Abstract
Vaccinating wildlife is becoming an increasingly popular method to reduce human disease risks from pathogens such as Borrelia burgdorferi, the causative agent of Lyme disease. To successfully limit human disease risk, vaccines targeting the wildlife reservoirs of B. burgdorferi must be easily distributable and must effectively reduce pathogen transmission from infected animals, given that many animals in nature will be infected prior to vaccination. We assessed the efficacy of an easily distributable oral bait vaccine based on the immunogenic outer surface protein A (OspA) to protect uninfected mice from infection and to reduce transmission from previously infected white-footed mice, an important reservoir host of B. burgdorferi. Oral vaccination of white-footed mice effectively reduces transmission of B. burgdorferi at both critical stages of the Lyme disease transmission cycle. First, oral vaccination of uninfected white-footed mice elicits an immune response that protects mice from B. burgdorferi infection. Second, oral vaccination of previously infected mice significantly reduces the transmission of B. burgdorferi to feeding ticks despite a statistically nonsignificant immune response. We used the estimates of pathogen transmission to and from vaccinated and unvaccinated mice to model the efficacy of an oral vaccination campaign targeting wild white-footed mice. Projection models suggest that the effects of the vaccine on both critical stages of the transmission cycle of B. burgdorferi act synergistically in a positive feedback loop to reduce the nymphal infection prevalence, and thus human Lyme disease risk, well below what would be expected from either effect alone. This study suggests that oral immunization of wildlife with an OspA-based vaccine can be a promising long-term strategy to reduce human Lyme disease risk.
Key Words: Borrelia burgdorferi, Lyme disease, Oral vaccine, Outer surface protein A, Peromyscus leucopus, Tick-borne disease, White-footed mice
Introduction
Infectious diseases are emerging and reemerging at an alarming rate (Daszak et al. 2000, Woolhouse et al. 2005, Jones et al. 2008). The majority of these diseases are zoonoses that are caused by pathogens residing in animal populations that can be transmitted to humans (Jones et al. 2008). An increasingly popular method to reduce human disease risks from zoonotic pathogens is to vaccinate the pathogen's natural reservoir hosts (Cross et al. 2007). However, the efficacy of wildlife vaccination campaigns can be limited by vaccine formulations that are practically difficult to administer to wild animals or by the prevalence of infected animals prior to the onset of the campaign. Thus, wildlife vaccines must be easily distributable to natural reservoir hosts and protect uninfected animals from infection as well as hinder transmission from infected animals to new hosts (Tompkins et al. 2009). The aim of this work was to test the efficacy of an easily distributable oral vaccine formulation in protecting wildlife from infection with Borrelia burgdorferi, the causative agent of Lyme disease, and in preventing transmission from infected animals to the tick vector.
Lyme borreliosis is a tick-borne zoonosis that is of significant public health concern in the Northern Hemisphere, where it ranks 7th among notifiable conditions, just below acquired immunodeficiency syndrome (AIDS) (Prevention 2011). In the northeastern United States, B. burgdorferi is transmitted among wildlife hosts, predominantly small mammals and ground-dwelling birds, by the immature stages (nymphs and larvae) of Ixodes scapularis ticks (Anderson 1988, LoGiudice et al. 2003, Brisson and Dykhuizen 2004, Brisson et al. 2008, Ogden et al. 2008, Brinkerhoff et al. 2010, Ogden et al. 2011). The human risk of contracting Lyme disease is strongly correlated with the prevalence of B. burgdorferi-infected nymphs (Piesman et al. 1987, Mather et al. 1996, Stafford et al. 1998, Diuk-Wasser et al. 2012). Thus, wildlife vaccination campaigns that reduce the prevalence of infected nymphs can be an effective method to control Lyme disease (Tsao et al. 2004). Because nymphal ticks can only become infected by feeding on an infected wildlife host during their larval blood meal (Magnarelli et al. 1987, Patrican 1997), wildlife vaccination campaigns must interrupt the transmission of B. burgdorferi between wildlife hosts and ticks to reduce human Lyme disease risk.
Lyme disease vaccines based on the immunogenic outer surface protein A (OspA) effectively protect uninfected laboratory mice from B. burgdorferi when delivered via parenteral (Fikrig et al. 1990, Fikrig et al. 1992b) or oral immunization (Fikrig et al. 1991, Dunne et al. 1995, Luke et al. 1997, Gomes-Solecki et al. 2006, Scheckelhoff et al. 2006, del Rio et al. 2008, Richer et al. 2011). Because the OspA protein is expressed by spirochetes, primarily in the tick midgut (Schwan et al. 1995), OspA-based vaccines work in an unconventional manner. Antibodies elicited by OspA vaccines in the mammalian host are injested by the tick during a blood meal and kill B. burgdorferi in the tick midgut and thus prevent transmission of the spirochete to vaccinated hosts (Fikrig et al. 1992b, de Silva et al. 1996). Furthermore, intraperitoneal immunization of previously infected mice can reduce subsequent transmission of B. burgdorferi to feeding larval ticks, likely by killing bacteria that migrate to the midgut (Tsao et al. 2001).
The reduction in B. burgdorferi transmission from infected and subsequently vaccinated mice was critical to the success of a recent field trial (Tsao et al. 2004). This intraperitoneal vaccination campaign targeted wild white-footed mice (Peromyscus leucopus), an important reservoir host of Lyme disease in the northeastern United States (Donahue et al. 1987, Mather et al. 1989), and resulted in significant reductions in the prevalence of infected nymphs and thus human Lyme disease risk (Tsao et al. 2004). However, immunizations by needle inoculation are not practical on scales relevant to public health (Cross et al. 2007). Thus, it is critical to develop an effective oral delivery system that is effective at both protecting wildlife and hindering transmission from infected reservoirs to the tick vector.
We developed an oral reservoir-targeted vaccine based on the immunogenic OspA protein of B. burgdorferi aimed at breaking the natural cycle of this spirochete. In the present study, we assessed the efficacy of oral immunization with an OspA protein in protecting uninfected mice from infection and reducing transmission from previously infected mice. Because most white-footed mice are infected prior to vaccination (Bunikis et al. 2004), it is critical to establish whether oral vaccination with an immunogenic protein can reduce B. burgdorferi transmission from infected wild mice to uninfected larval ticks. Importantly, we estimated critical pathogen transmission variables (tick-to-mouse and mouse-to-tick) in vaccinated mice and used these estimates to model the efficacy of an oral vaccination campaign targeting wild P. leucopus mice.
Materials and Methods
Mice, ticks, and bacteria
Adult outbred P. leucopus mice were obtained from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia). Mice were kept at 22°C with a 14:10 light:dark cycle. All mice were approximately 2 months old at the start of the experiments and Institutional Animal Care and Use Committee (IACUC) guidelines were followed. The uninfected larval ticks from 3 different adult female egg masses that were used for xenodiagnosis were purchased from I. scapularis colonies at Oklahoma State University. Infected I. scapularis nymphs were derived from wild P. leucopus mice, as described previously (Gomes-Solecki et al. 2006). Briefly, I. scapularis larvae were collected from naturally infected white-footed mice live-trapped in Elverson, Pennsylvania, and were allowed to molt into nymphs. We randomly selected a sample of 80 nymphs and determined that the frequency of infection was 80% by PCR as previously described (Brisson and Dykhuizen 2004).
Vaccine construction and formulations
Full-length ospA, including the lipidation site, from B. burgdorferi strain B31 was cloned into the pET45b plasmid (EMD Millipore) and transformed into Escherichia coli strain BL21. Incorporation of the complete recombinant ospA (rOspA) sequence into the plasmid was confirmed by Sanger sequencing, and protein expression was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and tandem mass spectrometry. Oral vaccinations used an agar-matrix formulation and an oral gavage formulation (Fikrig et al. 1991). The agar-matrix vaccine was prepared by suspending live OspA-producing E. coli at a final density of 2.5 billion cells/mL of Luria–Bertani agar (0.75% agar, 100 μg/mL ampicillin, and 80 mM isopropyl β-D-1-thiogalactopyranoside [IPTG]). OspA production was confirmed via total protein extraction and mass spectrometry. The concentration of rOspA produced by the E. coli was determined by purifying a histidine-tagged form of the protein from E. coli cells using standard methods (QIAexpressionist handbook). Bradford assays indicated that 1.25 * 1012 rOspA–E. coli cells yielded 5 mg of rOspA protein. The purified histidine-tagged rOspA proteins were used in the enzyme-linked immunosorbent assay (ELISA) assays described below.
Enzyme-linked immunosorbent assay
Total immunoglobulin G (IgG) against OspA in mouse blood was determined by ELISA, as previously described (Schwanz et al. 2011). Briefly, Nunc MaxiSorp 96-well plates were coated with purified rOspA protein (10 μg/mL) overnight at 4°C. The rOspA was removed and the plates were blocked using phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA) for 2 h at room temperature. Plates were washed three times with PBS+0.1% Tween 20 (PBS-T) and then incubated with 100 μL of 1:100 mouse sera (diluted in PBS) for 45 min. Plates were washed 3 times with PBS-T before adding the secondary antibody for 45 min followed by 3 more washes with PBS-T. Anti-P. leucopus secondary antibody conjugated to horseradish peroxidase was used at a 1:2500 dilution (KPL, Maryland) and the 1-Step Ultra TMB was added to initiate the color reaction. Optical density was read at 652 nm after 20 min. We conducted independent replicate ELISAs for both experiments to determine the precision in measuring the anti-OspA IgG immune response. The efficacy of the vaccine treatment, the effect size, was calculated by dividing the difference in optical density between the vaccine and control mice by the standard deviation of the optical density in the control group.
Xenodiagnosis
The efficacy of the vaccine to protect mice from B. burgdorferi challenge as well as to reduce transmission from infected mice was assessed by xenodiagnoses, as previously described (Donahue et al. 1987). Briefly, ∼50 uninfected larval ticks per mouse were allowed to feed to repletion and collected after naturally dropping off the host. Each blood-engorged larva was placed in its own Eppendorf tube with a strip of moistened paper towel and allowed to molt to the nymphal stage. Ticks were kept in the same room as the mice, and the temperature and humidity were strictly controlled. A random sample of 10–12 nymphs from each mouse were tested for the presence of B. burgdorferi DNA using nested ospC PCR (Brisson and Dykhuizen 2004, Gomes-Solecki et al. 2006, Brisson et al. 2008).
Vaccine-protection of uninfected mice
To determine the protective capacity of the vaccine, we challenged uninfected mice (both OspA- and control-vaccinated) with B. burgdorferi-infected nymphs. For these nymphal challenge experiments, 13 uninfected P. leucopus mice were randomly assigned to either the vaccine (n=9) or the control group (n=4). Mice were allowed to feed overnight on a 5-gram slice of agar containing ∼1010 cells of OspA-producing E. coli (or control E. coli strain BL21) 3 nights per week for 5 weeks (∼1010 cells per dose ≅40.5 μg of OspA protein per dose). On average, P. leucopus mice ate 91.0±5.7% (mean±standard error [SE]; n=13) of the agar slice over the 15 immunization nights. To compare the efficacy of the oral agar vaccine with parenteral immunization, 2 mice were immunized via intraperitoneal injection once per week for 5 weeks with 108 cells of OspA-producing E. coli (≅0.406 μg rOspA per dose). Blood was drawn from all mice at 14, 44, and 86 days after the initial vaccine dose to assess the immune response to OspA by ELISA. Mice were subsequently challenged with 10 B. burgdorferi-infected nymphal ticks 45 days after the initial vaccine dose. Blood-engorged nymphs were collected after naturally dropping off each mouse and subsequently tested for the presence of B. burgdorferi-infection. The infection status of mice was determined by xenodiagnosis 35 days after the nymphal challenge. Generalized linear models with a binomial error function were used to compare the proportion of infected ticks and the proportion of infected mice among treatments.
Vaccine-induced reduction of B. burgdorferi transmission from infected mice to feeding ticks
Infected mice (n=11) were subsequently vaccinated to estimate the effect of vaccination on mouse-to-tick transmission. Mice were infected with B. burgdorferi by infesting them with 10 B. burgdorferi-infected nymphs 30 days before the start of the vaccination schedule. Infected mice were randomly assigned to the vaccine group (n=6) or the control group (n=5). Mice were subsequently vaccinated using 50 million OspA-producing (≅0.203 μg rOspA per dose) or control E. coli (strain BL21) via oral gavage once per week for 4 weeks, a vaccination schedule that induced a strong anti-OspA IgG response in uninfected C3H/HeJ mice in a previous study (Fikrig et al. 1991).
The infection status of the mice and the capacity of the vaccine to reduce the transmission from infected mice to feeding larval ticks was assessed by xenodiagnosis 41 days after the initial vaccine dose. The blood-engorged, xenodiagnostic larvae were randomly assigned to be sacrificed on either day 30 (n=12 ticks per mouse) or day 150 (n=10 ticks per mouse) post blood meal to assess the long-term effect of the vaccine on the reduction of mouse-to-tick transmission. Blood was drawn 14 days prior to the initial vaccine dose and 57 days after the initial vaccine dose to assess the OspA immune response.
Natural vaccination coverage parameter estimates
To estimate the possibility of vaccinating wild mice, we analyzed a mark-recapture survey of wild populations of P. leucopus mice in 4 locations in the Crow's Nest Preserve, Elverson, Pennsylvania (April–September, 2009). Each trapping location (225×225 meters each) contained 64 trapping stations spaced every 15 meters with one Sherman live-trap per locality (Sherman Traps, Inc., Tallahassee, FL). Traps were baited (60 total trapping sessions) with rolled oats, set at 17:00, and checked the following morning before 10:00. Each captured mouse was fitted with a unique 4-digit ear tag to facilitate identification and the establishment of recapture histories. From these data, we estimated the probability each resident P. leucopus mouse would visit a baiting location given that bait was available as the number of times a mouse was captured divided by the total number of occasions that it was available to be captured.
Model of vaccine efficacy in wildlife
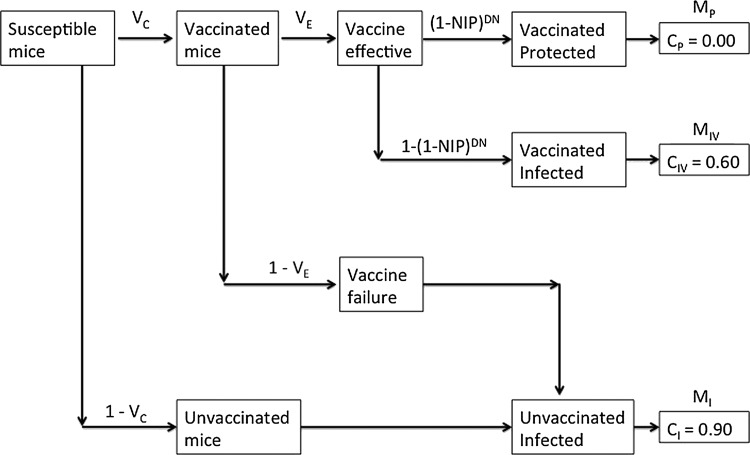
The efficacy of wildlife vaccination in reducing the nymphal infection prevalence (NIP), and thus human Lyme disease risk, was estimated using a deterministic model parameterized with empirical data from this study and from the literature (Fig. 1). The model calculates the expected NIP each year using the sum of the proportion of larval ticks that fed on mice protected due to vaccination (MP), infected mice that were subsequently vaccinated (MIV), infected mice that were not vaccinated (MI), and other wildlife hosts (W), as well as the rates at which B. burgdorferi is transmitted to feeding ticks from each class of host (CP, CIV, CI, and CW, respectively). The model estimates the proportion of mice that elicit a protective immune response prior to challenge by an infected tick as MP=VC * VE * (1−NIP)D * N, where VC is the vaccine coverage, VE is the vaccine efficacy, NIP is the current nymphal infection prevalence, D is the number of days between the first vaccine dose and full protection, and N is the number of nymphs that attach to each mouse per day. Vaccine coverage is the proportion of mice that successfully complete a vaccination schedule, whereas vaccine efficacy is the proportion of mice that effectively respond to the vaccine. Mice that elicit a protective immune response prior to challenge by an infected tick are not infected and thus do not transmit B. burgdorferi to feeding ticks (CP=0). The proportion of mice that are challenged by at least 1 infected tick prior to the vaccine taking full effect is given by MIV=VC*VE*[1−(1−NIP)D * N]. These mice are infected and subsequently vaccinated, resulting in a reduced rate of B. burgdorferi transmission to feeding ticks as estimated in this study (CIV=43/71 or 0.61). The remaining mice (1−VC*VE) include both unvaccinated mice and vaccinated mice for which the vaccine was not effective. These mice are infected and transmit B. burgdorferi to feeding ticks at a standard rate (CI=0.90), as estimated in this and previous studies (Donahue et al. 1987, LoGiudice et al. 2003, Brisson and Dykhuizen 2004, Brunner et al. 2008). Alternative wildlife hosts account for a fixed proportion of tick blood meals (W=0.70), as estimated from the literature (LoGiudice et al. 2003, Brisson et al. 2008), but do not transmit the infection to feeding ticks (CW=0). Thus, NIP in the year following a vaccination campaign can be calculated as MP * CP+MIV * CIV+MI * CI+W * CW. NIP was analyzed with this model by simulating across a wide parameter space for VC (11 levels: 0.00, 0.10, …, 1.00), VE (11 levels: 0.00, 0.10, …, 1.00), CIV (11 levels: 0.00, 0.10, …, 1.00), D (4 levels: 14, 28, 42, and 56 days), and N (5 levels: 0.05, 0.10, 0.20, 0.50 1.00 nymphs/day). For each of these 26,620 scenarios, the NIP at equilibrium was calculated.
FIG. 1.
Diagram of the model parameters that determine 3 classes of P. leucopus mice: Vaccinated and protected MP, infected and subsequently vaccinated (MIV), and infected and unvaccinated (MI). Important parameters include vaccination coverage (VC, the proportion of mice that are vaccinated), vaccination efficacy (VE, the proportion of mice in which the vaccine is effective), days to complete a vaccination schedule (D), nymphal attachment rate (N), and nymphal infection prevalence (NIP). NIP is determined by the proportions of infected, infected and vaccinated, and vaccine-protected mice and the rates at which B. burgdorferi are transmitted to feeding ticks from each mouse class.
Results
Oral vaccination protects uninfected mice from B. burgdorferi infection
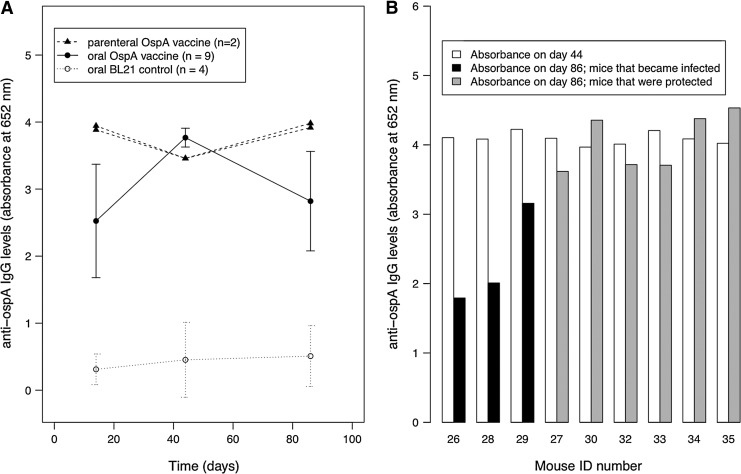
A vaccinated mouse was considered protected if all xenodiagnostic larvae tested negatively for B. burgdorferi following nymphal challenge. Vaccination protected 6 of the 9 OspA-vaccinated mice from infection, whereas none of the 4 control-vaccinated mice were protected (Table 1; Δ deviance=6.49, Δ degrees of freedom [df ]=1, p=0.011). Protection from infection was likely the result of the anti-OspA IgG immune response of the mice in the vaccine group, which was 6.3 standard deviations (SD) greater than that in the control group by day 42 (t=18.66, df=12, p<0.001; Fig. 2A). The anti-OspA IgG response was similar among the mice in the vaccine group prior to nymphal challenge (t=1.25, df=7, p=0.253; Fig. 2B). However, the anti-OspA IgG levels of the 3 mice that became infected were 1.73 SD lower than the 6 protected mice 41 days after nymphal challenge (t=4.66, df=7, p=0.002; Fig. 2B).
Table 1.
Efficacy of Vaccination Schedules in Uninfected and B. burgdorferi-Infected Mice
| Experiment | Infection statusa | Vaccine | Scheduleb | N | Effect sizec | Protectedd | Transmissione | Statisticalsignificanceg |
|---|---|---|---|---|---|---|---|---|
| Vaccine protection | Uninfected | OspA | 15 doses* 10×109 cells |
9 | 6.3 (p<0.001) | 6/9 | 0.80–0.90f | Tick-to-mouse transmission (p=0.011) |
| Uninfected | Control | 15 doses* 10×109 cells |
4 | 0/4 | 0.75–1.00 | |||
| Transmission reduction | Infected | OspA | 4 doses* 0.5×108 cells |
6 | 1.4 (p=0.574) | NA | 0.33–0.91 | Mouse-to-tick Transmission (p<0.001) |
| Infected | Control | 4 doses* 0.5 ×108 cells |
5 | NA | 0.75–1.00 |
The infection status of mice prior to the first vaccine treatment.
The number of doses and the number of cells per dose.
The effect size of the vaccination treatment on anti-OspA immune response.
The proportion of mice protected (tick-to-mouse transmission).
The minimum and maximum proportions of infected larvae (mouse-to-tick transmission).
For 3 nonprotected mice only.
Statistical significance of vaccination treatment on transmission
NA, not applicable.
FIG. 2.
Oral vaccination with Escherichia coli–expressing OspA suspended in an agar matrix results in a strong and rapid anti-OspA immunoglobulin G (IgG) immune response in uninfected P. leucopus mice. The anti-OspA IgG immune response was measured as the absorbance reading at 652 nm after 20 min using a 1:100 dilution of mouse serum. (A) The anti-OspA IgG immune response on day 42 in vaccinated, uninfected mice (solid circles; n=9 mice) was 6.3 standard deviations (SD) greater than the control mice (open circles; n=4 mice; t=18.66, [df ]=12, p<0.001). Orally vaccinated mice were immunized 15 times with 10 billion live cells per dose starting on day 0. Parenterally vaccinated mice (n=2) were immunized 5 times with 100 million cells per dose starting on day 0. Shown are the means and the 95% confidence limits. There are no confidence limits for the parenterally vaccinated mice because there were only 2 individuals. (B) B. burgdorferi infection reduced anti-OspA IgG levels in mice following the nymphal challenge. On day 86, the anti-OspA IgG response in rOspA-vaccinated mice that became infected following nymphal challenge (black bars; n=3) was 1.73 SD lower than the mice that remained uninfected following nymphal challenge (grey bars; n=6; t=4.66, df=7, p=0.002).
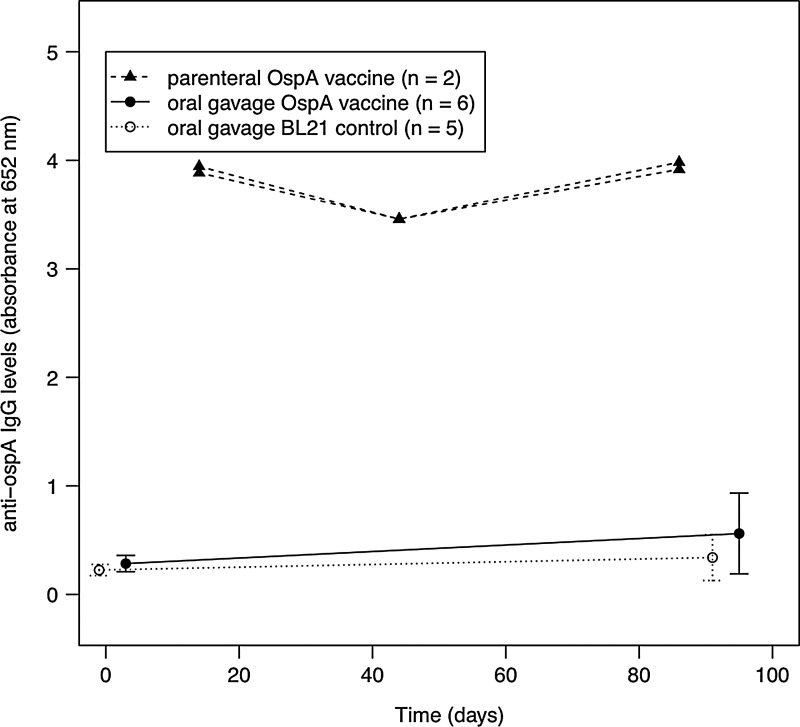
The anti-OspA IgG levels at day 42 were similar in the orally (n=9) and parentally (n=2) vaccinated mice (Fig. 2). Replicate measures of the anti-OspA IgG levels (absorbance at 652 nm at 20 min for 1:100 serum dilution) were highly correlated between independent ELISA assays (r=0.98, p<0.001), indicating a very low measurement error.
Oral vaccination reduces B. burgdorferi from feeding nymphs
At least 1 blood-engorged B. burgdorferi-infected nymph was recovered from all of the protected mice, indicating that all mice were exposed to at least 1 B. burgdorferi-infected nymph. However, the proportion of nymphs that remained infected after feeding on mice in the vaccine group (36.4%=20 infected/55 nymphs) was significantly lower than those nymphs that fed on mice in the control group (71.4%=20 infected/28 nymphs; Δ deviance=9.35, Δdf=1, p=0.002).
Oral vaccination reduces B. burgdorferi transmission from infected mice to feeding ticks
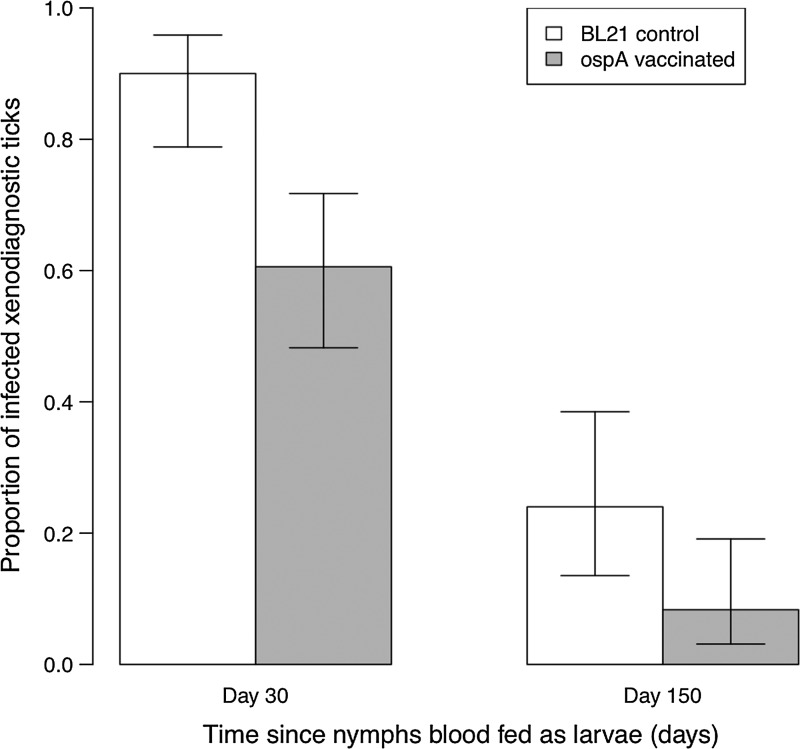
The anti-OspA IgG levels in the vaccinated mice were 1.4 SD greater than the control mice, a statistically nonsignificant difference (t=0.58, df=9, p=0.574). Replicate measures of the anti-OspA IgG levels (absorbance at 652 nm at 20 min for 1:100 serum dilution) were highly correlated between independent ELISA assays (r=0.74, p=0.005), indicating low measurement error. Comparison with the parenterally immunized mice from the previous experiment revealed that the oral gavage treatment induced a weak anti-OspA IgG immune response (Fig. 3). Despite this weak immune response, mouse-to-tick transmission of B. burgdorferi in infected and subsequently OspA-vaccinated mice (43 infected/71 ticks=60.6%; n=6 mice) was one-third lower than that of infected and control-vaccinated mice (54 infected/60 ticks=90%; n=5 mice), and this overall difference in the percentage of infected ticks was highly statistically significant (Δ df=1, Δ dev=20.48, p<0.001; Fig. 4). Among infected and OspA-vaccinated mice, there was considerable variation in the mouse-to-tick transmission rate (33–91% in Table 1). Despite this variation, the conservative independent 2-sample t-test (using mice as the units of replication) confirmed that the difference in the mean transmission rate between the vaccinated and control mice was statistically significant (t=2.98, df=9, p value=0.015). To test whether there was a relationship between mouse anti-OspA IgG antibody levels and the mouse-to-tick transmission rate, we used a logistic regression with a quasi-binomial error function. There was a negative, but not statistically significant, relationship between anti-OspA IgG levels and the transmission rate (slope on the logit scale=−0.97±0.426, F=1.88, p=0.203). When the analysis was restricted to the 6 vaccinated mice, the slope remained negative (−0.58±0.480) and not statistically significant.
FIG. 3.
Oral vaccination with E. coli-expressing OspA did not result in a strong anti-OspA immunoglobulin G (IgG) immune response in previously infected P. leucopus mice. The anti-OspA IgG levels were similar among the groups of infected mice prior to vaccination. Post vaccination, the anti-OspA IgG immune response in infected and subsequently vaccinated mice (n=6) was 1.4 standard deviations (SD) greater than in infected control mice (n=5), but this difference was not statistically significant (t=0.58, [df ]=9, p=0.574). Mice were first infected with B. burdorferi 31 days prior to the first of 4 vaccine doses of 50 million live cells per dose. For comparison, the anti-OspA IgG immune responses of the parenterally vaccinated mice (n=2) were included from the previous experiment. These mice were immunized 5 times with 100 million cells per dose via intraperitoneal injection. Shown are the means and the 95% confidence limits.
FIG. 4.
OspA vaccination reduces transmission of B. burgdorferi from previously infected P. leucopus mice to feeding larval ticks. For each of the 11 mice, xenodiagnostic larvae were collected and randomly assigned to be sacrificed on day 30 or day 150 after the blood meal. For each of the 11 mice, the prevalence of B. burgdorferi infection was estimated for 12 ticks sacrificed on day 30 and 10 ticks sacrificed on day 150. Nearly all of the day-30 nymphs that took their larval blood meal from control mice were infected (54 infected/60 ticks=90%; n=5 mice), whereas fewer day-30 nymphs from vaccinated mice were infected (43 infected/71 ticks=60.6%; n=6 mice; [df ]=1, Δ dev=20.48, p<0.001). Interestingly, the prevalence of B. burdorferi in ticks that were sacrificed 150 days after the transmission event (17 infected/110 ticks=15.5%; n=11 mice) was 4.8 times lower than ticks that were sacrificed 30 days after the transmission event (97 infected/131 ticks=74.0%; n=11 mice; df=1, Δ dev=95.95, p<0.001). However, the decrease in prevalence occurs at the same rate in the ticks that fed on vaccinated and unvaccinated mice such that the effect of the vaccine remains unchanged due to this phenomenon. The bars represent the means and the whiskers represent the 95% confidence intervals.
Interestingly, for both OspA-vaccinated and control mice, the prevalence of B. burgdorferi infection was significantly lower (df=1, Δ dev=95.95, p<0.001; Fig. 4) in the nymphs sacrificed on day 150 (17 infected/110 ticks=15.5%; n=11 mice) than the nymphs sacrificed on day 30 (97 infected/131 ticks=74.0%; n=11 mice). Because all nymphs (those sacrificed at day 30 and at day 150) were fed as larvae on the same 11 mice at the same time, the results suggest that infection prevalence in nymphal ticks can decrease over time. Importantly, the relative difference in the infection prevalence of nymphs from vaccinated and unvaccinated mice remained similar across time, indicating that the vaccine effect remains constant regardless of other biological factors.
Vaccine coverage
A total of 296 unique P. leucopus individuals were captured a total of 2089 times with an average daily capture probability of 0.70/day. Assuming that the capture rate is equivalent to the daily probability of a mouse encountering the vaccine, we can calculate the proportion of mice that will be vaccinated a specified number of times within a specified time period. For example, our data indicate that 47% of all P. leucopus mice would receive a complete vaccination schedule (15 doses) within 19 days and over 90% of mice would be completely vaccinated within 24 days. Thus, high vaccination coverage is readily achievable within a short period of time.
Reducing NIP through wildlife vaccination
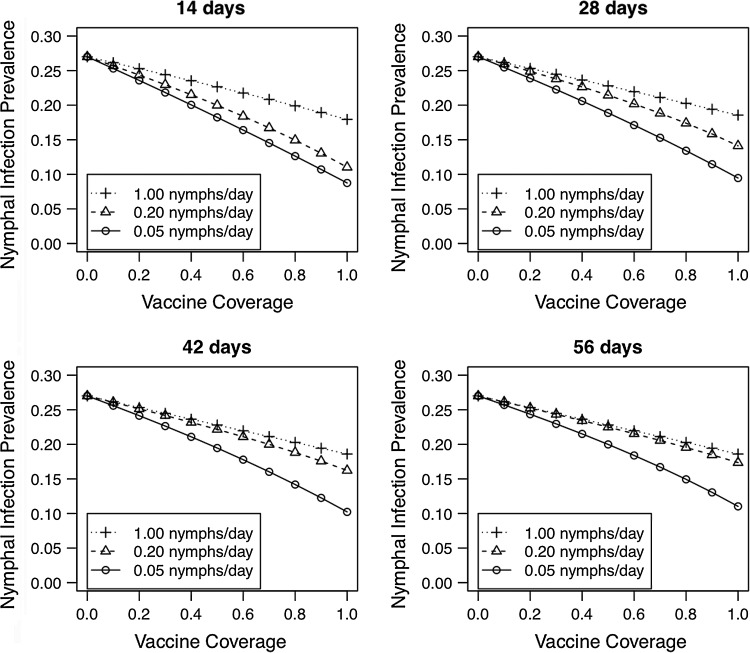
Oral vaccination of wild mouse populations can reduce the NIP both by preventing infection in uninfected mice and reducing the transmission from infected mice (Figs. 5 and 6). Importantly, reductions in current NIP reinforce lower future NIP by increasing the proportion of mice that can be vaccinated prior to exposure to a B. burgdorferi-infected tick, which in turn reinforces lower future NIP in this positive feedback loop. In the absence of vaccination, the equilibrium NIP is 0.270, which is similar to the NIP observed in natural systems (LoGiudice et al. 2003, Brisson and Dykhuizen 2004, Tsao et al. 2004, Hoen et al. 2009). Using the estimates of vaccination efficacy (VE=6/9=0.667) and the rate of B. burgdorferi transmission from infected and vaccinated mice (CIV=43/71=0.606) from this study, and optimistic vaccination parameters (100% vaccination coverage, 15 days to full vaccination, daily nymphal attachment rate=0.05 nymphs/day), our model predicts an equilibrium NIP of 0.097 (Fig. 5). The estimates of equilibrium NIP were highly sensitive to vaccine coverage and the daily nymphal attachment rate. More conservative estimates of these vaccination parameters led to greater equilibrium NIP.
FIG. 5.
Wildlife vaccination can result in dramatic reductions in the nymphal infection prevalence (NIP) by preventing infection in uninfected mice and reducing the transmission from infected mice. The equilibrium NIP decreases with vaccination coverage and increases with the nymphal attachment rate (N=0.05, 0.20, and 1.00 nymphs/day) and the time to complete vaccination (D=14, 28, 42, and 56 days). The vaccination efficacy parameter (VE=6/9=0.67) and the B. burgdorferi transmission rate from infected and vaccinated mice (CIV=43/71=0.60) were estimated from the data in the present study.
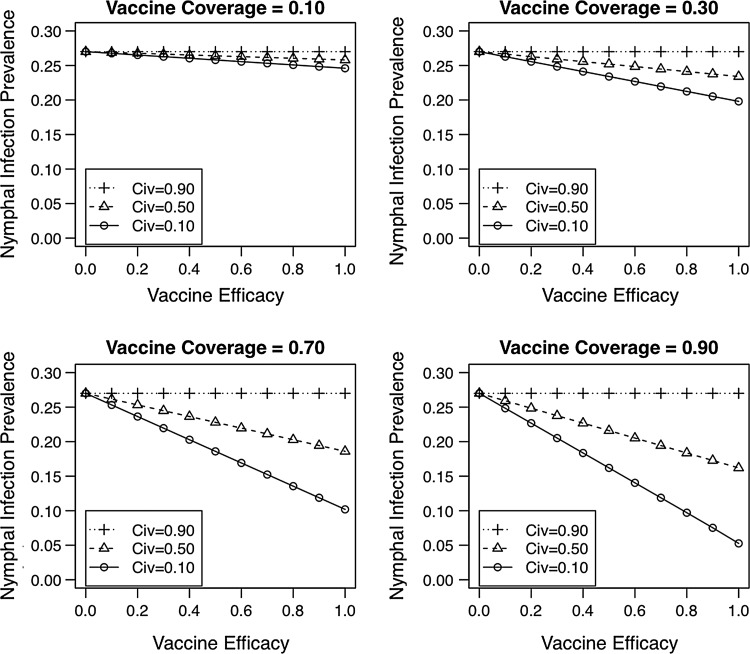
FIG. 6.
The nymphal infection prevalence depends strongly on the capacity of the vaccine to reduce B. burgdorferi transmission (CIV) from infected mice to feeding ticks. The equilibrium NIP decreases with higher vaccination coverage and vaccine efficacy (VE) and decreases at lower values of CIV. These simulations assumed the most conservative vaccination conditions with respect to the time to complete vaccination (D=56 days) and nymphal attachment rate (N=1.00 nymphs/day).
The importance of the vaccine's ability to reduce B. burgdorferi transmission from infected mice to feeding ticks (CIV) is readily apparent when the time to complete vaccination is long (D=56 days) and the nymphal attachment rate is high (1.00 nymphs/day) (Fig. 6). Under these circumstances, virtually all mice become infected prior to becoming vaccinated. Despite these challenging conditions, high vaccination coverage (VC=0.90) with an effective oral vaccine (VE>0.80) that reduces B. burgdorferi transmission from infected mice to feeding ticks (CIV<0.10) has the potential to reduce the equilibrium NIP to below 10%, even under otherwise challenging vaccination conditions.
Discussion
Oral vaccination of P. leucopus, an important wildlife host of B. burgdorferi, elicits an immune response that reduces transmission of B. burgdorferi at the two critical stages of the Lyme disease life cycle. Consistent with previous studies (Fikrig et al. 1991, Gomes-Solecki et al. 2006, Scheckelhoff et al. 2006, del Rio et al. 2008, Richer et al. 2011), oral vaccination of uninfected mice prevents transmission of B. burgdorferi from infected ticks to uninfected mice due to a strong immune response. Here we demonstrate that oral vaccination of previously infected mice with an OspA protein significantly reduces the transmission of B. burgdorferi to feeding larval ticks. Projection modeling suggests that the two effects of the vaccine on the transmission cycle of B. burgdorferi will act synergistically in a positive feedback loop to reduce the nymphal infection prevalence in wildlife communities well below what would be expected from either effect alone. This study suggests that oral immunization of wildlife with an OspA-based vaccine is a promising long-term strategy to reduce human Lyme disease risk.
Uninfected P. leucopus mice immunized with an easily distributable OspA vaccine were protected from B. burgdorferi infection when challenged with infected ticks (Table 1). As shown previously (Fikrig et al. 1992b), the protective feature of the vaccine was caused by the immune response of the vaccinated mice, which also cleared B. burgdorferi from a substantial proportion of the challenge nymphs. However, it is important to note that the “nymphal clearance” aspect of the vaccine is unlikely to affect its capacity to break the transmission cycle of Lyme disease in nature (Tsao 2009, Tsao et al. 2001, 2004). This is because nymphs molt to adults, which tend to feed on large hosts such as deer, which are not competent for transmitting B. burgdorferi (Bosler et al. 1984, Magnarelli et al. 1984, Telford et al. 1988, Jaenson and Talleklint 1992).
With respect to the capacity of the agar vaccine to protect mice, our results are directly relevant to field conditions because the experimental mice were challenged with ticks that were infected with a variety of natural strains. Furthermore, the number of infected ticks that fed on each mouse (mean=5.9 nymphs; range=1–8 nymphs), and thus the infectious dose of B. burgdorferi and the associated immunomodulatory tick salivary proteins (Randolph 2009), were greater in this study than what P. leucopus mice experience in nature (∼0.2 nymphs/day) (Mannelli et al. 1994, Daniels and Fish 1995, Lyon et al. 1996, Brisson and Dykhuizen 2004). Thus, wild mice may be better protected from B. burgdorferi than suggested by the present data if the inoculating dose of B. burgdorferi or the concentration of tick salivary proteins affects the probability of successful infection (Ribeiro et al. 1990, Nuttall and Labuda 2004). Further data are needed to determine if the infection of mice in this study, despite prior vaccination, resulted from the extreme dose of B. burgdorferi or salivary proteins that overwhelmed the vaccine protection, or if some strains of B. burgdorferi are less affected by the OspA immunogen.
Vaccinating previously infected mice effectively reduced mouse-to-tick transmission of B. burgdorferi from 90.0% to 60.6% (Fig. 4) despite a statistically nonsignificant immune response caused by the vaccine. Interestingly, the mouse-to-tick transmission efficiency varied substantially among infected, vaccinated mice, with 1 mouse showing no effect while others showed a 63% reduction (Table 1). Such variation can have important consequences for field vaccination strategies if ticks aggregate on rodent hosts that are the least responsive to the oral vaccination treatment. This scenario is plausible because ticks are often aggregated on their hosts (Randolph et al. 1996, Randolph et al. 1999, Brunner and Ostfeld 2008, Devevey and Brisson 2012) and tick saliva is known to inhibit the host immune response (Wikel 1999, Randolph 2009). Although there was a negative relationship between anti-OspA IgG levels and the transmission rate to feeding ticks, this relationship was not statistically significant, likely due to the very limited range of the immune responses among these mice. Thus, we cannot definitively say that the anti-OspA IgG levels are the mechanism that caused the transmission rate reduction in the vaccinated mice. One possibility is that the anti-OspA IgG levels were higher in the experimental mice at the time of the nymphal challenge (day 41) and then declined so that they were no longer different from the control mice after xenodiagnosis (day 90).
The effects of the OspA vaccine on mouse-to-tick transmission described here are conservative as the experimental mice received a very weak vaccination schedule (4 doses * 0.203 μg rOspA per dose) and had a correspondingly weak anti-OspA immune response (Fig. 3). More intense vaccination schedules, which are feasible in natural conditions, may further reduce mouse-to-tick transmission, although more experimental data are needed to confirm this hypothesis. Two sets of data suggest that more intense vaccination schedules are feasible under natural conditions. First, P. leucopus in this study ate >90% of the agar containing 1010 cells of live OspA-expressing E. coli over the 15 immunization sessions. Second, in an observational pilot study, field-captured P. leucopus mice willingly ate agar containing E. coli even in the presence of other food resources (pellet food or apple). In addition, PVC tubes containing agar that were distributed at our field site had evidence of rodent activity, including substantial reductions in agar, teeth marks in the agar, and rodent droppings in the PVC tube. Although observational, these data suggest wild P. leucopus willingly consume agar-based vaccines. Finally, our capture rate estimates suggest that it is possible to vaccinate a large proportion of the population (>90%) in a short period of time (24 days).
Previous studies demonstrate that parenteral immunization of previously infected P. leucopus mice with purified rOspA can reduce B. burgdorferi transmission to as little as 1% after just 3 immunizations. A recent experiment similarly demonstrated that vaccinating previously infected P. leucopus mice with a vaccinia virus-based OspA vaccine also significantly reduced transmission to feeding ticks, although not as effectively as parenteral immunizations (Bhattacharya et al. 2011). This vaccinia-based vaccine has the added advantage of requiring only a single immunization for inducing a strong immune response (Scheckelhoff et al. 2006, Bhattacharya et al. 2011). However, the mode of action of vaccinia-based vaccines differs considerably from the data shown here because the virus does not express the immunogen directly but infects host cells, which then express the immunogen (Scheckelhoff et al. 2006). This methodology has proven highly effective but has several potential regulatory hurdles because vaccinia-based vaccines are infectious to people and can be difficult to control in nature (McGuill et al. 1998, Rupprecht et al. 2001, Sepkowitz 2003). Thus, it is important to understand if oral immunization with a protein antigen is effective at reducing mouse-to-tick transmission despite promising data from needle- and virus-based delivery vehicles.
The reduction in the proportion of ticks testing positively for B. burgdorferi caused by the OspA immunization was apparent in both newly molted and 150-day-old nymphal ticks (Fig. 4). Interestingly, the proportion of B. burgdorferi-infected ticks was significantly lower in the 150-day-old ticks from both the control mice and the vaccinated mice (Fig. 4). These data may suggest that the duration of B. burgdorferi-survival in I. scapularis nymph midguts is limited. However, the experimental ticks were maintained at room temperature for 6 months, which is not analogous to the natural conditions that B. burgdorferi experiences in overwintering nymphs in the northeastern United States. Furthermore, the reduction in spirochete load in all ticks was not a product of vaccination and may not be biologically relevant. There are several experimental factors that may influence the survivorship of spirochetes in ticks, none of which were explored here. Future investigations into the effects of factors such as temperature, humidity, and tick activity-levels on the survivorship of B. burgdorferi in tick midguts are necessary to understand the relevance of these data. Future experiments should keep replicate samples of ticks in different incubators.
The anti-OspA IgG levels from the mice that were not protected by OspA vaccination decreased significantly after these mice became infected with B. burgdorferi. Although there was no difference in the anti-OspA IgG levels among vaccinated mice prior to nymphal challenge, the anti-OspA IgG levels were significantly reduced in the mice that became infected, suggesting that infection with B. burgdorferi may have caused a reduction in the standing anti-OspA IgG antibody levels relative to the mice that were successfully protected (Fig. 2). The transmission rate of B. burgdorferi to feeding larval ticks from these infected mice was also very high and similar to the transmission rate from control mice (Table 1). These results may suggest that, despite a strong immune response and apparent success of the vaccination, there was neither a protective effect nor an effect on transmission suggesting vaccination failure. Additionally, these data may suggest that infection with B. burgdorferi may hinder an immune response against an oral OspA-based vaccine, a topic that should be investigated explicitly in future studies. Regardless, vaccinating previously infected mice significantly reduces mouse-to-tick transmission and thus could have a substantial impact on the prevalence of infected ticks in natural systems (Figs. 4–6).
Our model assumes that vaccinated mice remain protected for the duration of their lifetimes. This assumption was validated by a previous study that found that uninfected mice gained yearlong protection following oral OspA immunization (Richer et al. 2011). Further studies are necessary to determine the duration of transmission reduction following oral OspA immunization of B. burdorferi-infected mice. However, the agar-matrix–based oral vaccine described in this study could be easily distributed to wildlife throughout the nymphal activity season and thus provide a continual booster to increase vaccine efficacy. Agar has little digestible material and thus adding agar-matrix–based baits to a natural ecosystem is unlikely to affect P. leucopus population demography dramatically. In addition, our bait vaccine formulations are readily eaten by wildlife and remain immunogenic for several days under environmental conditions representative of the summers in the northeastern United States (Brisson, unpublished data). Restricted distribution of oral vaccines to the primary natural reservoirs of B. burgdorferi (small mammals) can be achieved via bait palatability or by bait repositories that exclude larger animals, as shown in our preliminary field trials. Hence, any shortcomings in the duration of vaccine efficacy could be overcome using an extended vaccination schedule with an easily distributable bait vaccine.
The inhibitory effects of OspA vaccination on both tick-to-mouse and mouse-to-tick transmission will benefit wildlife vaccination campaigns aimed at reducing the prevalence of infected nymphal ticks and thus human Lyme disease risk (Figs. 5 and 6). The reductions in mouse-to-tick transmission are imperative in practice because a majority of mice in Lyme disease endemic areas cannot be vaccinated prior to becoming infected (Bunikis et al. 2004). Our projection models suggest that the reductions in NIP achieved by vaccinating infected mice will increase the probability of successfully vaccinating uninfected mice prior to exposure with infected ticks, which further reduces NIP. Thus, the inhibition of mouse-to-tick and tick-to-mouse transmission works synergistically to reduce NIP below levels that would be expected from either effect alone. The model results suggest that the equilibrium NIP achieved through a vaccination campaign is sensitive to the proportion of animals vaccinated, the efficacy of the vaccine in protecting animals from infection, and the ability of the vaccine in reducing transmission from infected mice.
Although vaccine coverage in P. leucopus mice is relatively easy to achieve, future studies should investigate whether a more aggressive oral vaccination regime could improve the protection efficacy and reduce the transmission rates from infected animals to levels similar to those reported from needle vaccination studies (Tsao et al. 2001). Combining B. burgdorferi-targeted and I. scapularis-targeted oral vaccines will also likely amplify these synergistic effects by further reducing the number of ticks that feed on mice (Maritz-Olivier et al. 2007). Although the data and models presented have focused on P. leucopus mice, a major wildlife reservoir of B. burgdorferi, these results can be extended to include other wildlife reservoirs as data on the effect of the vaccine on other species become available. These results suggest that wildlife vaccination can be an effective and long-term strategy to reduce human Lyme disease risk.
Acknowledgments
The study was supported by grant CK000170 from the Centers for Diseases Control and Prevention and AI076342 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIAID). We thank Rick Ostfeld and Maria Gomes-Solecki for their input on this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson JF. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann NYAcad Sci. 1988;539:180–191. doi: 10.1111/j.1749-6632.1988.tb31852.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D. Bensaci M. Luker KE. Luker G, et al. Development of a baited oral vaccine for use in reservoir-targeted strategies against Lyme disease. Vaccine. 2011;29:7818–7825. doi: 10.1016/j.vaccine.2011.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosler EM. Ormiston BG. Coleman JL. Hanrahan JP, et al. Prevalence of the Lyme-disease spirochete in populations of white-tailed deer and white-footed mice. Yale J Biol Med. 1984;57:651–659. [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ. Bent SJ. Folsom-O'Keefe CM. Tsao K, et al. Genotypic diversity of Borrelia burgdorferi strains detected in Ixodes scapularis larvae collected from North American songbirds. Appl Environ Microbiol. 2010;76:8265–8268. doi: 10.1128/AEM.01585-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D. Dykhuizen DE. ospC Diversity in Borrelia burgdorferi: Different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D. Dykhuizen DE. Ostfeld RS. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc R Soc Biol Sci Series B. 2008;275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner JL. Ostfeld RS. Multiple causes of variable tick burdens on small-mammal hosts. Ecology. 2008;89:2259–2272. doi: 10.1890/07-0665.1. [DOI] [PubMed] [Google Scholar]
- Brunner JL. LoGiudice K. Ostfeld RS. Estimating reservoir competence of Borrelia burgdorferi hosts: Prevalence and infectivity, sensitivity, and specificity. J Med Entomol. 2008;45:139–147. doi: 10.1603/0022-2585(2008)45[139:ercobb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bunikis J. Tsao J. Luke CJ. Luna MG. Fish D. Barbour AG. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: A longitudinal study in an area where Lyme borreliosis is highly endemic. J Infect Dis. 2004;189:1515–1523. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- Cross ML. Buddle BM. Aldwell FE. The potential of oral vaccines for disease control in wildlife species. Vet J. 2007;174:472–480. doi: 10.1016/j.tvjl.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Daniels TJ. Fish D. Effect of deer exclusion on the abundance of immature Ixodes scapularis (Acari, Ixodidae) parasitizing small and medium-sized mammals. J Med Entomol. 1995;32:5–11. doi: 10.1093/jmedent/32.1.5. [DOI] [PubMed] [Google Scholar]
- Daszak P. Cunningham AA. Hyatt AD. Wildlife ecology—Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- de Silva AM. Telford SR., III Brunet LR. Barthold SW, et al. Borrelia burgdorferi ospA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio B. Dattwyler RJ. Aroso M. Neves V, et al. Oral immunization with recombinant Lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin Vaccine Immunol. 2008;15:1429–1435. doi: 10.1128/CVI.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devevey G. Brisson D. The effect of spatial heterogeneity on the aggregation of ticks on white-footed mice. Parasitology. 2012;139:915–925. doi: 10.1017/S003118201200008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA. Hoen AG. Cislo P. Brinkerhoff R, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hygiene. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JG. Piesman J. Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hygiene. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- Dunne M. Al-Ramadi BK. Barthold SW. Flavell RA, et al. Oral vaccination with an attenuated Salmonella typhimurium strain expressing Borrelia burgdorferi ospA prevents murine Lyme borreliosis. Infect Immun. 1995;63:1611–1614. doi: 10.1128/iai.63.4.1611-1614.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E. Barthold SW. Kantor FS. Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant ospA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E. Barthold SW. Kantor FS. Flavell RA. Protection of mice from Lyme borreliosis by oral vaccination with Escherichia coli expressing ospA. J Infect Dis. 1991:1641224–1227. doi: 10.1093/infdis/164.6.1224. [DOI] [PubMed] [Google Scholar]
- Fikrig E. Barthold SW. Kantor FS. Flavell RA. Long-term protection of mice from Lyme disease by vaccination with ospA. Infect Immun. 1992a;60:773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E. Telford SR., III Barthold SW. Kantor FS, et al. Elimination of Borrelia burgdorferi from vector ticks feeding on ospA-immunized mice. Proc Natl Acad Sci USA. 1992b;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Solecki MJC. Brisson DR. Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disese spirochete can be delivered via bait. Vaccine. 2006;24:4440–4449. doi: 10.1016/j.vaccine.2005.08.089. [DOI] [PubMed] [Google Scholar]
- Hoen AG. Rollend LG. Papero MA. Carroll JF, et al. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 2009;9:431–438. doi: 10.1089/vbz.2008.0155. [DOI] [PubMed] [Google Scholar]
- Jaenson TGT. Talleklint L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol. 1992;29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- Jones KE. Patel NG. Levy MA. Storeygard A, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K. Ostfeld RS. Schmidt KA. Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003b;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke CJ. Huebner RC. Kasmiersky V. Barbour AG. Oral delivery of purified lipoprotein ospA protects mice from systemic infection with Borrelia burgdorferi. Vaccine. 1997;15:739–746. doi: 10.1016/s0264-410x(97)00219-3. [DOI] [PubMed] [Google Scholar]
- Lyon SM. Edman JD. VanDriesche RG. Field estimates of numbers of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs per hectare successfully feeding on Peromyscus leucopus in Massachusetts. J Med Entomol. 1996;33:812–818. doi: 10.1093/jmedent/33.5.812. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA. Anderson JF. Chappell WA. Antibodies to spirochetes in white-tailed deer and prevalence of infected ticks from foci of Lyme-disease in Connecticut. J Wildlife Dis. 1984;20:21–26. doi: 10.7589/0090-3558-20.1.21. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA. Anderson JF. Fish D. Trans-ovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari, Ixodidae) J Infect Dis. 1987;156:234–236. doi: 10.1093/infdis/156.1.234. [DOI] [PubMed] [Google Scholar]
- Mannelli A. Kitron U. Jones CJ. Slajchert TL. Influence of season and habitat on Ixodes scapularis infestation on white-footed mice in northwestern Illinois. J Parasitol. 1994;80:1038–1042. [PubMed] [Google Scholar]
- Maritz-Olivier C. Stutzer C. Jongejan F. Neitz AWH, et al. Tick anti-hernostatics: Targets for future vaccines and therapeutics. Trends Parasitol. 2007;23:397–407. doi: 10.1016/j.pt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Mather TN. Wilson ML. Moore SI. Ribeiro JMC, et al. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Mather TN. Nicholson MC. Donnelly EF. Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- McGuill MW. Kreindel SM. DeMaria A. Robbins AH, et al. Human contact with bait containing vaccine for control of rabies in wildlife. J Am Vet Med Assn. 1998;213:1413–1417. [PubMed] [Google Scholar]
- Nuttall PA. Labuda M. Tick-host interactions: Saliva-activated transmission. Parasitology. 2004;129:S177–S189. doi: 10.1017/s0031182004005633. [DOI] [PubMed] [Google Scholar]
- Ogden NH. Lindsay LR. Hanincova K. Barker IK, et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. 2008;74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH. Margos G. Aanensen DM. Drebot MA, et al. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol. 2011;77:3244–3254. doi: 10.1128/AEM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrican LA. Absence of Lyme disease spirochetes in larval progeny of naturally infected Ixodes scapularis (Acari: Ixodidae) fed on dogs. J Med Entomol. 1997;34:52–55. doi: 10.1093/jmedent/34.1.52. [DOI] [PubMed] [Google Scholar]
- Piesman J. Mather TN. Dammin GJ. Telford SR, et al. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–1189. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- Prevention. Summary of Notifiable Diseases—United States, 2009. Morbid Mortal Weekly Rep. 2011;58:1–100. [PubMed] [Google Scholar]
- Randolph SE. Tick-borne disease systems emerge from the shadows: The beauty lies in molecular detail, the message in epidemiology. Parasitology. 2009;136:1403–1413. doi: 10.1017/S0031182009005782. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Gern L. Nuttall PA. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996;12:472–479. doi: 10.1016/s0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Miklisova D. Lysy J. Rogers DJ, et al. Incidence from coincidence: Patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177–186. doi: 10.1017/s0031182098003643. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC. Weis JJ. Telford SR. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- Richer LM. Aroso M. Contente-Cuomo T. Ivanova L, et al. Reservoir targeted vaccine for Lyme borreliosis induces a year long, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011:1809–1816. doi: 10.1128/CVI.05226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht CE. Blass L. Smith K. Orciari LA, et al. Brief report: Human infection due to recombinant vaccinia-rabies glycoprotein virus. N Engl J Med. 2001;345:582–586. doi: 10.1056/NEJMoa010560. [DOI] [PubMed] [Google Scholar]
- Scheckelhoff MR. Telford SR. Hu LT. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006;24:1949–1957. doi: 10.1016/j.vaccine.2005.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG. Piesman J. Golde WT. Dolan MC, et al. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanz LE. Voordouw MJ. Brisson D. Ostfeld RS. Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector Borne Zoonotic Dis. 2011;11:117–124. doi: 10.1089/vbz.2009.0215. [DOI] [PubMed] [Google Scholar]
- Sepkowitz KA. How contagious is vaccinia? N Engl J Med. 2003;348:439–446. doi: 10.1056/NEJMra022500. [DOI] [PubMed] [Google Scholar]
- Stafford KC. Cartter ML. Magnarelli LA. Ertel SH, et al. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR. Mather TN. Moore SI. Wilson ML, et al. Incompetence of deer as reservoirs of the Lyme-disease spirochete. Am J Trop Med Hygiene. 1988;39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- Tompkins DM. Ramsey DSL. Cross ML. Aldwell FE, et al. Oral vaccination reduces the incidence of tuberculosis in free-living brushtail possums. Proc R Soc B Biol Sci. 2009;276:2987–2995. doi: 10.1098/rspb.2009.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet Res. 2009;40:36. doi: 10.1051/vetres/2009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J. Barbour AG. Luke CJ. Fikrig E, et al. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:65–74. doi: 10.1089/153036601750137705. [DOI] [PubMed] [Google Scholar]
- Tsao JI. Wootton T. Bunikis J. Luna MG, et al. An ecological approach to preventing human infection: Vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci USA. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK. Tick modulation of host immunity: An important factor in pathogen transmission. Int J Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ. Haydon DT. Antia R. Emerging pathogens: The epidemiology and evolution of species jumps. Trends Ecol Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]