Abstract
Objective
The aim of this study was to assess the impact of transcriptional induction on thyroid follicular cell (TFC) differentiation from endodermally matured embryonic stem (ES) cells. The thyroid transcription factors—NKx2 homeobox 1 (NKx2-1, formerly called TTF-1) and Paired box gene 8 (Pax8)—are known to associate biochemically and synergistically in the activation of thyroid functional genes including the sodium/iodide symporter (NIS), thyrotropin (TSH) receptor (TSHR), thyroglobulin (Tg), and thyroid peroxidase (TPO) genes. In this study, we investigated the ability of ectopically expressed Pax8 and NKx2-1 to further the induction and differentiation of murine ES cells into potential TFCs.
Methods
ES cells were stably transfected with either the Pax8 gene, the NKx2-1 gene, or both genes to study the induction of NIS, TSHR, Tg, and TPO genes as assessed using both quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and protein expression. The derived cells were cultured with or without the presence of activin A to allow their differentiation into multipotent endodermal cells.
Results
The four thyroid-specific genes NIS, TSHR, Tg, and TPO were all significantly activated by expressing both transcription factors within the same ES cell. In contrast, significant but much lower transcriptional activity of the TSHR, Tg, and TPO genes was detected in cells expressing just NKx2-1, and only the NIS and TSHR genes responded to Pax8 alone. No Tg protein expression could be detected prior to their development into endodermal derivatives. However, after further differentiation of postembryoid body ES cells with activin A and TSH into endodermal cell lines, those cells with dual transfection of Pax8 and NKx2-1 demonstrated greatly enhanced expression of the NIS, TSHR, Tg, and TPO genes to such a degree that it was similar to that found in control thyroid cells. Furthermore, these same cells formed three-dimensional neofollicles in vitro and expressed Tg protein, but these phenomena were absent from lines expressing only Pax8 or NKx2-1.
Conclusion
These findings provide further evidence that co-expression of Pax8 and NKx2-1 in murine ES cells may induce the differentiation of thyroid-specific gene expression within endodermally differentiated ES cells and commit them to form three-dimensional neofollicular structures.
Introduction
Genes expressed in a cell type–specific manner are usually regulated by promoters containing recognition sequences for both tissue-specific and ubiquitous transcription factors. It is the functional interaction between these various regulating proteins and the regulatory DNA sequences that enables individual cell types to play their specific role. Hence, tissue-specific transcriptional regulation is mediated by a set of transcription factors whose combination is unique to individual cell types such as the thyroid follicular cell (TFC). TFCs, the most abundant cell population of the thyroid gland, are characterized by the expression of a specific set of genes, including (i) thyroglobulin (Tg) and thyroperoxidase (TPO), which are exclusively expressed in this cell type; (ii) thyroid-stimulating hormone receptor (TSHR) and the sodium/iodide symporter (NIS), which also exhibit extrathyroidal expression (1,2); and (iii) transcription factors such as NKx2 homeobox 1 (NKx2-1, formerly called TTF-1), forkhead box protein E1 (Foxe1), and Paired box gene 8 (Pax8), which only co-express in TFCs and appear to be the factors most responsible for the thyroid-specific expression of the Tg and TPO genes (3,4). While the specific role of Foxe1 in the development and differentiation of the thyroid gland is less clear, the roles of NKx2-1 and Pax8 have been extensively studied (2), and the differentiation program of TFCs clearly relies on the interplay between these sequence-specific transcription factors and transcriptional coregulators with the basal transcriptional machinery of the cell. Furthermore, embryonic stem (ES) cells, even when persuaded to differentiate into thyrocyte-like cells, including the use of activin A and TSH but without the high transcriptional expression of these two important genes, have proven to be epigenetically refractory to maturation into stable and functional thyrocytes (5–7).
The simultaneous expression of Pax8 and NKx2-1 in thyroid cells suggested the existence of a functional interaction between these two transcription factors. Accordingly, it has been demonstrated that Pax8 and NKx2-1 associate biochemically and synergistically to activate transcription from the TPO and the Tg gene promoters (8). Indeed, the functional interaction of Pax8 and NKx2-1 has been shown to activate thyroid-specific promoter/enhancer elements even in Morris hepatoma cells (8). Therefore, using the NKx2-1 and Pax-8 genes, it is possible to probe the mechanisms responsible for commitment of undifferentiated endodermal precursor cells toward the thyroid phenotype. In this study, we demonstrate that the thyroid-specific genes NIS, TSHR, Tg, and TPO were significantly activated in ES cells that were ectopically expressing both Pax8 and NKx2-1 transcription factors, while only low transcriptional activation of these genes was observed in cells expressing either Pax8 or NKx2-1 alone. After further differentiation toward the endodermal lineage, these double transfected ES cells developed into three-dimensional thyroid follicles and expressed abundant thyroglobulin protein.
Methods
Growth and maintenance of ES cells
W9.5 mouse ES cells were maintained as previously described on gelatin-coated dishes in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies, Inc.) supplemented with 15% fetal calf serum (STEMCELL Technologies, Inc.), penicillin-streptomycin (100 U/mL; Invitrogen Life Technologies, Inc.), 1.5×10−4 M monothioglycerol (Sigma-Aldrich Corp.), and 10 ng/mL leukemia inhibitory factor (LIF; STEMCELL Technologies, Inc.). Cells were cultured in a humidified chamber in a 5% CO2–air mixture at 37°C. ES cell cultures were passaged at 1:3–5 ratios every two days.
Generation of Pax8+, or NKx2-1+, or Pax8+/NKx2-1+ expressing ES cell lines
Two vectors, kindly provided by Dr. Uwe Haberkorn of the German Cancer Research Center, Heidelberg, Germany (8), were as follows:
(A) M48EF1αhPax8IVREShyg: 5′LTR–EF1α–hPax8–IVS–IRES–hyg–3′LTR
(B) M48EF1αdNKx2-1IVRESneo: 5′LTR–EF1α–dNkx2-1–IVS–IRES–neo–3′LTR
The M48 bicistronic retroviral vectors were constructed based on the Moloney murine leukemia virus. The vectors were designed to transfer the human Pax8 (hPax8) or the dog NKx2-1 (dNKx2-1) (95% homology with human TTF-1) genes, and the hygromycin (hyg) or the neomycin (neo) genes by use of an internal ribosomal entry site (IRES) from the encephalomyocarditis virus. A synthetic intron (IVS) was inserted to stabilize the mRNA. Gene expression was regulated by the elongation factor 1α gene (EF1α) promoter. LTR indicates the long terminal repeat.
These vectors, alone or together, were electroporated into ES cells (Neon transfection system; Invitrogen Life Technologies, Inc.), and after two days, the cells were cultured in hygromycin (0.5 mg/mL) or G418 (0.8 mg/mL) or both for at least four weeks until resistant clones were established. A large number of clones were then selected and characterized for their gene expression and high-expressing clones chosen for expansion into stable lines.
Differentiation of ES cell
Embryoid bodies (EBs) were differentiated as described previously (7). In brief, single ES cell suspensions were plated in 60-mm bacterial grade dishes to induce EB formation in the absence of LIF. EBs were cultured for one day in the same culture medium as ES cells except for the lack of LIF. The following day, EBs were harvested and allowed to settle by gravity in a 50-mL tube and transferred to new dishes and cultured in fresh medium supplemented with or without 50 ng/mL human activin A (R&D Systems, Inc.) for five days. To induce the differentiation of ES cells into thyroid cells, EBs were collected and embedded in growth factor-restricted Matrigel (BD Biosciences) (9,10) and placed into six-well plates in differentiation medium that containing Dulbecco's modified Eagle's medium supplemented with penicillin/streptomycin, 15% KnockOut serum replacement medium (Invitrogen Life Technologies, Inc.), 5% protein-free hybridoma medium (PFHM-II; Invitrogen Life Technologies, Inc.), 1.5×10−4 M monothioglycerol, and 1000 μU/mL human recombinant TSH (Fitzgerald Industries). Cells were harvested for analysis at days 5 and 21 (total time) of culture (Fig. 1).
FIG. 1.
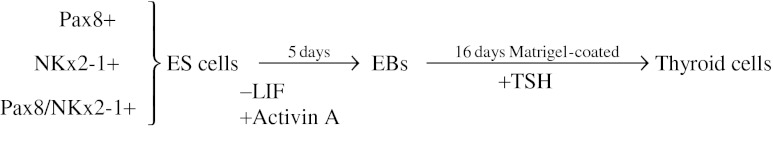
Protocol summary.
RNA isolation and reverse-transcription polymerase chain reaction
Total RNA was extracted from cultured cell lines using the RNeasy Mini Kit Isolation System (Qiagen Ltd.), which included a digestion step with DNase I. RNA quantity and quality were assessed by UV spectrophotometry. cDNA synthesis was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen Corp.). Semi-quantitative reverse-transcription (RT) polymerase chain reaction (PCR) was carried out with 25–30 cycles of amplification depending on the target. The number of cycles used was determined to be in the log-linear phase of the amplification reaction. The PCR products were separated by 2% agarose gel electrophoresis and visualized with ethidium bromide staining. The real-time quantitative RT-PCR (qRT-PCR) was carried out using SYBR green qPCR Master Mix (Applied Biosystems) and employing the StepOnePlus Real-time PCR System (Applied Biosystems). Relative expression levels of each gene in real time were analyzed using the 2−ΔΔCt method and normalized to the expression of the housekeeping gene GAPDH. Data presented (mean) are from three independent experiments in which all sample sets were analyzed in triplicate.
Immunodetection
Cells were grown in Delta T culture dishes, fixed for 15 min with 4% paraformaldehyde (PFA), rinsed three times (5 min each) with phosphate-buffered saline (PBS), and blocked for 60 min at room temperature in 3% bovine serum albumin (BSA), 5% horse serum in PBS. Depending on the analysis marker, 0.3% Triton X-100 was added into the blocking buffer for cell permeabilization to detect intracellular antigens. Cells were incubated with appropriate primary antibodies diluted in a solution of PBS containing 1% BSA, 1% horse serum, and 0.1% Triton X-100 overnight at 4°C. Cells were rinsed three times (5 min each) with PBS and incubated with the appropriate secondary antibody for one hour at room temperature. After that, cells were rinsed three times with PBS and mounted using hard set mounting media containing DAPI (Vector Laboratories).
Results
Generation and characterization of stable Pax8- and NKx2-1– expressing ES cell lines
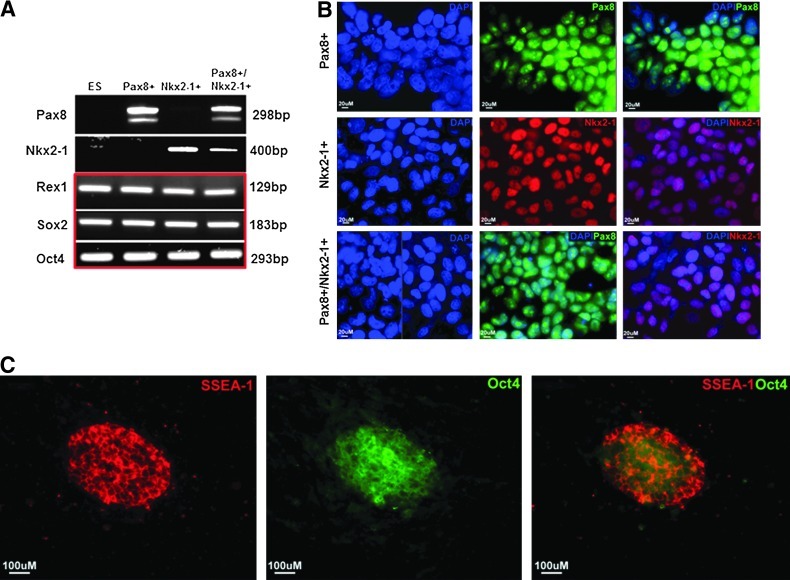
To test whether overexpression of Pax8 and NKx2-1 in ES cells could induce or promote thyroid-specific gene expression, undifferentiated mouse W9.5 ES cells were electroporated with the M48EF1αhPax8IVSIREShyg vector and/or M48EF1αdNKx2-1IVSIRESneo vector. After selection with the respective antibiotic drug, stable resistant cell lines, including Pax8+, NKx2-1+, and Pax8+/NKx2-1+ ES cell lines, were established as evidenced by their gene expression profiles (Fig. 2A) and by immunodetection of Pax8 and NKx2-1 nuclear expression (Fig. 2B). However, overexpression of these transcription factors at this stage did not change the pluripotent state of the ES cells, since the stemness markers Rex1, Sox2, and Oct4 (Fig. 2A) and SSEA-1 (Fig. 2C) continued to be expressed to the same degree as in the original ES cells.
FIG. 2.
Generation and characterization of stable Pax8+, NKx2-1+, and Pax8+/NKx2-1+ mouse ES cell lines. (A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of selected Pax8-, NKx2-1–, or Pax8/NKx2-1–expressing mouse embryonic stem (ES) cell lines for thyroid transcription factors Pax8 and NKx2-1 and pluripotent markers Rex1, Sox2, and Oct4 after the cells were cultured in selection medium for up to four weeks. (B) Immunofluorescence analysis of Pax8 and NKx2-1 in the selected double transfected cell line. Scale=20 μm. (C) Representative immunodetection analysis of pluripotent markers SSEA-1 and Oct4 expression in Pax8+/NKx2-1+ cells. Scale=100 μm.
Activation of thyroid-specific genes in Pax8+, NKx2-1+, and Pax8+/NKx2-1+ ES cells
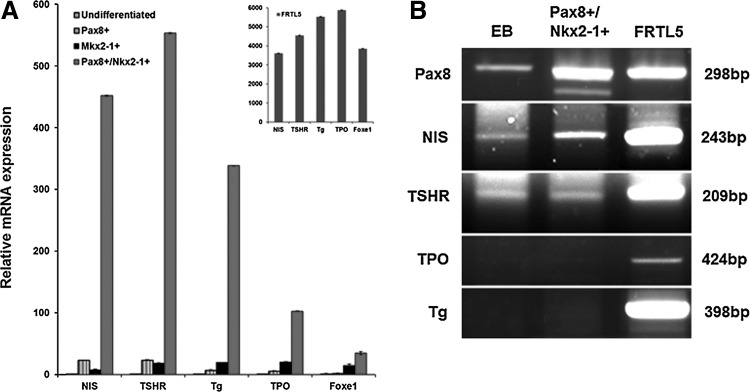
The individual cell lines expressing Pax8, or NKx2-1, or Pax8/NKx2-1 were subsequently analyzed with regard to the activation of thyroid-specific genes (NIS, TSHR, Tg, and TPO) using qRT-PCR and compared to control rat thyroid cells (FRTL-5; Fig. 3A). Stable cell lines of single transfected genes showed only a 5–10-fold increase in expression of thyroid-specific genes compared to the baseline expression. Pax8+ cells showed minor induction of NIS and TSHR gene expression, while NKx2-1+ cells showed minor induction of TSHR, Tg, and TPO gene expression. However, only the double transfected (Pax8+/NKx2-1+) cells showed robust induction of NIS, TSHR, Tg, and TPO gene transcription with a 30–55-fold increase, as well as induction of the transcription factor Foxe1 (Fig. 3A). Nevertheless, when compared to the control thyroid cells (FRTL5), the degree of gene induction remained limited (Fig. 3A, inset). Hence, co-expression of Pax8 and NKx2-1 in these untreated ES stable lines initiated a change in the cell's fate, driving their differentiation toward a thyroid follicular cell lineage, but appeared to be insufficient for full thyrocyte fate determination. This conclusion was also supported by the fact that immunodetection of Tg protein expression was unsuccessful in the Pax8+/NKx2-1+ cells in keeping with the relatively low level of gene transcription observed (data not shown). Therefore, it appeared that further differentiation was required to drive protein translation of Tg and to commit the cells to a definite and permanent thyrocyte fate.
FIG. 3.
(A) qRT-PCR analysis for thyroid-specific gene activity and Foxe1 expression in Pax8+, NKx2-1+, and Pax8+/NKx2-1+ ES cell lines. Inset: The quantitative reverse-transcription polymerase chain reaction (qRT-PCR) data for rat thyroid (FRTL5) cells. Relative expression of each transcript is presented as fold change compared to ES cells (mean±SEM, n=3), and represent one of three separate experiments. (B) RT-PCR analysis for thyroid-specific gene expression in untreated Pax8+/NKx2-1+ embryoid bodies (EB) cells. The gel images represent one of three separate experiments.
Thyroid-specific gene expression in embryoid bodies cells
In order to determine if the immature non-three-dimensional state of the ES cells was limiting thyroid-specific gene transcription, the stable Pax8+/NKx2-1+ ES cells were matured into embryoid bodies (EB) cells by removal of LIF and the cells cultured for a total of 21 days. During this time, we again saw only limited or absent Tg gene expression (Fig. 3B) and no TPO gene expression.
Influence of activin A on thyroid-specific gene expression in nontransfected EB cells
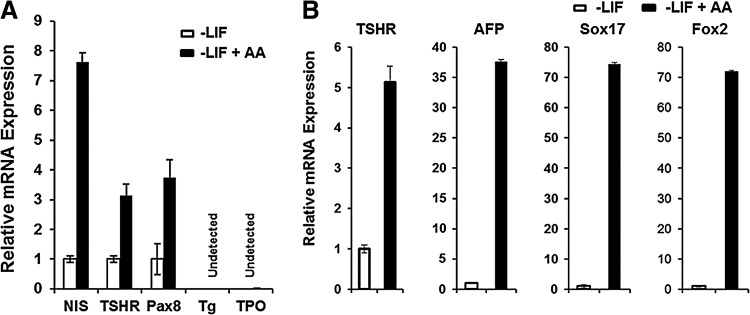
Thyroid tissue develops from the endodermal lineage of cells. Thus, using the EB differentiation model we have previously shown the potential of activin A to induce ES cells into the thyroid endoderm (7). Although in this model we clearly observed an increase in the transcription of certain thyroid-specific genes (TSHR, NIS, and Pax8), after five days of activin A treatment (Fig. 4A), we were still unable to detect NKx2-1, Tg, or TPO gene expression. It was evident, therefore, that the induction of ES cells into thyroid cells expressing NIS and TSHR and Pax8 was helped by a sophisticated schedule including the sequential application of activin A but that these conditions alone were not sufficient for successful thyroid cell fate determination.
FIG. 4.
(A) qRT-PCR analysis of NIS, TSHR, Pax8, Tg, and TPO genes in EB cells and in EB cells treated for five days with activin A. Although there was significant expression of NIS, TSHR, and Pax8 after activin treatment, there was no induction of Tg or TPO in these cells. (B) qRT-PCR analysis for TSHR and endoderm markers (AFP, Sox17, and Fox2) in EB cells formed from activin A treated and untreated Pax8+/NKx2-1+ ES cells. Data were expressed as mean±SEM, and represent one of three separate experiments. All the data were significantly different from –LIF cells (p<0.01). LIF, leukemia inhibitory factor; AA, activin A.
Further differentiation of Pax8/NKx2-1-expressing ES cells
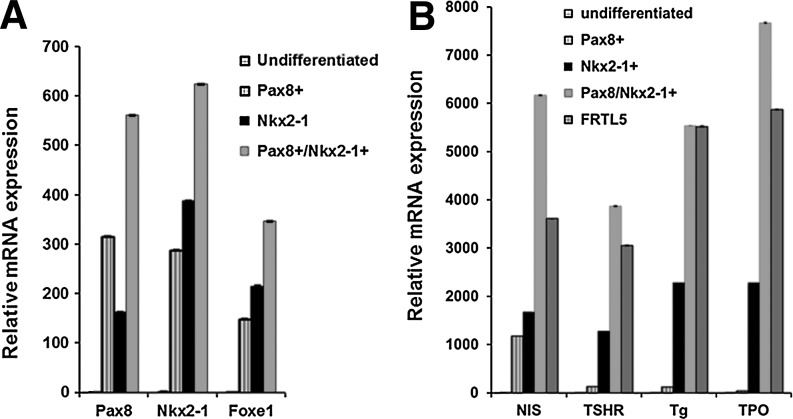
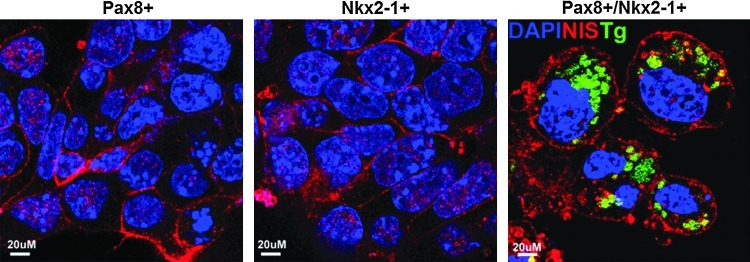
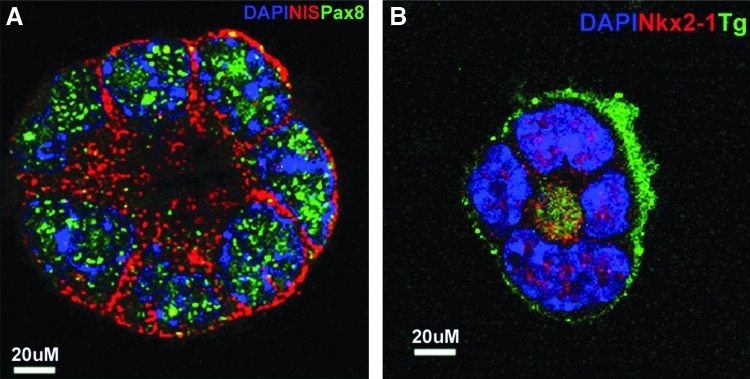
Since these data indicated that at least the NIS and the TSHR thyroid-specific genes showed a marked increase in expression with activin A, we proceeded to combine this approach with overexpression of the critical transcription factors Pax8 and NKx2-1. We induced EB formation in single and double transfected stable ES cell lines by withdrawal of LIF and then treated the resulting EB cells with activin A for five days to induce endoderm differentiation. Examination after activin A exposure showed the development of endoderm by expression of endodermal markers AFP, Sox 17, and Fox2, and the expected increased upregulation of TSHR gene expression (Fig. 4B). Further qPCR analysis of these activin A–stimulated Pax8+/NKx2-1+ cells after another 16 days of culture (on day 21 after EB formation) showed a significant increase in Pax8 and NKx2-1 transcription, as well as the induction of endogenous Foxe1 (Fig. 5A). More importantly, there was an even more robust transcriptional activation of all four of the thyroid-specific genes NIS, TSHR, Tg, and TPO to the same or even higher levels as seen in control thyroid cells (Fig. 5B). Immunofluorescent analysis confirmed abundant NIS expression in all three cell lines and co-expression of Tg and NIS was seen in Pax8+/NKx2-1+ cells but not in Pax8+ or NKx2-1+ expressing cells (Fig. 6). Such cells appeared to be fully committed to their thyrocyte fate because of clear basolateral surface expression of NIS and the presence of intracellular Tg. On continued culture, this led to convincing three-dimensional thyroid follicle formation with Tg present in the follicular lumen (Fig. 7).
FIG. 5.
Differentiation of Pax8-, NKx2-1–, or Pax8/NKx2-1–expressing mouse ES cell lines into thyroid cells. (A) qRT-PCR analysis for thyroid transcriptional factors (Pax8, NKx2-1, and Foxe1) in differentiated Pax8+, NKx2-1+, and Pax8+/NKx2-1+ ES cells at day 21. (B) qRT-PCR analysis for thyroid functional genes (NIS, TSHR, Tg, and TPO) in differentiated Pax8+, NKx2-1+, and Pax8+/NKx2-1+ ES cells at day 21. The relative expression of each transcript is presented as fold change compared to undifferentiated ES cells (mean±SEM) and represent one out of three separate experiments. All data were significant (p<0.01) when compared to control ES cells.
FIG. 6.
Immunodetection of NIS (red) in the differentiated Pax8+, NKx2-1+, and Pax8+/NKx2-1+ EB cells at day 21 with DAPI (blue) nuclear staining. Immunodetection of Tg (green) expression was only seen in the Pax8+/NKx2-1+ cells. Scale=20 μm.
FIG. 7.
Immunostaining of endoderm-derived thyroid neofollicles. (A) Immunodetection of Pax8 (green) and NIS (red) expression in a thyroid neofollicle derived from differentiated Pax8+/NKx2-1+ EB cells at day 21. Note that the NIS expression is seen on the cell surface, and the staining for transcription factor Pax8 is seen within the nucleus. Scale bar=20 μm. (B) Immunodetection of Tg (green) and NKx2-1 (red) expression in a thyroid neofollicle derived from differentiated Pax8+/NKx2-1+ EB cells at day 21. Note that the Tg is seen in the intrafollicluar lumen and the staining for transcription factor NKx2-1 is seen within the nucleus. Extravasation of Tg is seen around this three-dimensional structure within the extracellular matrix support. Scale bar=20 μm.
Discussion
The thyroid gland includes two endocrine cell types: (i) thyroid follicular cells (TFC) making up 95% of the gland, which synthesize and secrete thyroid hormones, and are derived from foregut endoderm; and (ii) parafollicular C cells that produce calcitonin and are derived from neuroectoderm. It is now clear that the cooperative action of the transcription factors Nkx2 (TTF1) and Pax8, together with downstream Foxe1 (TTF2), is crucial for many stages of thyroid development. Until now, insight into this complex biological process has been gained by studying snapshots of the signaling process during embryonic development in wild type and mutant mice (1–3). Although these studies have shown that early steps leading to the formation and growth of the thyroid are independent of TSH signaling (1) they have also failed to reveal all the factors that initiate expression of thyroglobulin (Tg), the signature molecule produced only by the thyroid gland.
Pax8, NKx2-1, and Foxe1 are three well-characterized transcription factors that in combination have been specifically shown to regulate thyroid-specific gene expression. None of these transcription factors are expressed exclusively in the thyroid, but their co-expression is unique to the thyroid and appears to form a thyroid-specific gene expression program (1) responsible for initiation and differentiation of thyrocytes. It has been shown that Pax8 and NKx2-1 directly interact and synergistically activate thyroid-specific transcription even in nonthyroid cells (3,4,8) and therefore provide a major part of a model system for the differentiation of ES cells into thyroid cells. While our current studies were under way, Antonica et al. have taken advantage of this information to generate functional thyroid cells in vivo using an inducible expression system employing both of these transcription factors (11).
In this study, we successfully established stable ES cell lines overexpressing either Pax8 or NKx2-1 alone or together. We found significant expression of the NIS and TSHR genes in all three cell lines, but the induction of TPO and Tg gene expression was only seen in Pax8+/NKx2-1+ cells. Nevertheless, these double-positive cells were still unable to translate Tg protein successfully, and the degree of thyroid-specific gene expression remained low when compared to control thyroid cells. However, after exposure to activin A and the successful development of an endodermal phenotype, we found abundant Tg expression in the Pax8+/NKx2-1+ cells, and these cells had gained the potential for forming thyroid neofollicles. These cells not only gained the three-dimensional structure of neofollicles but also expressed Pax8 and NKx2-1 within their nuclei, NIS on their basolateral surfaces, and intracytoplasmic and intraluminal Tg.
These data indicate the importance of the cooperation between Pax8 and NKx2-1 in the activation of Tg gene expression (12) but also support the concept that the developmental stage of the cell and its progenitor program are equally important for full differentiation. Such a differentiation milieu can clearly be provided in vivo, and these data indicate that, in vitro, activin A appears to be capable of imitating some of this influence. Activin is a member of the TGFβ superfamily, which is critical in the regulation of endoderm formation in vitro and in vivo, and acts as a mimetic for native nodal (13,14). We have previously succeeded in recapitulating the development of thyroid endodermal progenitor cells in vitro using embryoid body transformation and a chemically defined differentiation protocol, which resulted in a small population of cells expressing Pax-8, the TSHR, and NIS (7). Our present studies have successfully taken advantage of these observations in order to produce cells capable of neofollicle formation and abundant Tg expression. The use of stable ES cell lines overexpressing both Pax8 and NKx2-1 is in sharp contrast to the reported transient approach (11), and will provide opportunities to characterize the detailed molecular events leading to thyroid cell speciation and a predictable supply of thyroid progenitor cells for in vivo studies. However, our studies have been limited to mouse ES cells, and it is uncertain at this point whether such proof of principle defines the exact sequential steps and molecular events in the development of human thyrocytes from human ES cells or induced pluripotent stem cells.
Acknowledgments
This work was supported in part by DK080459, DK069713, and DK052464 from the National Institutes of Health and the VA Merit Review Program.
Author Disclosure Statement
The authors declare that no competing interests exist.
References
- 1.Damante G. Di Lauro R. Thyroid-specific gene expression. Biochim Biophys Acta. 1994;1218:255–266. doi: 10.1016/0167-4781(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 2.Damante G. Tell G. Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 3.Di Palma T. Nitsch R. Mascia A. Nitsch L. Di Lauro R. Zannini M. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem. 2003;278:3395–3402. doi: 10.1074/jbc.M205977200. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza CR. Schmitt TL. Loos U. Thyroid transcription factor 1 and Pax8 synergistically activate the promoter of the human thyroglobulin gene. J Mol Endocrinol. 2001;27:59–67. doi: 10.1677/jme.0.0270059. [DOI] [PubMed] [Google Scholar]
- 5.Lin RY. Kubo A. Keller GM. Davies TF. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology. 2003;144:2644–2649. doi: 10.1210/en.2002-0122. [DOI] [PubMed] [Google Scholar]
- 6.Arufe MC. Lu M. Kubo A. Keller G. Davies TF. Lin RY. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology. 2006;147:3007–3015. doi: 10.1210/en.2005-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma R. Latif R. Davies TF. Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin A. Endocrinology. 2009;150:1970–1975. doi: 10.1210/en.2008-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altmann A. Schulz RB. Glensch G. Eskerski H. Zitzmann S. Eisenhut M. Haberkorn U. Effects of Pax8 and TTF-1 thyroid transcription factor gene transfer in hepatoma cells: imaging of functional protein-protein interaction and iodide uptake. J Nucl Med. 2005;46:831–839. [PubMed] [Google Scholar]
- 9.Martin A. Valentine M. Unger P. Lichtenstein C. Schwartz AE. Friedman EW. Shultz LD. Davies TF. Preservation of functioning human thyroid organoids in the scid mouse: 1. System characterization. J Clin Endocrinol Metab. 1993;77:305–310. doi: 10.1210/jcem.77.2.8345031. [DOI] [PubMed] [Google Scholar]
- 10.Valentine M. Martin A. Unger P. Katz N. Shultz LD. Davies TF. Preservation of functioning human thyroid “organoids” in the severe combined immunodeficient mouse. III. Thyrotropin independence of thyroid follicle formation. Endocrinology. 1994;134:1225–1230. doi: 10.1210/endo.134.3.8119163. [DOI] [PubMed] [Google Scholar]
- 11.Antonica F. Kasprzyk DF. Opitz R. Iacovino M. Liao XH. Dumitrescu AM. Refetoff S. Peremans K. Manto M. Kyba M, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascia A. De Felice M. Lipardi C. Gentile R. Cali G. Zannini M. Di Lauro R. Nitsch L. Transfection of TTF-1 gene induces thyroglobulin gene expression in undifferentiated FRT cells. Biochim Biophys Acta. 1997;1354:171–181. doi: 10.1016/s0167-4781(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 13.Tam P.P. Kanai-Azuma M. Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393–400. doi: 10.1016/s0959-437x(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 14.Kubo A. Shinozaki K. Shannon JM. Kouskoff V. Kennedy M. Woo S. Fehling HJ. Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]