasal IL-8 level, pg/mL | |||
| Acute phasea | 290 ± 216 | 331 ± 255 | NS |
| Day 10-12 | 372 ± 258 | 354 ± 350 | NS |
| Day 28 | 322 ± 258 | 289 ± 267 | NS |
| Nasal MIP-1α level, pg/mL | |||
| Acute phasea | 20.2 ± 28.1 | 32.3 ± 40.6 | .068 |
| Day 10–12 | 12.6 ± 24.6 | 16.8 ± 23.4 | NS |
| Day 28 | 7.7 ± 17.7 | 6.0 ± 7.9 | NS |
Data are no. of patients or mean value ± SD. Outpatients had mild disease, whereas hospitalized patients had severe disease.
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; IL-6, interleukin 6; IL-8, interleukin 8; MIP-1α, macrophage inflammatory protein 1α; NS, not significant; PFU, plaque-forming units.
a At the time of diagnosis of RSV infection.
Unexpectedly, serum IgG titers to RSV envelope glycoproteins and RSV-neutralizing antibody titers in acute-phase specimens were slightly, but significantly, higher in hospitalized persons. Because hospitalized persons had been symptomatic nearly twice as long as mildly ill outpatients at evaluation, we analyzed antibody kinetics in the outpatient group to assess the effect of timing on acute-phase antibody titers. This indicated that the neutralizing antibody level rises rapidly between days 2 and 5 after illness onset, and by day 5–6 titers more than doubled (Figure 1). Thus, the delay in obtaining serum may have contributed to slightly higher acute-phase RSV-specific serum antibody titers in hospitalized subjects.
Figure 1.
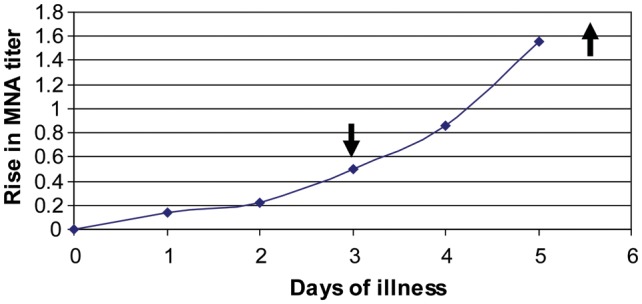
Kinetics of serum microneutralization titer to group A respiratory syncytial virus (RSV; MNA) in RSV-infected outpatients with mild disease. Down arrow indicates mean time of evaluation for the outpatient group, and up arrow indicates mean time of evaluation for the hospitalized group.
In contrast, mean RSV-specific nasal IgA antibody titers in acute-phase specimens were significantly lower in the hospitalized group despite a longer illness duration at evaluation, although the absolute difference was small (approximately 2-fold). We did not collect nasal samples before illness in the prospective cohort and therefore could not assess the kinetics of nasal IgA during infection.
Despite marked differences in disease severity between groups, acute- and convalescent-phase serum IL-6 levels were similar (Table 2). However, nasal IL-6 levels were greater at each time point in hospitalized subjects. Mean nasal MIP-1α levels were also higher in acute-phase specimens from hospitalized persons. For both groups, MIP-1α levels fell substantially from the acute-phase time point to day 12, suggesting that levels peak early after symptom onset. When only data from samples obtained on days 1–4 after illness onset were considered, hospitalized subjects had ∼2-fold higher mean MIP-1α levels (±SD) than less ill outpatients (33.8 ± 30.7 vs 19.1 ± 26.7 pg/mL; P = .05).
Relationship Between Virus Titer and Antibody
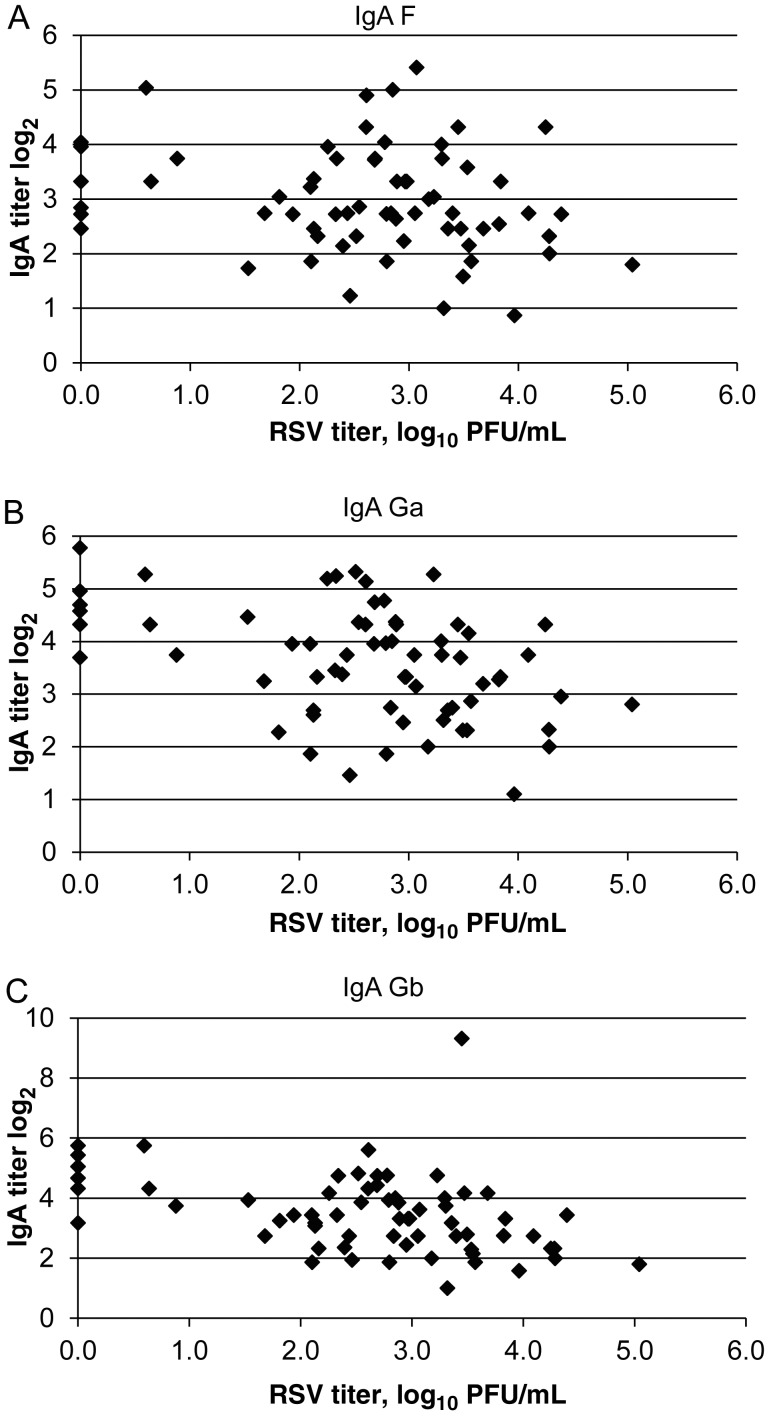
We also explored the relationship between nasal and sputum virus titers and antibody levels. Because the serum antibody level increases rapidly after illness onset, the analysis was limited to persons with symptoms for <5 days at evaluation. There was a weak inverse correlation between peak nasal virus titer and serum IgG to RSV envelope glycoproteins (F: r = −.21; Ga: r = −.09; and Gb: r = −.18) but a stronger inverse correlation with nasal IgA titers (F: r = −.22, P = .07; Ga: r = −.44, P = .0002; and Gb: r = −.40, P = .001; Figure 2A–C). The strongest relationship was noted when antibody titer was matched to infecting virus group (IgA to Ga protein and RSV A titer: r = −.62, P = .0004; data not shown).
Figure 2.
Relationship between nasal respiratory syncytial virus (RSV)–specific immunoglobulin A (IgA) and viral titers in subjects ill for ≤4 days at the time of evaluation. A, IgA to F: r = −.22, P = .07. B, IgA to Ga: r = −.44, P = .0002. C, IgA to Gb: r = −.40, P = .001.
Age-Related Effects
Because older persons were disproportionately represented in the hospitalized group and had more underlying medical conditions that could influence disease severity, we analyzed the less ill outpatients separately to better assess age-related differences in virus shedding and antibody and cytokine responses (Table 3). Subjects ≥65 years of age had higher nasal titers (log10 2.8 vs 2.0 PFU/mL; P = .01) and shed virus approximately 3 days longer in both upper and lower respiratory tract secretions (nasal: 11.3 vs 8.7 days; sputum: 10.5 vs 7.2 days; P = .04 for both comparisons). Older subjects with mild illness also had a greater rise in antibody titers after infection. We did not find significant age-related differences in cytokine levels, except for higher nasal IL-8 levels on day 12. However, as noted previously for the entire population, when the analysis was limited to nasal samples collected early in infection (days 1–4 of illness), older subjects had significantly higher mean MIP-1α levels (±SD) than younger subjects (28.4 ± 35.9 vs 12.2 ± 12.6 pg/mL; P = .04).
Table 3.
Viral and Antibody Comparisons for Respiratory Syncytial Virus (RSV)–Infected Outpatients Aged <65 Years or ≥65 Years
| Variable | <65 years (n = 37) | ≥65 years (n = 24) | P |
|---|---|---|---|
| RSV titer, log10 PFU/mL | |||
| Nasal | 2.0 ± 1.3 | 2.8 ± 1.0 | .01 |
| Sputum | 2.3 ± 1.9 | 2.6 ± 1.8 | NS |
| Duration of RSV shedding, d | |||
| Nasal | 8.7 ± 4.3 | 11.3 ± 5.2 | .04 |
| Sputum | 7.2 ± 4.0 | 10.5 ± 3.8 | .04 |
| Rise in serum IgG titer, log2 | |||
| To RSV F | 1.2 ± 1.2 | 2.1 ± 1.6 | . 01 |
| To RSV Ga | 1.5 ± 1.5 | 1.6 ± 1.4 | NS |
| To RSV Gb | 1.2 ± 1.1 | 2.2 ± 1.5 | .009 |
| Rise in serum neutralizing antibody titer, log2 | |||
| To RSV group A | 1.9 ± 1.4 | 2.8 ± 1.9 | .04 |
| To RSV group B | 1.6 ± 1.4 | 2.9 ± 2.0 | .004 |
| Rise in nasal IgA titer, log2 | |||
| To RSV F | 1.7 ± 1.9 | 2.8 ± 1.5 | .02 |
| To RSV Ga | 1.9 ± 1.7 | 3.1 ± 1.5 | .008 |
| To RSV Gb | 1.8 ± 1.6 | 2.8 ± 1.9 | .03 |
| Serum IL-6 level, pg/mL | 4.5 ± 11.2 | 7.8 ± 14.0 | NS |
| Nasal IL-6 level, pg/mL | |||
| Acute phasea | 3.4 ± 3.2 | 3.8 ± 3.6 | NS |
| Day 12 | 2.5 ± 4.2 | 2.0 ± 2.1 | NS |
| Day 28 | 1.7 ± 2.0 | 1.8 ± 1.6 | NS |
| Nasal IL-8 level, pg/mL | |||
| Acute phasea | 278 ± 198 | 309 ± 245 | NS |
| Day 12 | 312 ± 205 | 454 ± 304 | NS |
| Day 28 | 301 ± 218 | 351 ± 309 | .04 |
| Nasal MIP-1α level, pg/mL | |||
| Acute phasea | 17.0 ± 23.6 | 25.3 ± 33.0 | NS |
| Day 12 | 13.0 ± 25.6 | 12.0 ± 24.2 | NS |
| Day 28 | 7.9 ± 16.9 | 7.4 ± 19.3 | NS |
Data are mean value ± SD. All outpatients had mild disease.
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; IL-6, interleukin 6; IL-8, interleukin 8; MIP-1α, macrophage inflammatory protein 1α; NS, not significant; PFU, plaque-forming units.
a At the time of diagnosis of RSV infection.
T-Cell Proliferation and Activation During RSV Infection
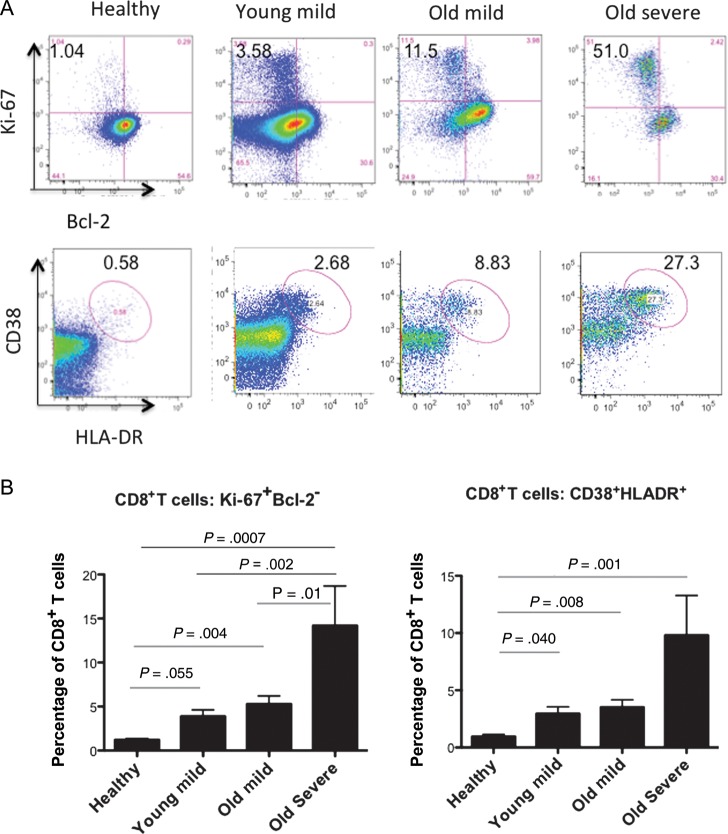
We analyzed samples for an activated CD8+ T-cell phenotype, identifiable as CD38+HLA-DR+ or Ki-67+Bcl-2− cells. These cells have been shown to directly correlate with virus-specific CD8 responses during live virus exposure [24]. We randomly selected subjects who had not received corticosteroids prior to blood sampling, from 3 groups: persons <40 years of age with mild disease (young mild; n = 10), persons ≥65 years of age with mild disease (old mild; n = 13), and older persons with severe disease (old severe; n = 10). PBMCs from 5 uninfected asymptomatic healthy controls (mean age [±SD], 38 ± 16 years) were also analyzed. Multicolor flow cytometry findings of CD8+ T cells with a profile of activation and proliferation (CD38+HLA-DR+ and Ki-67+Bcl-2−) from a representative subject are shown in Figure 3A. The highest frequency of activated Ki-67+Bcl-2− CD8+ T cells during RSV infection was found in the old severe group (mean [±SD], 14.2% ± 13.5%), significantly greater than in the old mild group (P = .01), the young mild group (P = .002), and the control group (P = .0007; Figure 3B). The young mild and old mild groups also had a greater percentage of activated Ki-67+Bcl-2− CD8+ T cells than the control group, although only the latter reached statistical significance. The CD38+HLA-DR+ CD8+ T-cell activated phenotype frequency was also greater for each of the RSV-infected groups, compared with the uninfected controls, although differences between each of the RSV-infected groups did not reach statistical significance. Backgating indicated that >90% of the CD38+HLA-DR+ cells were Ki-67+Bcl-2–, and conversely 70%–90% of Ki-67+Bcl-2– cells were CD38+HLA-DR+.
Figure 3.
CD8+ T-cell responses during acute respiratory syncytial virus (RSV) infection (4 groups): healthy controls (n = 5), persons <40 years of age with mild disease (young mild; n = 10), persons ≥65 years of age with mild disease (old mild; n = 13), and older persons with severe disease (old severe; n = 10). A, The top panel shows CD8+Ki-67+Bcl-2+ CD8+ T cells (ie, proliferating CD8+ T cells), and the bottom panel shows CD8+CD38+HLA-DR+ CD8+ T cells (ie, activated CD8+ T cells). B, Mean percentages (±SD) of CD8+ T cells that were Ki-67+Bcl-2+ (left) or CD38+HLA-DR+ (right) from each group. The mean time of blood collection (±SD) in relation to symptom onset was similar for each group (mild young, 4.8 ± 1.8 days; mild old, 5.5 ± 1.4 days; and severe old, 5.9 ± 2.1 days). All times were expected to coincide with peak viral shedding.
Proliferating CD4+ T-cell frequencies during acute RSV infection were also significantly greater in the old severe group as compared to the old mild group (P = .03), young mild group (P = .007), and control group (P = .08; data not shown).
Multivariate Analysis
Multivariate logistic regression was used to determine variables, especially age, that are independently associated with disease severity in adults. Since the T-cell analysis was only performed on a subset of subjects, these data were not included in this analysis, nor were serum antibody data included, owing to the delay in collection of serum in the hospitalized group. In bivariate analysis with each of the other variables, age remained significant, except when paired with the total number of underlying medical conditions. By using forward selection, the best model to predict severity was determined (Table 4). In order of increasing odds ratio, the number of underlying comorbidities, female sex, nasal IL-6 level, and duration of virus shedding were each independently associated with increased severity. The level of nasal MIP-1α was borderline significant. Age, however, did not reach statistical significance as an independent predictor of illness severity.
Table 4.
Results of Multivariate Analysis of Factors Associated With Severe Disease in Adults
| Variable | OR (95% CI) | P |
|---|---|---|
| Age, per 10 y increase | 1.4 (.96–2.1) | .08 |
| Nasal shedding duration >7 d | 2.2 (1.0–4.9) | .03 |
| Acute-phasea log2 IL-6 level | 2.2 (1.2–4.2) | .01 |
| MIP-1α level ≥ 40 ng/mL | 9.1 (.95–87.6) | .06 |
| Underlying conditions | 18.4 (5.1–65.9) | <.0001 |
| Female sex | 5.4 (1.2–23.8) | .03 |
Abbreviations: CI, confidence interval; IL-6, interleukin 6; MIP-1α, macrophage inflammatory protein 1α; OR, odds ratio.
a At the time of diagnosis of RSV infection.
DISCUSSION
Although RSV infection primarily considered a disease of infants and young children, it is increasingly appreciated as a cause of serious illness in various adult populations. Although studies have documented the incidence and clinical outcome of RSV infection in these populations, few have examined viral and immune factors associated with RSV.
In this analysis of 111 RSV-infected adults age 24–95 years, the duration of virus shedding averaged approximately 10–13 days in nasal secretions, with shedding in some lasting for ≥20 days. Interestingly, in available sputum specimens, we consistently found viral RNA, with levels slightly above nasal titers, suggesting that virus replication may commonly involve lower airways in adults. This finding is consistent with our recent report that detection rates for many respiratory viruses are greater when sputum is tested [18]. However, it is possible that viral RNA in expectorated sputum might reflect upper respiratory tract contamination, because we did not qualitatively examine sputum microscopically. We rarely detected RSV RNA in respiratory condensates, similar to results reported for influenza virus infections [25].
Despite relatively prolonged viral shedding in many subjects, peak viral titers were substantially lower in comparison to those in infants [11]. Older subjects had higher nasal titers, shed longer, and, consistent with our prior studies, had greater serum and nasal antibody responses to RSV infection than younger subjects [26]. The quantitative increase in neutralizing antibody directly correlated with both peak nasal titer and duration of virus shedding. Recently, we reported that adults with prolonged virus shedding also had a longer duration of detectible RSV-specific antibody-secreting cells in blood [23]. These results suggest that the greater humoral immune response in older persons, compared with younger individuals, may be due to the prolonged and heightened antigenic stimulation associated with viral shedding in older persons.
We were particularly interested to learn whether disease severity was associated with heightened innate and inflammatory cytokine responses. We noted that more severely ill subjects had significantly higher nasal MIP-1α levels early in infection and that nasal IL-6 levels were higher at each time point in severely ill hospitalized subjects, compared with less ill outpatients, similar to findings in infants. The difference for IL-6 remained significant in multivariate analysis, while difference for MIP-1α almost reached significance. Others have also reported that levels of MIP-1α, a chemotactic protein for NK and T lymphocytes secreted by macrophages, T cells, and dendritic cells, correlated with the quantity of leukocytes in respiratory secretions and RSV disease severity in infants [27, 28]. Our finding that MIP-1α levels peak early and fall rapidly in nasal secretions is also similar to the kinetics described in infants with RSV infection [29]. Whether these innate chemokine responses are causally related or simply a marker of disease severity is not known.
Previously, we and others noted an inverse correlation between antibody titer and both infection and disease severity in adults with RSV infection [16, 21, 30]. Our failure to reproduce this may relate to a different study design, resulting in later initial blood draws in the hospitalized group. The early rapid anamnestic rise in serum antibody may have obscured differences between the 2 groups. Nevertheless, we noted an inverse relationship between nasal RSV-specific IgA and peak viral titers, which was strongest for antibody specific to the RSV G protein. Since the G protein serves as one of the viral attachment proteins and also has potentially important immunomodulatory effects, it is plausible that antibody to G protein may be most efficient at reducing viral titers [31].
The unexpected finding that female sex was associated with more severe disease is interesting but not readily explained. Perhaps women are more frequently exposed to RSV-infected infants, thus resulting in a higher viral inoculum. Among inpatients, ages were similar by sex, but women were significantly less likely to have COPD than men (21% vs 58%; P = .006), with a similar trend among outpatients. Although older age was associated with more severe disease in univariate and, in most cases, bivariate analyses, multivariate logistic regression did not support an independent effect of age on severity. Rather, viral factors such as duration of shedding, innate chemokine responses, and the number of chronic medical conditions had the greatest association with disease severity.
Finally, we were interested in exploring T-cell responses to infection. Previously, we reported an age-related decrease in interferon γ production in antigen-stimulated PBMCs and a decrease in the ratio of CD8+/CD4+ memory T cells expressing interferon γ after in vitro stimulation with RSV [32, 33]. Others have also reported that the number of RSV-specific memory CD8+ T cells in peripheral blood diminishes with advancing age [34, 35]. In this study, we noted an increase in activated and proliferating CD8+ T cells in older persons with both mild and severe illness due to RSV, compared with mildly ill younger persons. Since the assay does not use antigen stimulation, it is unclear whether the activated CD8+ T cells are all RSV specific, and it is possible that bacterial coinfection or generalized activation by cytomegalovirus in the oldest and most ill group could play a role in activating CD8+ T cells. Some have proposed that CD8+ T cells with these phenotypes may be a more accurate measure of the total antiviral T-cell response than methods such as tetramer staining, which will underestimate the total viral T-cell response by using single epitopes [24, 36]. If so, this study might suggest that CD8+ T-cell responses may not be specifically deficient in older persons, although their functional capacity, which was not measured, could be impaired. Our results suggest that older persons, especially those with more severe disease, have increased T-cell activation, and it is possible these cells are causally implicated in disease severity. However, our data do not address whether differences in T-cell responses contribute to prolonged and higher virus shedding in older persons. Further analysis of the T-cell responses in these subjects is currently in progress.
This study has a number of limitations. The foremost limitation is the study's inability to precisely determine the time of virus inoculation and duration of infection prior to evaluation as it relates to measurements of cytokine, antibody, and T-cell responses. We chose to use time from symptom onset, acknowledging that this may be an imprecise measurement, especially in older and severely ill subjects, although the consistent shedding curves for outpatient and inpatient groups (Supplementary Figure 1) suggest that this is a reasonable approach. In addition, despite our use of logistic regression analysis, a larger study may have been required to identify age as a risk for severe disease, given the degree of heterogeneity in our subject population.
In summary, older adults and those with more severe disease shed virus longer and at slightly higher levels and have greater mucosal innate chemokine responses during RSV infection than younger adults or those with mild disease. However, the greatest influence on disease severity is underlying medical comorbidities, a factor less amenable to modification. Thus, prevention with an effective vaccine would be desirable.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants UO1 AI-045969 and K23 AI67501-01A1 [to F. E. L.]).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 4.Henderson FW, Collier AM, Clyde WA, Denny FW. Respiratory syncytial-virus infections, reinfections and immunity. N Engl J Med. 1979;300:530–4. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–6. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 6.Whimbey E, Couch RB, Englund JA, et al. Respiratory syncytial virus pneumonia in hospitalized adult patients with leukemia. Clin Infect Dis. 1995;21:376–9. doi: 10.1093/clinids/21.2.376. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–15. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 10.Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–8. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 12.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukens MV, van de Pol AC, Coenjaerts FE, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–83. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan CB, Walsh EE, Peterson DR, Lee FE, Falsey AR. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200:1242–6. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189:233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 17.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–24. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Formica MA, Walsh EE. Yield of sputum for viral detection by reverse transcriptase PCR in adults hospitalized with respiratory illness. J Clin Microbiol. 2012;50:21–4. doi: 10.1128/JCM.05841-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63:191–6. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78:1493–7. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- 21.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–8. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 22.Walsh EE, Falsey AR. A simple and reproducible method for collecting nasal secretions in frail elderly adults, for measurement of virus-specific IgA. J Infect Dis. 1999;179:1268–73. doi: 10.1086/314726. [DOI] [PubMed] [Google Scholar]
- 23.Lee FE, Falsey AR, Halliley JL, Sanz I, Walsh EE. Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults. J Infect Dis. 2010;202:1659–66. doi: 10.1086/657158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 25.St George K, Fuschino ME, Mokhiber K, Triner W, Spivack SD. Exhaled breath condensate appears to be an unsuitable specimen type for the detection of influenza viruses with nucleic acid-based methods. J Virol Methods. 2010;163:144–6. doi: 10.1016/j.jviromet.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–9. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 27.Garofalo RP, Hintz KH, Hill V, Patti J, Ogra PL, Welliver RC., Sr A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol. 2005;75:282–9. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]
- 28.Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–22. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 29.McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191:1225–32. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 30.Luchsinger V, Piedra PA, Ruiz M, et al. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis. 2012;54:905–12. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Choi Y, Haynes LM, et al. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduced pulmonary inflammation and virus replication in mice. J Virol. 2010;84:1148–57. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee FE, Walsh EE, Falsey AR, et al. The balance between influenza- and RSV-specific CD4 T cells secreting IL-10 or IFNgamma in young and healthy-elderly subjects. Mech Ageing Dev. 2005;126:1223–9. doi: 10.1016/j.mad.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Looney RJ, Falsey AR, Walsh E, Campbell D. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis. 2002;185:682–5. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 34.Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. Age related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun Ageing. 2010;7:14. doi: 10.1186/1742-4933-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bree GJ, Heidema J, van Leeuwen EM, et al. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–8. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 36.Heidema J, Lukens MV, van Maren WW, et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179:8410–7. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.