Abstract
In hospitals and clinics worldwide, medical device surfaces have become a rapidly growing source of nosocomial infections. In particular, patients requiring mechanical ventilation (and, thus, intubation with an endotracheal tube) for extended lengths of time are faced with a high probability of contracting ventilator-associated pneumonia. Once inserted into the body, the endotracheal tube provides a surface to which bacteria can adhere and form a biofilm (a robust, sticky matrix that provides protection against the host immune system and antibiotic treatment). Adding to the severity of this problem is the spread of bacterial genetic tolerance to antibiotics, in part demonstrated by the recent and significant increase in the prevalence of methicillin-resistant Staphylococcus aureus. To combat these trends, different techniques in biomaterial design must be explored. Recent research has shown that nanomaterials (materials with at least one dimension less than 100 nm) may have the potential to prevent or disrupt bacterial processes that lead to infections. In this study, polyvinyl chloride (PVC) taken from a conventional endotracheal tube was embedded with varying concentrations of zinc oxide (ZnO) nanoparticles. S. aureus biofilms were then grown on these nanocomposite surfaces during a 24-hour culture. Following this, biofilms were removed from the surfaces and the number of colony forming units present was assessed. Bacterial proliferation on the samples embedded with the highest concentration of ZnO nanoparticles was 87% less when compared to the control, indicating that this technique is effective at reducing biofilm formation on PVC surfaces without the use of antibiotics.
Keywords: nanomaterials, endotracheal tube, biofilm, zinc oxide, nanoparticles, Staphylococcus aureus
Introduction
Just three types of medical device-related infections account for over 60% of all reported nosocomial infections: urinary tract infections (catheter-associated), bloodstream infections (intravascular device-associated), and pneumonia (ventilator-associated).1 Contamination can occur from the presence of just a small number of microorganisms due to surgical procedure, improper sterilization, and, more commonly, the simple migration of bacteria from the skin into the body after an operation.2 In addition, medical device surfaces are especially prone to bacterial infections since they are commonly composed of materials that do nothing to inhibit the growth of bacteria. Almost immediately after attaching to a device surface, bacteria begin to secrete and collect proteins, polysaccharides, and DNA in order to form a complex aggregate of cells, known as a biofilm.3 This protected state guards the bacteria against antibiotics and the host immune system, making the infection especially difficult to treat and often necessitating device removal.4
The scope of this important clinical problem is vast and widespread. Specifically, around 2 million patients contract nosocomial infections per year in the US alone, and around half of these are device-related.5 This places a tremendous burden upon the health care system, both economically and socially. It is estimated that device-related infections cause up to 100,000 excessive deaths every year (as compared with 13,000 in 1992), which costs the US health care system over US$35 billion.6
Out of all medical device infections, ventilator-associated pneumonia (VAP) is the most common nosocomial infection among patients requiring extended mechanical ventilation, and is caused by pathogenic microorganisms in the oropharyngeal, gastrointestinal, and respiratory system of an intubated patient.7 The insertion and continuing presence of an endotracheal tube (ETT) can interfere with the body’s natural respiratory clearing mechanisms, as well as provide a surface for bacterial adherence, proliferation, and entry into the respiratory system.8 VAP is a serious condition, especially because patient groups requiring mechanical ventilation often already have weakened immune systems (such as elderly patients, premature infants, etc). The mortality rate of VAP is estimated to be in the range of 24%–50% and a positive diagnosis can increase hospital costs by over US$40,000 per patient.9,10
The increase in bacterial genetic tolerance to antibiotics is another rapidly growing problem for the treatment of medical device-related infections. Staphylococcus aureus is a prolific biofilm former and the most common Gram-positive (and second most common overall) bacterial pathogen associated with ETT fouling and VAP.11 The recent and swift rise in the prevalence of strains of methicillin-resistant S. aureus (MRSA) has caused great concern amongst the medical community about the spread of antibiotic-resistant bacteria. Implant infections were found to be the third most common cause of potential MRSA infections leading to hospitalization between 1999 and 2005, and an even more recent study concluded that 56% of all device-related infections caused by S. aureus between 2006 and 2007 were reported as MRSA.12,13 The International Nosocomial Infection Control Consortium further found that 84.1% of all isolated S. aureus worldwide contained MRSA.14 The financial side of the situation is just as dire, as MRSA infections are costing the health care system up to US$9.7 billion in excess costs.12,14 This problem shows no sign of abating and, in fact, the percentage of infections with MRSA is predicted to continue to rise.12,13 Thus, there is an urgent clinical need to develop new ways of preventing device-related (and, more specifically, ETT-related) infections without resorting to the use of antibiotics.
In this regard, the use of nanotechnology (either by creating nanoparticles or medical device surfaces with nanostructured features) has shown great promise as a novel approach to combat biofilm-mediated, drug-resistant, device-centric infections.15 Materials that have morphological features on the scale of <100 nm (called nanomaterials) are beginning to be used for a wide variety of biomedical applications due to their unique surface properties which have the ability to control initial protein adsorption and subsequent cell behavior.8,16 This “nanoroughness” gives nanomaterials a greater functional surface area than conventional materials, which do not have features on the nanoscale. In addition, it is theorized that nanoparticles may also have general mechanisms of toxicity towards bacteria that do not cause problems for mammalian cells. For example, nanoparticles can bind to bacterial cell walls and cause membrane disruption through direct interactions or through free radical production, whereas mammalian cells are able to limit free radical damage due to their ability to phagocytose and subsequently degrade nanoparticles by lysozomal fusion.17,18
For the above reasons, the objective of the present in vitro study was to inexpensively reduce S. aureus density on conventional polyvinyl chloride (PVC) ETTs by embedding the polymer with ZnO nanoparticles. Results demonstrated that this approach significantly decreased bacterial density and biofilm formation on ETTs without resorting to the use of antibiotics, thus, providing much promise for their clinical use.
Materials and methods
ZnO/PVC nanocomposite preparation
In order to make ZnO/PVC nanocomposites, PVC taken from a commercial Sheridan® 6.0 mm ID uncuffed endotracheal tube (Hudson RIC, Temecula, CA, USA) was first chemically dissolved using tetrahydrofuran (Mallinckrodt Chemicals, St Louis, MO, USA) at a ratio of 1 g PVC:4 mL tetrahydrofuran. Once the PVC was completely dissolved, ZnO nanoparticles with a diameter of 20 nm (mkNANO, Williamsville, NY, USA) were added to the mixture at weight percentages of 0% (control), 10%, 20%, and 30%. The PVC/ZnO mixture was left to stir for 1 hour until completely homogenous, and then placed in a sonicator for 15 minutes to ensure an even distribution of the nanoparticles. The mixture was then pipetted onto 12 mm circular glass coverslips and was allowed to dry overnight in a chemical hood.
Material characterization
Scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDAX)
The surface topographies and compositions of the nanocomposite materials were visualized at four different magnifications using a scanning electron microscope (Hitachi S4800 SEM, 3.0 kV accelerating voltage; Hitachi Ltd, Tokyo, Japan) according to standard operating procedures. Prior to imaging, samples were coated with a 3 nm layer of palladium (Pd) using a sputter coater (Cressington 208; Cressington Scientific Instruments, Watford, UK) to prevent interference. EDAX spectra were also obtained for the 0% and 10% samples to confirm the preservation of conventional ETT surface chemistry and to ensure the absence of any unexpected elements in the control sample.
Surface energy
The surface energies of the nanocomposite samples were compared using a water droplet technique. Samples of each weight percentage were sterilized in 70% ethanol for 1 minute and ensured free of dust and other particulate matter. A 5 μL drop of deionized water was carefully placed with a micropipette in the center of each sample. After 20 seconds, the diameter of the water droplet was measured.
Bacterial culture
For the bacterial culture, S. aureus (strain #25923; American Type Culture Collection [ATCC], Manassas, VA, USA) was hydrated and streaked for isolation on a tryptic soy agar (Thermo Fisher Scientific, Waltham, MA, USA) plate. Following growth, a single isolated colony was selected and used to inoculate 3 mL of tryptic soy broth media (MP Biomedicals, Solon, OH, USA). The bacteria culture was grown on a shaking incubator set at 200 rpm for 18 hours at 37°C. The resulting suspension was then adjusted to have an optical density at 562 nm (OD562) of 0.52, corresponding to a bacterial density of 109 colony forming units (CFU) per mL. Following this, the solution was serially diluted over a 3-log range to a bacterial density of 106 CFU/mL using simulated body fluid supplemented with 10% fetal bovine serum.
The ZnO/PVC nanocomposites were removed from the glass coverslips with forceps and were sterilized with three rounds of alternating 30 second rinses in 70% ethanol (Fischer Scientific) and phosphate-buffered saline ([PBS] Thermo Fisher Scientific). Two samples of each weight percentage were placed into separate wells of a 24-well plate. 1 mL of the 106 CFU/mL solution of S. aureus in simulated body fluid was pipetted into each well, taking care to ensure complete submersion of the sample. The well plate was then placed in a stationary incubator at 37°C with 5% carbon dioxide to allow for biofilm formation.
After 24 hours, the excess bacteria solution was carefully aspirated from the wells, and the samples were thoroughly washed three times with PBS to ensure removal of all media residue and non-adherent bacteria. Samples were then extracted from the well plate with sterile forceps and placed in 1.5 mL microcentrifuge tubes (Thermo Fisher Scientific) with 1 mL of PBS. Great effort was made to minimize handling of the samples as much as possible during the transfer to avoid disrupting the biofilms on the surfaces. The microcentrifuge tubes were vortexed at 3000 rpm for 15 minutes in order to strip all adherent bacteria from the sample into solution. This suspension was then serially diluted in PBS over a 3-log range and 200 μL of the final solution was spread on tryptic soy agar plates and placed in a stationary incubator at 37°C with 5% carbon dioxide for 24 hours. Finally, the number of S. aureus colonies that formed on each plate was recorded, and the number of CFU/mL was calculated by multiplying the result by five to account for only 200 μL of solution being plated.
Statistical analysis
For the bacterial culture experiments, numerical data were analyzed for significance using a Student’s two-sample t-test, comparing each sample group (10%, 20%, and 30% ZnO) against the control group (0% ZnO). Two samples from each weight percentage were utilized during each run of the experiment, which was repeated in triplicate (n = 2, N = 3). Values are reported as the mean ± standard deviation.
For the surface energy comparison, numerical data were analyzed for significance using a Student’s two-sample t- test, comparing each sample group (10%, 20%, and 30% ZnO) against the control group (0% ZnO). Five samples from each weight percentage were tested (n = 5). Values are reported as the mean ± standard deviation.
Results
Material characterization
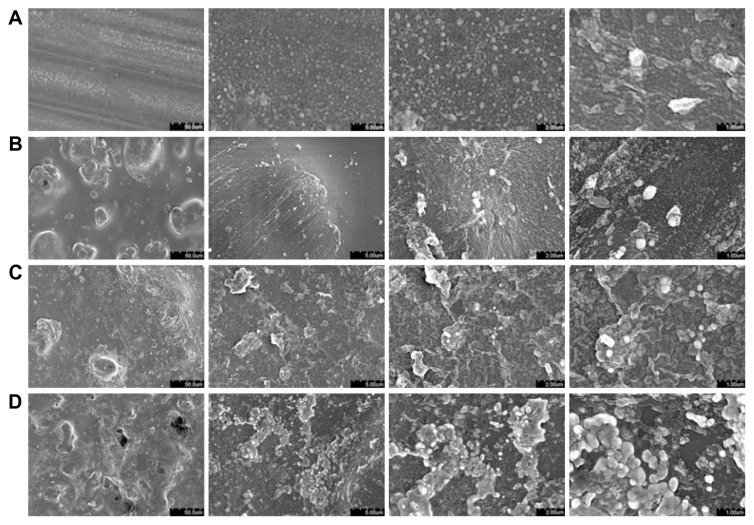
The surface roughness and features of the ZnO/PVC nanocomposites were visualized using SEM (Figure 1). The images confirm the presence of surface features on the nanoscale and show that surface roughness increased with the addition of larger quantities of ZnO nanoparticles. The images also reveal some agglomerations of nanoparticles, particularly in the samples with higher nanoparticle concentrations. The authors believe that sonicating the liquid ZnO/PVC mixture for a longer time period would help increase the uniformity of the surfaces, and may lead to even greater antibacterial activity through additional nanofeatures and exposed ZnO surface area.
Figure 1.
Scanning electron micrographs of the nanocomposite samples.
Notes: Images reveal an increase in surface roughness and features at greater ZnO nanoparticle concentrations. The (A) 0%, (B) 10%, (C) 20%, and (D) 30% samples are shown at magnifications of (from left to right) ×1000, ×10,000, ×20,000, and ×40,000.
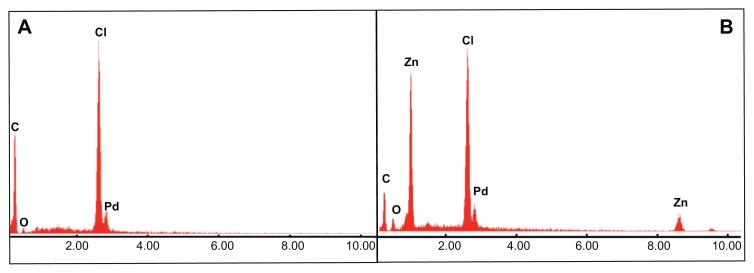
EDAX data (Figure 2) indicated the preservation of conventional endotracheal tube surface chemistry. The apparent chlorine and carbon peaks are characteristic of PVC. In addition, EDAX data confirmed the presence of zinc in the 10% sample, and the absence of zinc in the 0% sample. No unexpected elements were found in either sample (the presence of Pd is explained by the preparation of the nanocomposites for microscopy, refer to the “Materials and methods” section for more detail).
Figure 2.
EDAX spectra of the 0% and 10% nanocomposite samples.
Notes: Images confirm the preservation of conventional ETT surface chemistry in the 0% (A) and 10% (B) samples, as well as the lack of any Zn in (A). The presence of Pd is explained by the preparation of samples for microscopy (refer to “Materials and methods” section).
Abbreviations: EDAX, energy dispersive X-ray analysis; ETT, endotracheal tube.
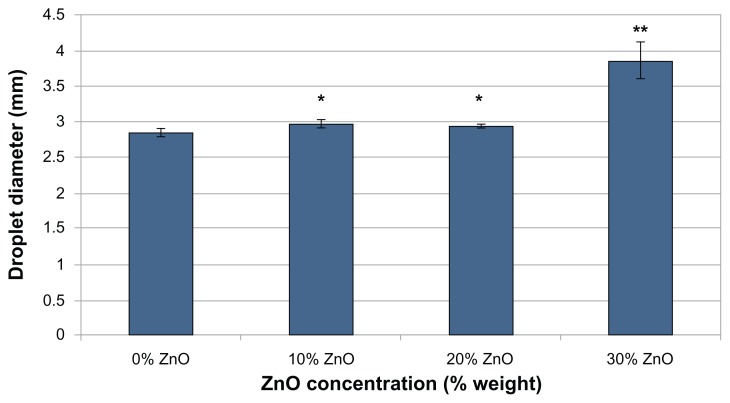
Results of the surface energy comparison (Figure 3) showed a general trend of increased surface energy as ZnO nanoparticle concentration increased. An increase in surface energy indicates increased hydrophilicity.
Figure 3.
Droplet diameter versus ZnO nanoparticle concentration.
Notes: Diameter of a 5 μL droplet of deionized water 20 seconds after being placed on different weight percentages of ZnO nanoparticle-embedded PVC. Data represents mean ± SD; n = 5. Larger droplet diameters were observed when compared to the control (0% ZnO) sample at confidence levels of *P < 0.001, **P < 0.0001. The difference in droplet diameter between the 10% ZnO and 20% ZnO samples was not statistically significant.
Abbreviations: PVC, polyvinyl chloride; SD, standard deviation.
Bacterial culture
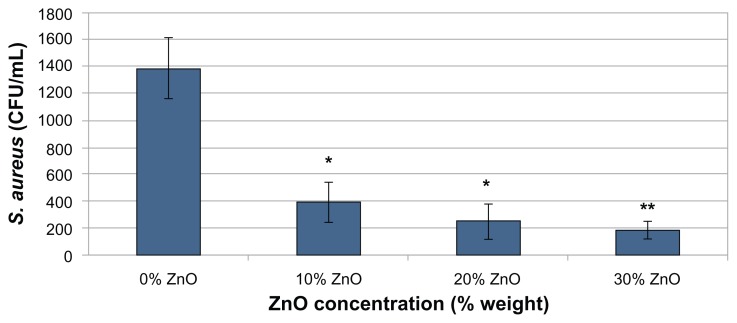
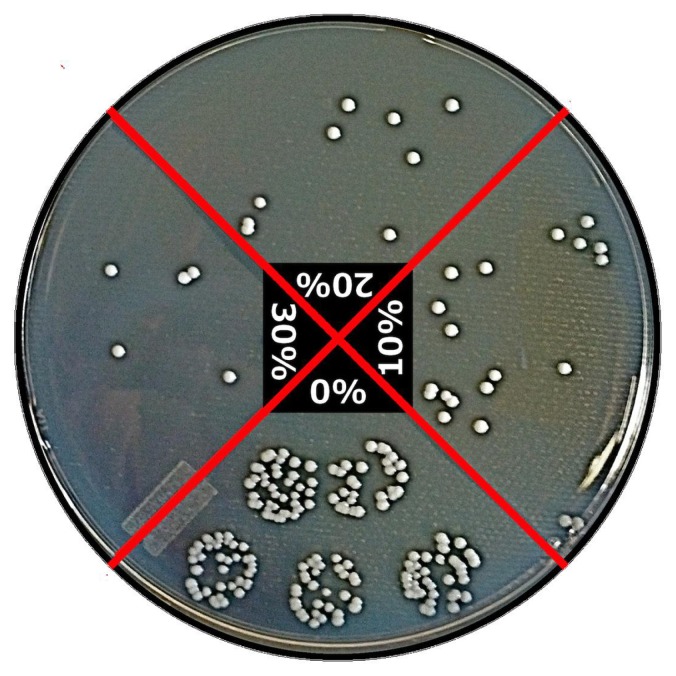
The colony counts from the bacterial culture experiments showed that the ZnO/PVC nanocomposites significantly reduced viable bacterial growth (Figure 4). Bacterial adherence significantly decreased through the addition of just 10% ZnO nanoparticles to the polymer (representing a 71% reduction from the control) and further decreased with greater concentrations of ZnO nanoparticles (representing an 82% and 87% reduction from the control for the 20% and 30% ZnO concentrations, respectively). A visual confirmation of these results was obtained by pipetting five 20 μL dots of solution from each sample onto the same tryptic soy agar plate rather than on separate plates (Figure 5).
Figure 4.
Staphylococcus aureus colonies formed versus ZnO nanoparticle concentration.
Notes: Anti-biofilm activity of different weight percentages of ZnO nanoparticle-embedded PVC against S. aureus after 24 hours. Data represents mean ± SD. The experiment was repeated in triplicate (N = 3), using two samples of each ZnO weight percentage for each trial (n = 2). Fewer bacterial CFU were observed when compared to the control (0% ZnO) sample at confidence levels of *P < 0.001, **P < 0.0001.
Abbreviations: CFU, colony forming units; PVC, polyvinyl chloride; SD, standard deviation.
Figure 5.
Combined plate: Staphylococcus aureus colonies formed versus ZnO nanoparticle concentration.
Notes: Tryptic soy agar plate with five 20 μL drops of bacteria solution taken from each sample (bottom: 0% ZnO; right: 10%; top: 20%; left: 30%).
Discussion
Various metal oxides, such as TiO2, ZnO, MgO, and CaO, have been of particular interest to nanomaterial scientists due to their stability under harsh process conditions and the fact that they are generally regarded as safe to humans (in fact, MgO and CaO are essential minerals for human health, and ZnO and TiO2 have been used extensively in the formulation of personal care products).17 ZnO in particular has shown great promise in reducing infections due to its inherent antimicrobial properties. These properties have been proven to be enhanced when the particles are on the nanoscale rather than the microscale.15 For example, in a recent study, researchers found that the antibacterial activity of ZnO was directly correlated with particle size (which was calculated from surface area).19 When tested with a methicillin sensitive strain of S. aureus (MSSA), the percentage of viable bacterial cells recovered after 24 hours of incubation at 37°C fell from ~85% when using ZnO particles 212 nm in diameter, to ~5% when using ZnO particles 12 nm in diameter.19 The researchers also noted that when the ZnO particle diameter was >100 nm, the bacteria were not completely killed, meaning that the ZnO functioned only as a bacteriostatic agent. In contrast, the 12 nm diameter ZnO particles not only limited MSSA growth, but also effectively killed them.18 When tested against MRSA and a high biofilm-producing strain of Staphylococcus epidermidis, a 4–7 mM colloidal suspension of the 12 nm diameter ZnO particles inhibited 95% of the bacterial growth.19
While the mechanism of the observed antibacterial activity of ZnO nanoparticles is not completely understood, several studies have suggested two possible mechanisms: the production of increased levels of reactive oxygen species (ROS) such as hydroxyl radicals and singlet oxygen, and/or the accumulation of the nanoparticles in either the cytoplasm or the periplasmic region of the bacterial cells causing disruption and disorganization of cellular functions and membranes.20–22 ROS production can be increased due to metal ion release or through the interaction with ultraviolet light, both of which are dependent on particle size.20–22 Two studies in 2009, published by Lipovsky et al and Applerot et al, provided a very detailed analysis of ROS generation by ZnO nanoparticles. The authors used electron paramagnetic resonance coupled with the spin-trapping technique to show the formation of hydroxyl radicals and singlet oxygen resulted from water suspensions of ZnO nanoparticles, the production of both of which has been strongly linked to antibacterial activity.23,24
Additionally, it has been shown that materials with surface features on the nanoscale may also have properties that are effective at reducing microbial adhesion, proliferation, and biofilm growth. Studies have indicated that incorporating nanofeatures onto medical device surfaces can enhance surface energy, increase select protein adsorption, and promote protein bioactivity.25 Not only has this been shown to improve tissue-forming cell functions, but many researchers also hypothesize that these same mechanisms simultaneously prevent bacterial colonization.26,27 It has also been theorized that material surface roughness at the nanoscale may minimize flush contact between bacterial cell walls and the surface itself. This could inhibit subsequent electrostatic interactions necessary for initial adhesion to the surface.3 In a recent study performed by Seil et al in 2011,8 PVC taken from a conventional ETT was given a nanorough topography through enzymatic treatment (without the addition of any nanoparticles or antimicrobial agents). The bacterial adherence of Pseudomonas aeruginosa, another prolific biofilm-former, was reduced from 0.89 ± 0.27 CFU/μm2 on the untreated samples to 0.54 ± 0.13 CFU/μm2 on the nanorough samples (a reduction of ~60%).8
The author s believe that the explorat ion of nanocomposites as described in this study shows great promise for the creation of a safer, inexpensive, antibacterial ETT, through the combination of both the addition of ZnO nanoparticles, and features on the nanoscale, to the device surface. This approach offers several key advantages over VAP treatments and modified ETTs that are currently on the market, and does not preclude the incorporation of other ETT advances such as the use of cuffs and double lumens. It is also important to note that many other medical devices are also constructed of PVC (catheters, medical tubing, etc) and should be able to be modified using this technique as well.
First, and perhaps most importantly, this method is preventative and not curative and, thus, does not employ the use of antibiotics. Slowing the spread of MRSA and bacterial genetic tolerance to antibiotics in general should be a top priority for those in the health care field. Second, the procedure to create the nanocomposite material is simple and inexpensive. The authors envisage that the ZnO nanoparticles could be added directly to the liquid PVC before the ETT is extruded, eliminating the need for many extra steps to be added to the manufacturing process. Not only would this lower production cost, but it would also ensure that the ETT is covered both intraluminally and extraluminally.
Current popular antimicrobial ETTs on the market can cost around $100 (US dollars) per tube, making them ~50× more expensive than a conventional ETT.28 This cost could be prohibitive to patients in low-income areas, where access to such a device would provide great therapeutic benefit. Finally, using an embedding technique rather than a coating technique is promising for greater retention of antibacterial effects over time.
Conclusion
The material surfaces of implanted medical devices are especially prone to bacterial colonization and adhesion, leading to infections that are resilient and difficult to treat. In this study, the biofouling of endotracheal tubes by the pathogen S. aureus, leading to VAP, was explored. S. aureus activity on PVC taken from a conventional ETT embedded with different concentrations of ZnO nanoparticles was quantified and compared. After an incubation time of 24 hours, it was found that bacterial cell counts were significantly reduced on all of the ZnO-containing nanocomposites, with an increase in nanoparticle concentration corresponding to a decrease in viable bacterial growth. An increase in nanoparticle concentration was also found to be correlated with an increase in surface roughness and surface energy. The mechanism(s) underlying this bacterial reduction will be further investigated in future studies.
Acknowledgments
The authors would like to thank Northeastern University, Boston, MA, USA for their funding and support.
Footnotes
Disclosure
The authors report no conflicts of interest in this paper.
References
- 1.Burke JP. Infection control – a problem for patient safety. N Engl J Med. 2003;348(7):651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious disease. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 3.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74(2):470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto M. Staphylococcus epidermidis – the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating heath care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fears R, van der Meer JW, ter Meulen V. The changing burden of infectious disease in Europe. Sci Transl Med. 2011;3(103):103cm30. doi: 10.1126/scitranslmed.3002556. [DOI] [PubMed] [Google Scholar]
- 7.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 8.Seil JT, Rubien N, Webster TJ, Tarquinio KM. Comparison of quantification methods illustrates reduced Pseudomonas aeruginosa activity on nanorough polyvinyl chloride. J Biomed Mater Res B Appl Biomater. 2011;98(1):1–7. doi: 10.1002/jbm.b.31821. [DOI] [PubMed] [Google Scholar]
- 9.Fagon JY, Chastre J, Domart Y, Trouillet JL, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis. 1996;23(3):538–542. doi: 10.1093/clinids/23.3.538. [DOI] [PubMed] [Google Scholar]
- 10.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120(2):555–561. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]
- 12.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008 issued June 2009. Am J Infect Control. 2010;38(2):95–104. e2. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomedicine. 2011;6:1463–1473. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Webster TJ. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials. 2007;28(2):354–369. doi: 10.1016/j.biomaterials.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) J Nanopart Res. 2007;9(3):479–489. [Google Scholar]
- 18.Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18(6):383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 19.Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 20.Sawai J, Kawada E, Kanou F, et al. Detection of active oxygen generated from ceramic powders having antibacterial activity. J Chem Eng Jpn. 1996;29(4):627–633. [Google Scholar]
- 21.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279(1):71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 22.Touati D. Iron and oxidative stress in bacteria. Arch Biochem Biophys. 2000;373(1):1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 23.Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R. EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J Phys Chem C. 2009;113(36):15997–16001. [Google Scholar]
- 24.Applerot G, Lipovsky A, Dror R, et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater. 2009;19(6):842–852. [Google Scholar]
- 25.Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4(1):66–80. [Google Scholar]
- 26.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 27.Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006;78(3):595–604. doi: 10.1002/jbm.a.30789. [DOI] [PubMed] [Google Scholar]
- 28.Shorr AF, Zilberberg MD, Kollef M. Cost-effectiveness analysis of a silver-coated endotracheal tube to reduce the incidence of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2009;30(8):759–763. doi: 10.1086/599005. [DOI] [PubMed] [Google Scholar]