Abstract
Genetic studies indicate that protein homeostasis is a major contributor to metazoan longevity1. Collapse of protein homeostasis results in protein misfolding cascades and the accumulation of insoluble protein fibrils and aggregates, such as amyloids2. A group of small molecules, traditionally used in histopathology to stain amyloid in tissues, bind protein fibrils and slow aggregation in vitro and in cell culture3,4. We proposed that treating animals with such compounds would promote protein homeostasis in vivo and increase longevity. Here we show that exposure of adult Caenorhabditis elegans to the amyloid-binding dye Thioflavin T (ThT) resulted in a profoundly extended lifespan and slowed ageing. ThT also suppressed pathological features of mutant metastable proteins and human β-amyloid-associated toxicity. These beneficial effects of ThT depend on the protein homeostasis network regulator heat shock factor 1 (HSF-1), the stress resistance and longevity transcription factor SKN-1, molecular chaperones, autophagy and proteosomal functions. Our results demonstrate that pharmacological maintenance of the protein homeostatic network has a profound impact on ageing rates, prompting the development of novel therapeutic interventions against ageing and age-related diseases.
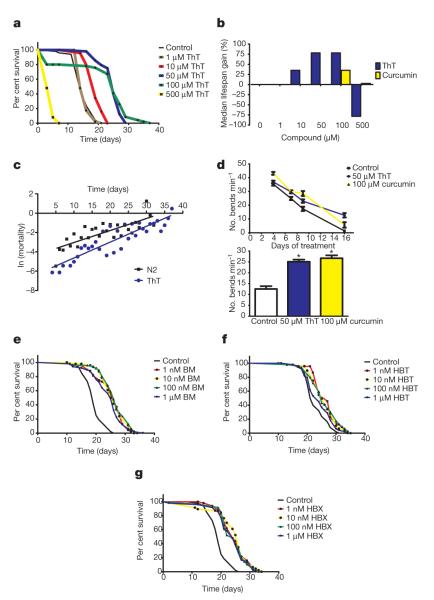
The longevity of the nematode Caenorhabditis elegans is influenced by hundreds of genes including an insulin-like signalling pathway (ILS) that regulates the activities of the transcription factors FOXO-like DAF-16 (ref. 5) and Nrf2-like SKN-1 (ref. 6). Together with the stress response transcription factor HSF-1, DAF-16 also regulates protein homeostasis and influences lifespan7–9, indicating that chemical modulation of protein homeostasis might slow ageing. We reasoned that compounds that have protein-fibril- and protein-aggregate-binding properties may affect age-related changes to protein homeostasis and tested a series of amyloid-binding proteins for lifespan effects. We found that exposing sterilized wild-type (N2) nematodes to the fibril-binding flavonoid ThT (4-(3,6-dimethyl-1,3-benzothiazol-3-ium-2-yl)-N,N-dimethylaniline chloride)10 at either 50 or 100 μM throughout adult life leads to an increase in median (60%) and maximal lifespan (43–78%; Fig. 1a, b, Supplementary Fig. 2 and Supplementary Table 1). The compound reduced age-specific mortality at all ages (P < 0.001, Fig. 1c) and slowed age-related decline in spontaneous movement (Fig. 1d), indicating improved health throughout adulthood. At higher doses (500 μM) ThT is toxic and shortens lifespan (Fig. 1a, b). Other compounds with protein-aggregate-binding properties, including curcumin and rifampicin, increased lifespan to a lesser extent (up to 45%) (Supplementary Figs 3, 4). When ThT and curcumin treatments were combined, we did not observe additive effects on lifespan (Supplementary Fig. 5).
Figure 1. Amyloid-binding compounds extend C. elegans lifespan.
a, Dose–response Kaplan–Meier survival curves of synchronously ageing hermaphrodite wild-type (N2) populations exposed to 0 μM (control) to 500 μM ThT at 20 °C. b, Per cent change in median lifespan of N2 populations cultured on 0–500 μM ThT and curcumin. c, ln-linear plot of age-specific mortality rate with age for control and 50 μM ThT-treated C. elegans. d, Effect of 50 μM ThT and 100 μM curcumin on motility of N2 worms evaluated as the mean number of body bends in a 20-s period in 15 individual worms throughout life (upper panel) and after 12 days of treatment (lower panel) with ThT and curcumin. Data are presented as bends min−1 and represent the average of three independent experiments. *P < 0.0001. e–g, Dose–response Kaplan–Meier survival curves of synchronously ageing hermaphrodite N2 populations exposed to 0 μM (control) to 1 μM of BM (e), HBT (f) and HBX (g) at 20 °C. Plots are representative of three independent experiments.
We then tested several compounds with similar structural features to ThT, but with different pharmacological properties: 2-(2-hydroxyphenyl)-benzoxazole(HBX),2-(2-hydroxyphenyl) benzothiazole (HBT) and 2-(2-aminophenyl)-1H-benzimidazole (BM)11 (Supplementary Fig. 6). These compounds also extended the lifespan of adult nematodes (up to 40%) but at concentrations significantly lower than ThT (Fig. 1e–g), indicating that the bioavailability and/or pharmacological properties of ThT-like compounds influence lifespan.
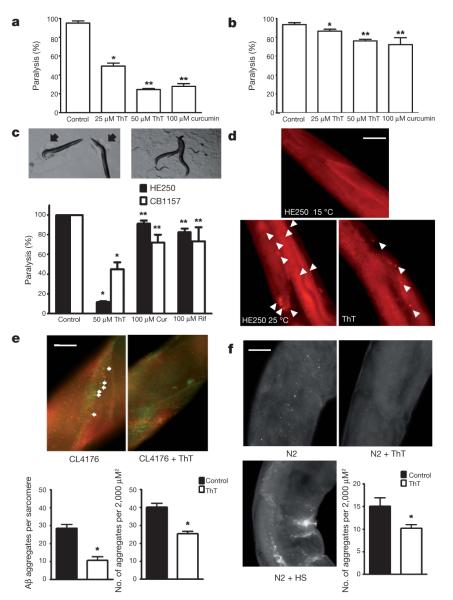
To test the effects of ThT on protein homeostasis we exploited two C. elegans models of human proteotoxic disease: the strain CL4176 (dvIs27[myo-3::Aβ3–42 let 3′UTR(pAF29); pRF4 (rol-6(su1006))])12, which expresses an aggregating amyloid-β(3–42) peptide (Aβ(3–42)) in muscle tissue13 and AM140 (rmIs132[P(unc-54) Q35::YFP]), which expresses a polyglutamine (polyQ) protein. Amyloid-β aggregates are associated with lesions in Alzheimer’s disease, and polyQ aggregation is a feature in several neurological conditions14. When raised at 25°C, nematodes expressing these proteins in muscle accumulate protein aggregates and become paralysed. We found that 50 μM ThT and 100 μM curcumin decreased the proportion of paralysed worms (Fig. 2a, b). By immunohistochemistry we found that ThT reduced Aβ(3–42) aggregation in vivo and preserved muscle integrity in CL4176 (Fig. 2e). We also found that ThT rescued Aβ(3–42) aggregation-induced paralysis even when nematodes were treated 18 h after the induction of aggregate formation, indicating that ThT can ameliorate detrimental effects during the development of the aggregate-related pathology (Supplementary Fig. 7).
Figure 2. ThT and curcumin rescue a paralysis phenotype and slow protein aggregation in vivo.
a, b, The paralysis phenotype associated with protein aggregation is suppressed by 25 μM ThT, 50 μM ThT, 100 μM curcumin in CL4176 (*P < 0.001, **P < 0.0001) expressing Aβ(3–42) (a) and AM140 (*P < 0.05, **P < 0.01) expressing polyQ (b) after 1 and 8 days at 25 °C, respectively. Error bars represent the mean ± s.e.m. of four independent experiments. c, Temperature-sensitive strain HE250 [unc-52(e669su250)II] after 36 h at 25 °C showing the typical paralysis phenotype (left upper panel) and the rescue elicited by 50 μM ThT (right upper panel). Arrows indicate the halos of clearance in the bacterial lawn characteristic of paralysed worms. Lower panel shows protection (± s.e.m.) of the HE250 paralysis phenotype by 50 μM ThT, 100 μM curcumin (Cur) and 100 μM rifampicin (Rif).
*P < 0.0001, **P < 0.01. n = 4 independent experiments. d, Perlecan immunolocalization showing disruption/aggregation pattern after 24 h at 25 °C, as compared with worms raised at the permissive temperature (upper panel), and the suppression of disruption by 50 μM ThT treatment. Sixteen of twenty worms showed similar perlecan distribution in three independent experiments. Arrows indicate perlecan aggregates. Scale bar, 30 μm.
e, Immunolocalization of aggregation-prone soluble oligomeric protein (A11 antibody, red) and Aβ(3–42) (green) in the presence or absence of 50 μM ThT in CL4176. Scale bar, 10 μm. Error bars represent the mean ± s.e.m., 11 worms per group in 3 independent experiments. *P < 0.0001. f, Immunolocalization of aggregation-prone soluble oligomeric protein (A11 antibody) in the presence or absence of 50 μM ThT and under heat shock (HS) in 11 days old wild-type N2 worms. Scale bar, 20 μm. Error bars represent the mean ± s.e.m., 11 worms per group, of 3 independent experiments. *P < 0.0001. Scale bar, 10 μm.
If amyloid-binding compounds extended lifespan through improved protein homeostasis, then we expected that they would influence not only heterologous disease-related models but also nematode proteins. We tested ThT and curcumin on mutant worms that express metastable proteins previously exploited as indicators of the protein homeostatic network capacity15. Strains carrying mutations in the genes unc-52 (HE250 [unc-52(e669su250)II]) and unc-54 (CB1157 [unc-54(e1157)I]) produce temperature-sensitive muscle proteins UNC-52 (perlecan) and UNC-54 (myosin class II heavy chain), respectively, that exhibit altered structure and cause paralysis at 25 °C15,16,17. We found that ThT suppressed paralysis of these mutants (Fig. 2c), prevented the disruption of the muscle sarcomeres (Supplementary Fig. 8) and restored perlecan organization (Fig. 2d). We extended these observations to other temperature-sensitive missense protein-folding mutations expressed in the neuromuscular junction and in the nervous system18. We found that ThT suppressed ethanol sensitivity in a strain carrying the gas-1(fc21) mutation in a subunit of mitochondrial complex I and levamisole resistance in a strain carrying unc-63(x26), an α-subunit of the nicotinic acetylcholine receptor (Supplementary Fig. 9), indicating that ThT could act in a variety of tissues including the nervous system.
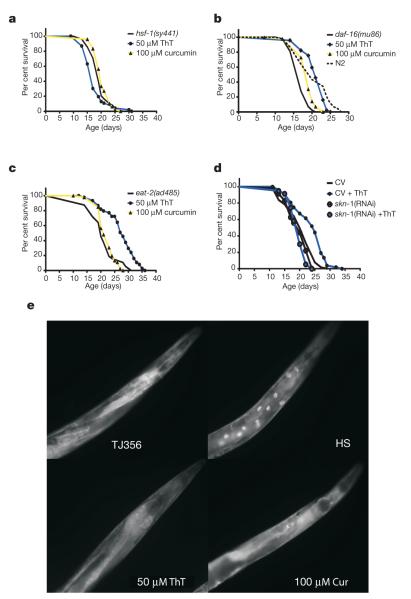
Because certain forms of dietary restriction suppress protein aggregation and increase lifespan, we asked whether ThT acts as a dietary restriction mimetic. We observed that ThT produces a small decrease in pharyngeal pumping rate (~15%) after 3 days of treatment, which could slightly decrease food intake. No difference was detected after 6 days of ThT treatment (Supplementary Fig. 16a). It is very unlikely that this small difference could promote the major ThT-mediated lifespan extension we observe. ThT also increased the lifespan of a strain carrying the eat-2(ad1116) mutation (Fig. 4c) that causes a major defect in pharyngeal pumping, thereby inducing a dietary restriction lifespan extension19. Dilution of the bacterial food source also leads to lifespan extension by dietary restriction20. ThT at 50 μM was detrimental to lifespan in this dietary restriction model but 1 and 10 μM ThT increased lifespan by 24% (Supplementary Fig. 16b). As ThT increases lifespan in both genetic and nutrient-based models of dietary restriction, ThT-induced lifespan extension is at least in part independent from dietary restriction.
Figure 4. ThT enhancement of lifespan depends on HSF-1 and SKN-1 transcription factors but not on DAF-16.
a–c, Effect of 50 μM ThT and 100 μM curcumin on Kaplan–Meier survival curves of synchronously ageing PS3551 [hsf-1(sy441)I] (a), CF1038 [daf-16(mu86)I] (b) and DA465 [eat-2(ad465)II] (c) lifespan. d, Effect of reducing SKN-1 by skn-1 RNAi on the increase in lifespan elicited by ThT. Plots are representative of three independent experiments. e, ThT and curcumin (Cur) treatment does not result in DAF-16::GFP relocalization as compared to control strain [daf-16::daf-16-gfp + rol-6] (upper left). Control strain under heat shock (HS) produces a clear relocalization of DAF-16::GFP to the nuclei of intestinal cells.
We then considered whether ThT was interacting more directly with homeostatic mechanisms. We exploited ThT fluorescence to visualize the compound in vivo and observed a variable co-distribution of ThT with Aβ(3–42) aggregates detected by immunolocalization, indicating a direct interaction between ThT protein misfolding cascades (Supplementary Fig. 10). Consequently, we tested for the presence of amino acid sequence-independent oligomers of protein or peptides prone to aggregation. We found that protein detected by an antibody specific for such oligomers (A11) accumulated during normal ageing and after heat shock, but was significantly decreased in both CL4176 (Fig. 2e) and N2 strains (Fig.2f) after ThT treatment, consistent with ThT affecting protein misfolding cascades.
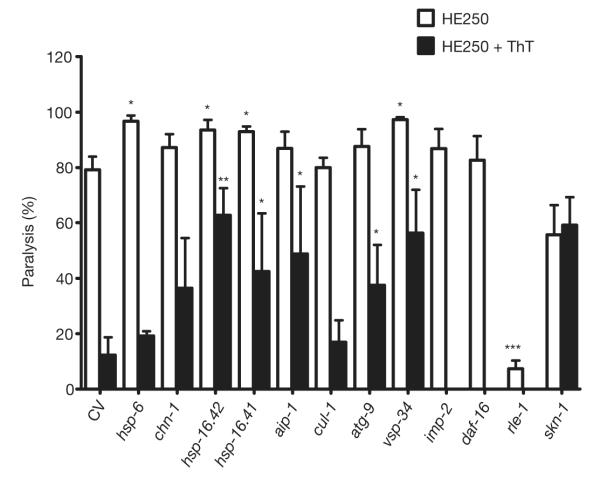
We reasoned that ThT may also require components of the protein homeostatic network activated by DAF-16 and HSF-1 to influence protein aggregation and lifespan8,21. We undertook a targeted pharmacogenetic RNA interference (RNAi) screen of genes encoding several components of the ubiquitin/proteasome system, autophagy/lysosomal machinery and molecular chaperones. First, we asked whether reducing the expression of genes encoding these proteins modulated the paralysis of metastable perlecan mutant in the absence of ThT (Fig. 3). RNAi of small chaperones known to positively modulate lifespan in C. elegans, HSP-16.2 and HSP-16.41 (refs 8, 22), and the mitochondrial HSP-70 (hsp-6) increased paralysis of the HE250 strain. An autophagy gene (vps-34) also influenced the HE250 mutant paralysis phenotype (Fig. 3). Interestingly, RNAi targeting rle-1, an E3 ubiquitin ligase that influences lifespan in C. elegans by determining the rate of DAF-16 degradation23, produced a remarkable reduction in the paralysis phenotype. This led us to test daf-16 (RNAi), but no change in the paralysis phenotype was observed, indicating that other proteins regulated by rle-1 can influence protein homeostasis.
Figure 3. Dependency of ThT suppression of protein aggregation-associated paralysis on protein homeostasis factors.
RNAi by feeding was used to knockdown the expression of genes encoding proteostatic factors in HE250 in the presence or absence of 50 μM ThT and the paralysis phenotype was scored after 36 h. Proportion of worms paralysed is plotted (mean ± s.e.m.). *P < 0.01, **P < 0.001, ***P < 0.0001 versus control vector (CV).
We then tested for interactions between these protein homeostasis factors and the protective effect elicited by ThT on the HE250 paralysis phenotype, and found that ThT protection was decreased when combined with RNAi for several stress genes (for example, hsp-16.2, hsp-16.41), consistent with a concerted action between chaperones and ThT to maintain protein conformation. Similarly, ubquitin/proteasome (aip-1) and autophagy/lysosomal (atg-9 and vps-34) functions were required for the beneficial effects of ThT. Interestingly, LMP-2, a protein involved in lysosome function, suppresses the ThT effect on paralysis. As lmp-2 knockdown itself has no effect on paralysis it is possible that ThT is cleared from the cell by a lysosomal mechanism, such that lmp-2 RNAi results in increased ThT bioactivity.
Next, we explored the dependency of DAF-16 for ThT action. The ThT suppression of the paralysis phenotype was potentiated by daf-16 (RNAi), indicating that some proteins activated by DAF-16 interfere with the mechanism elicited by ThT to promote protein homeostasis. This is consistent with the previous report of a reduction of amyloid-β aggregation by daf-16 (RNAi)9. In contrast, skn-1 (RNAi), encoding a transcription factor that positively modulates stress resistance and longevity, was required for the ThT effect on the HE250 paralysis phenotype (Fig. 3), indicating that some SKN-1 target genes are necessary for ThT action. However, ThT does not seem to induce constitutive nuclear localization of a SKN-1 fusion protein CF2189 [Is001(skn-1::GFP; rol-6(su1006)] as occurs with certain toxins (Supplementary Fig. 15).
We returned then to the question of whether ThT was extending lifespan by a mechanism related to protein homeostasis and initially focused on the transcription factor genes hsf-1 and daf-16; mutation of either one shortens normal lifespan and suppresses the beneficial effects of a daf-2 mutation on the amyloid-β-aggregation model in C. elegans9. We found that ThT does not increase lifespan in a strain carrying the hsf-1(sy441) mutation—resulting in non-functional HSF protein (Fig. 4a and Supplementary Fig. 11)—indicating that the ThT effect on lifespan requires the participation of HSF-1-regulated machinery. Consistent with this idea, we found that the protein levels of HSP-16.2 and HSP-70, the mRNA levels of a mitochondrial (hsp-6) and a cytosolic (chn-1) hsp-70 isoform are upregulated by ThT treatment (Supplementary Fig. 12a). We also detected a slight increase in the levels of HSF-1 protein under ThT treatment (Supplementary Fig. 12b).
ThT treatment did extend the lifespan of daf-16(mu86) worms lacking functional DAF-16 (Fig. 4b) and in a long-lived ILS mutant, age-1(hx546), which is hypomorphic for the p110 catalytic subunit of a phosphoinositide 3-kinase (Supplementary Fig. 13). In addition, ThT treatment did not alter the localization of a DAF-16 fusion protein TJ356 (zIs356IV[daf-16::daf-16-gfp; pRF4(rol-6(su1006))]), in contrast to the effect of stress (Fig. 4e; Supplementary Fig. 14). We conclude that ThT lifespan extension is independent of ILS.
Because ThT suppression of the paralysis phenotype on the HE250 strain required the skn-1 transcription factor, we tested if it was required for lifespan extension. We found that ThT does not increase the lifespan of nematodes exposed to skn-1 RNAi and conclude that this transcription factor is, along with HSF-1, required for the increase in lifespan elicited by ThT (Fig. 4d).
We have observed that compounds traditionally used to stain amyloid-β deposits confer a large increase in lifespan to C. elegans. ThT is capable of suppressing protein-aggregation-associated paralysis in toxic protein models in multiple tissues. ThT reduces Aβ(3–42) aggregation, decreases the levels of soluble aggregation-prone oligomeric proteins and localizes with these aggregates in vivo. The mechanism of aggregation suppression depends on molecular chaperones, autophagy and proteosomal functions. Finally, the extent of the ThT-mediated lifespan increase depends on the transcription factors HSF-1 and SKN-1. We propose that amyloid-binding compounds act as stress response mimetics and activate the C. elegans stress-response pathways regulated by the HSF-1 and SKN-1 transcription factors, leading to stabilization of misfolded proteins and increased lifespan. Given the known ability of these compounds to bind to amyloid, we propose that they may also directly interact with aggregating proteins, promoting proper folding and possibly activating stress response pathways by an unknown mechanism (Supplementary Fig. 1). This modulation of protein homeostasis and protein aggregation pathways has beneficial effects for healthspan and lifespan. Small stress response mimetic molecules that target protein homeostatic mechanisms may provide opportunities for intervention in ageing and age-related disease.
METHODS
Nematode growth and strains
Strains were cultured under standard laboratory conditions in USP agar. Strains used in this work include N2, HE250 [unc-52(e669su250)II], CB1157 [unc-54(e1157)I], CF1038 [daf-16(mu86)I], DA465 [eat-2(ad465)II], TJ1052 [age-1(hx546)II], PS3551 [hsf-1(sy441)I], TJ356 [zIs356 IV (daf-16::daf-16-gfp; pRF4 (rol-6(su1006))], CL4176 [dvIs27[myo-3::Aβ(3–42)-let 3′UTR(pAF29); pRF4 (rol-6(su1006))], AM140 (rmIs132[P(unc-54) Q35::YFP]), ZZ26 (unc-63(x26)I), CW152 (gas-1(fc21) X), CF2189 (Is001[skn-1::gfp rol-6(su1006)]).
Lifespan assay
Lifespan assays were performed as described previously24. Briefly, the nematode growth media (NGM, USP agar) plates were prepared under sterile conditions. One-hundred microlitres of concentrated stocks of each of the compounds used in this study were added onto a previously prepared NGM small plate (3 ml volume) immediately spread over the surface of the plate. The final concentrations quoted in the text assume an even distribution of compound throughout the 3 ml of agar in the plate. The plates were then placed in a laminar flow hood at room temperature (22 °C) for 30 min and then 60 μl of a concentrated suspension of E. coli OP50 was spotted to form a circular lawn on the centre of each plate. Thirty late L4 larvae growing at 20 °C were transferred to fresh NGM plates with FUdR (75 μM, unless otherwise stated) in the presence or absence of the specified compounds and incubated at 20 °C. The first day of adulthood is day 3 in survival curves. We noted between-experiment variation in the magnitude of the lifespan extension observed with ThT, which appeared to correlate with different suppliers and batches. ThT concentration should be optimized depending on batch and purity and stability of the compound. We noted that a darkening in the appearance of the stock resulted in loss of lifespan extension activity and even early death. The optimal range for lifespan extension was between 25 and 75 μM.
Nematodes were scored as alive, dead or lost every second day. Nematodes that failed to display touch-provoked movement were scored as dead. Nematodes that died from causes other than ageing, such as sticking to the plate walls, internal hatching of eggs (‘bagging’) or gonadal extrusion were censored as were lost worms. Nematodes were transferred to fresh plates every 3–6 days. All lifespan experiments were performed at 20 °C unless otherwise stated. Survival curves were plotted and statistical analyses (log-rank test) were performed using Prism 4 software (Graphpad Software).
Dietary restriction
Plates were prepared as described25 but bacterial concentration was adjusted to 1.0 × 1012 c.f.u. ml−1 and diluted to achieve a bacterial concentration of 1.0 × 109 c.f.u. ml−1. Diluted bacterial cultures were spotted onto dietary restriction agar plates, which were modified from the standard nematode growth media (NGM) plates by excluding peptone and increasing agar from 1.7% to 2.0%. Carbenicillin (50 mg ml−1) was added to the agar plates to further prevent bacterial growth. Synchronized L4 larvae grown under standard laboratory conditions (NGM plates with OP50 food, 20 °C) were transferred to fresh dietary restriction agar plates in the presence or absence of 1, 10, 25, 50 and 100 μM ThT, and lifespans scored as described earlier.
Demographic analysis
Estimates of initial mortality rate and rate of increase with age and model fitting were made using WinModest. Gompertz mortality curves, ln(ux) = ln(a) + bx, where ux defines the age-specific hazard, were fitted with log-likelihood ratios used to examined the effects of constraining the intercept (a) or gradient (b) variables.
Worm paralysis assays
Populations of CL4176 dvIs2[pCL12(unc-54/human Aβ3–42 minigene) + pRF4] or AM140 (rmIs132[P(unc-54) Q35:YFP]) worms were grown at 20 °C for 48 h and then exposed to 50 μM ThT and 100 μM curcumin at 25 °C in presence of FUdR (10 μg ml−1) for AM140. Scoring for paralysis was initiated 2 and 8 days after temperature upshift for CL4176 and AM140, respectively. Nematodes were scored as paralysed if they failed to move during observation and exhibited ‘halos’ of cleared bacteria around their heads (indicative of insufficient body movement to access food), eggs accumulated close to the body or if they failed to respond to a touch-provoked movement with a platinum wire. For sensitivity to ethanol or levamisole resistance, CW152 gas-1(fc21 X) and ZZ26 (unc-63(x26)I) worms were picked into 0.4 M ethanol or 50 μM levamisole, respectively, equilibrated for 5 min, and scored for paralysis as described earlier. Treated and untreated worms were compared with an unpaired t-test (implemented in Prism 4, Graphpad Software).
Immunostaining and photomicroscopy
For fluorescent microscopy, TJ356 [zIs356 IV (daf-16::daf-16-gfp; pRF4 (rol-6(su1006))] or CF2189 Is001(skn-1::gfp + rol-6(su1006)) nematodes were paralysed with 1 mM levamisole mounted on 1% agarose pads and imaged using Olympus BX51 (×60 objective) and HCImage software (Hamamatsu). For positive controls, TJ356 worms were exposed for 3 h to 33 °C and CF2189 worms were exposed to 5 mM NaAsO2 for 15 min. For immunofluorescence, N2, HE250 unc-52(e669su250), CB1157 unc-54(e1157) or CL4176 dvIs27[myo-3::Aβ(3–42)-let 3′UTR(pAF29); pRF4 (rol-6(su1006))] worms were treated for 24–36 h with or without 50 μM ThT at 25 °C. After this period the worms were collected, rinsed and fixed in 4% paraformaldehyde overnight. After fixation, worms were rinsed twice with 1 ml of 10 mM Tris-HCl pH 7.5 and then permeabilized by 24 h exposure to β-mercaptoethanol at 37 °C followed by collagenase treatment (2 mg ml−1 for 1–1.5 h at 37 °C) to allow for digestion of the cuticle. Paramyosin and perlecans were detected with primary monoclonal antibodies 5-23 and MH3 (developed by H. Epstein and R. H. Waterston and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology) and AlexaFluor 633 goat anti-mouse (Molecular Probes) as secondary antibody. Soluble oligomers and amyloid-β peptide were detected with anti polyclonal A11 (Invitrogen) and 6E10 monoclonal (Covance) primary antibiodies, respectively, with AlexaFluor 568 goat anti-rabbit and AlexaFluor 488 goat anti-mouse (Molecular Probes) as secondary anti-bodies. Image analysis was performed in Image Analyst MKII 2.0.49 (Image Analyst Software) as follows. Aggregates were counted by image segmentation. To this end, wide-field epifluorescence and confocal micrographs were pre-processed by high pass Butterworth filtering at ωcuton = 0.85 cycles μm−1 (order 1.5) to amplify small (<1.2 μm) punctate details, smoothed by Wiener filtering and rescaled with a gamma level 0.7. Image segmentation was performed by a modified Watershed method from seeds defined by being brighter than the 99.9 percentile of the rescaled image. Pixels brighter than 10% of the peak intensity for each aggregate were taken as positive. Because the high-pass filtering subtracts local mean, the segmentation resulted in objects outlined close to their original half maximal intensities, and therefore the size of segmented objects truly reflected the size of the aggregates. Objects larger than 30 pixels (0.56 μm2) were rejected. The high-pass filtering also eliminated the blur of wide-field images, therefore these images were handled in the same way as confocal images. Images of different conditions were handled with the same algorithm, and no subjective threshold levels were applied. The number of aggregates was determined in same-size areas close to the vulva of at least 10 worms under the same conditions.
We explored ThT distribution and potential colocalization with proteins prone to aggregate by using two-photon excitation of ThT at 800 nm and emission at 435–485 nm in combination with anti-oligomers and amyloid-β peptide immunodetection described earlier. In this spectral range worms exhibited negligible autofluorescence, therefore the signal was highly specific for ThT. Considering that image acquisition was performed after immunostaining probably only the protein-bound form of ThT was imaged. Pearson’s coefficient values for ThT and amyloid-β were calculated by using Image Analyst MKII 2.0.49 (Image Analyst Software).
Western blot
Peptide corresponding to amino acids 110–145 (NLSEDGKL SIEAPKKEAVQGRSIPIQQAIVEEKSAE) of HSP-16.2 was used to commercially synthesize antiserum (Invitrogen). Briefly, KLH-peptide was emulsified by mixing with an equal volume of Freund’s adjuvant and injected into three subcutaneous dorsal sites for a total of 0.1 mg of peptide for immunization. The animals (rabbits) were bled, the blood allowed to clot and the serum collected by centrifugation. Monoclonal HSP-70 and polyclonal HSF-1 primary antibodies were from Stressgen (N27F3-4 and SPA-901, respectively).
For immunoblot analysis, 3-day-old adult hermaphrodites were treated with 50 μM ThT or 100 μM curcumin as described earlier and replicates of 25 nematodes were collected for each treatment. Worms were transferred to siliconized eppendorf tubes, washed once in S-basal and frozen in liquid N2. Standard SDS–PAGE was performed using (4–12%) NOVEX gels and MES running buffer. Following transfer PVDF (BioRad) membranes were incubated with antisera (1:10,000) or primary antibodies (1:1,000) diluted in blocking buffer and then with secondary, goat anti-rabbit IgG antibody/horseradish peroxidase conjugate (Pierce), diluted 1:25,000. Detection was undertaken with chemiluminescent reagents (SuperSignal, Pierce) and standard autoradiography.
RNAi knockdown of gene expression
RNAi bacterial strains expressing double-stranded RNA that inactivates specified genes were cultured and used as previously described26. Briefly, eggs isolated from synchronous populations of cultures were placed on fresh RNAi plates and allowed to grow at 15 °C; 3 days later, L4 moult nematodes were transferred to new plates seeded with the same bacteria in the presence or absence of compounds and switched to 25 °C. In all cases, 1 mM isopropyl-b-d-thiogalactopyranoside (IPTG) was used for induction of doublestranded RNA. In all cases the identity of the clones was confirmed by sequencing.
Real-time qPCR analysis
Twelve single adults from control or 50 μM ThT populations were picked after 3, 6 and 12 days of treatment into 5 μl of distilled water and flash frozen until extraction. Individual worms were extracted and analysed on the QIAcube robot as described previously27. The housekeeping genes gpd-1 and gpd-4 were found to have invariant steady-state levels across treatments and were used to derive calibrated normalized relative quantities (CNRQs) for each gene of interest as previously described27.
Supplementary Material
Acknowledgements
We thank A. A. Gerencser for expert assistance with the confocal microscopy; A. M. Cuervo, M. S. Gill, M. Lucanic, J. Campisi, S. Melov, V. Lunyak and P. Kapahi for suggestions on the manuscript, members of the G.J.L. and P. Kapahi laboratories for helpful discussion and members of the Paper Polishing Club. Nematode strains were provided by the Ceanorhabditis Genetics Center, funded by the National Institutes of Health (NIH) National Center for Research Resources. CF2189 was a gift from C. Kenyon’s laboratory. This work was supported by grants from the Larry L. Hillblom Foundation and the NIH (UL1024917, supporting the Interdisciplinary Research Consortium on Geroscience and 1R01AG029631-01A1). G.J.L. is supported by the NIH AG21069, AG22868, AG029631-01A1, ES016655, the Larry L. Hillblom Foundation and UL1 RR024917. S.A. was supported by the U19AGO231222 from the Longevity Consortium.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions S.A. planned and designed the project with consultation and support from G.J.L. All the data were collected by S.A. and M.C.V., with assistance from D.J.S.Z. and I.M.K. S.A. and G.J.L. wrote the paper with contribution from all authors.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 3.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 4.Frid P, Anisimov SV, Popovic N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Brain Res. Rev. 2007;53:135–160. doi: 10.1016/j.brainresrev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Tullet JM, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 9.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 10.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils—current status. J. Chem. Biol. 2009;3:1–18. doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Rodríguez C, et al. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J. Am. Chem. Soc. 2009;131:1436–1451. doi: 10.1021/ja806062g. [DOI] [PubMed] [Google Scholar]
- 12.Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 13.McColl G, et al. The Caenorhabditis elegans Aβ1–42 model of Alzheimer’s disease predominantly expresses Aβ3–42. J. Biol. Chem. 2009;284:22697–22702. doi: 10.1074/jbc.C109.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temussi PA, Masino L, Pastore A. From Alzheimer to Huntington: why is a structural understanding so difficult? EMBO J. 2003;22:355–361. doi: 10.1093/emboj/cdg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 16.Zengel JM, Epstein HF. Identification of genetic elements associated with muscle structure in the nematode Caenorhabditis elegans. Cell Motil. 1980;1:73–97. doi: 10.1002/cm.970010107. [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Brenner S. A selection for myosin heavy chain mutants in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1984;81:4470–4474. doi: 10.1073/pnas.81.14.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl Acad. Sci. USA. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 22.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Gao B, Lee SM, Bennett K, Fang D. RLE-1, an E3 ubiquitin ligase, regulates C. elegans aging by catalyzing DAF-16 polyubiquitination. Dev. Cell. 2007;12:235–246. doi: 10.1016/j.devcel.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.McColl G, et al. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem. 2008;283:350–357. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 27.McColl G, et al. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 2010;12:260–272. doi: 10.1016/j.cmet.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.