Abstract
Hospital-acquired infections remain a costly clinical problem. Barium sulfate (BaSO4, in micron particulate form) is a common radiopacifying agent that is added to catheters and endotracheal tubes. Due to the recently observed ability of nanostructured surface features to decrease functions of bacteria without the aid of antibiotics, the objective of this in vitro study was to incorporate nano-barium sulfate into pellethane and determine the antimicrobial properties of the resulting composites. The results demonstrated for the first time that the incorporation of nano-barium sulfate into pellethane enhanced antimicrobial properties (using Staphylococcus aureus and Pseudomonas aeruginosa) compared to currently used pellethane; properties that warrant further investigation for a wide range of clinical applications.
Keywords: antimicrobial polymer, hospital-acquired infections, surface roughness, radiopaque agent, dynamic contact microbial testing
Introduction
Each year, there are approximately 1.7 million hospital-acquired infections in the USA, 90,000 of which are fatal.1 More than half of these infections can be attributed to contamination of life-sustaining medical devices such as endotracheal tubes, bladder catheters, and central venous catheters, as well as other medical implants.2 These infections can prolong hospital stay, increase medical costs, and result in the death of the patient.3 In the United States alone, as many as 28,000 patients die each year due to catheter-related bloodstream infections.4,5 Each blood infection can cost the health care system more than US$35,000 per case, with a resulting total potential burden of US$35 billion.6 Clearly, there is a great need to develop more efficient antimicrobial and bacterial static products for the medical device community.
The main challenge of preventing bacteria adhesion and proliferation leading to medical device infection is to catch bacteria early, before a biofilm has formed, since, once the bacteria biofilm is formed, bacteria become profoundly more resistant to host defenses as well as to antibiotic treatment.2 Costerton et al defines biofilms as a collection of matrix-enclosed bacteria populations, adherent to each other and/or surfaces or interfaces.7 Once bacteria develop a biofilm, it is very difficult for antibiotics to penetrate the polysaccharide slime layer and effectively kill the bacteria.8 One study, by Nichols et al, showed that Pseudomonas aeruginosa (a common biofilm-forming bacteria) was 15 times more susceptible to antibiotic treatment when dispersed from a biofilm than when in a biofilm.9
Currently, silver is an actively researched antimicrobial active ingredient being developed for numerous medical device applications; however, there remains a need for more cost-effective, less toxic, solutions (Foster Corporation, email communication, 2011). Specifically, some studies cite the emergence of silver resistant bacteria and provide indications of mammalian cell toxicity.10 In contrast, there has been an emergence of studies which demonstrated slowed or stopped growth of bacteria on materials with nanostructured surface features which alter surface energetics or nanoscale surface roughness to repel bacteria.10
Along this line, barium sulfate (BaSO4) is a common agent added to catheters or endotracheal tubes which make such medical tubing radiopaque. In addition, BaSO4 polymeric formulations have been shown to exhibit some antimicrobial activity (Foster Corporation, email communication, 2011). Since studies have shown that the use of nanostructures on medical device surfaces can create surfaces resistant to bacteria proliferation,11 the goal of this study was to add BaSO4 nanoparticles to pellethane to create nanoscale surface features. In this manner, this study demonstrated that nano-BaSO4 pellethane composites effectively minimized bacteria interactions while still remaining radiopaque.
Material and methods
Sample preparation
Pellethane pellets (Foster Corp., Putnam, CT, USA) were mixed with various weight percentages of BaSO4 powder (either 73 nm diameter for nano-BaSO4 or 7 micron diameter for conventional BaSO4). Seven different sample groups were made (0% BaSO4, 20% nano-BaSO4, 30% nano-BaSO4, 40% nano-BaSO4, 20% BaSO4, 30% BaSO4, and 40% BaSO4). 20% BaSO4 was chosen due to its commercial use. These mixtures were mixed, melted, and extruded into tapes. Tapes were then cut into disks to fit within the 12-well plate (approximately 22 mm in diameter). These disks were then sterilized through successive 5 minute soaks in 100% ethanol, 70% ethanol, and sterile deionized water (deionized water soaks were done at least twice to ensure complete removal of ethanol). For sterilization, samples were then exposed to UV light for 1 hour and were allowed to dry under sterile conditions. Dry samples were stored at room temperature under sterile conditions.
Contact angle test
Contact angle measurements were made on a Krüss Easy Drop contact angle instrument (Krüss GmbH, Hamburg, Germany) connected to an image analysis program (Drop Shape Analysis v1.8; Krüss). The Krüss Easy Drop apparatus was used to measure the contact angles that resulted when a 10 μL drop of either water, glycerol, or ethylene glycol was placed on the surface of a sample disk.12
Radiopacity
Polymer samples were labeled and X-ray properties determined using an Infinity XMA HF-30AP (UMG/Del Medical, Harrison, NY, USA) set to manual technique mode with an exposure time of 0.016 seconds and milliampere per second at 6.1 and 70 kV. Images were taken on each sample individually and s-values (the numerical value of exposure received by the receptors in the digital system) were recorded.13 For analysis, the s-value for the 0% BaSO4 sample was used as a base and subtracted from all the other values to normalize the results.
Atomic force microscopy (AFM) surface analysis
AFM images of polymer samples were obtained on an Agilent 5500 AFM/SPM microscope (Agilent Technologies, Santa Clara, CA, USA) under acoustic AC mode using Si probes operating at a resonant frequency of 154 kHz. All measurements were carried out at room temperature and the acquired images had a resolution of 512 × 512 pixels collected at a speed of one line/minute. Post image processing of AFM images were done using the Pico Image software provided with the instrument. The images were subjected to standard image processing techniques that included line correction, form removal, leveling, and threshold adjusting. Root mean square (RMS) roughness values were also obtained.
Bacteria culture
Stock solutions of Staphylococcus aureus (#25923) and P. aeruginosa (Schroeter) Migula (#27853) were obtained from the American Type Culture Collection ([ATCC] Manassas, VA, USA). Stock solutions were diluted and frozen. Bacteria from stock solutions were streaked out for isolation on agar plates. Approximately 3 mL of sterile tryptic soy broth (TSB) (Sigma-Aldrich, St Louis, MO, USA) were inoculated with one colony of the desired bacteria and then were incubated at 37°C while on a shaker set to 200 rpm. These solutions were incubated for 18 hours to reach the stationary phase and were then diluted to a density of 1 × 107 bacteria/mL (as estimated by the McFarland scale which corresponded to an optical density of 0.52 at 562 nm and was then further diluted at a ratio of 1:100).14
Bacteria growth
All bacterial growth trials with polymer samples were completed in 12-well tissue culture plates. For all trials, polymer samples were used as sterile polymer disks placed in separately labeled wells of a 12-well tissue culture plate. They were then covered in 1 mL of the bacteria solution. The plates were then sealed with parafilm and then incubated at 37°C while shaking at 200 rpm for 1.5 hours. Next, 10 μL samples of each solution were added to separate 990 μL of fresh sterile TSB in a micro-centrifuge tube, creating a 1:100 dilution. The tubes were then vortexed and 100 μL was removed from each tube and added to 900 μL of sterile TSB in a micro-centrifuge tube, creating a 1:1000 dilution. To quantify the colony forming units, five separate 20 μL samples were plated in TSB-Agar plates, and were allowed to incubate overnight. In addition to experimental trials, a set of control growth trials were conducted where bacteria were grown under similar conditions to the experimental samples, but without the polymer disk, and were diluted and plated at 15-minute intervals to track the bacteria growth rate at room temperature. The next day the colony forming units were counted and this count was used to calculate the total colony forming units in each solution.
All experiments were completed in triplicate and repeated at three different times. Results were analyzed using ANOVA followed by Student’s t-tests.
Results
Contact angle test
No significant contact angle differences were observed between the polymer samples (Figure 1). Such results suggest that neither the nano- nor micron-BaSO4 were exposed on the surface as that would have changed surface energy and, thus, contact angles.
Figure 1.
Contact angle measurements on pellethane samples.
Note: Data = mean ± standard error of the mean.
Abbreviations: BaSO4, barium sulfate; dH2O, distilled water.
Radiopacity
These trials indicated that the nano-BaSO4 samples were radiopaque. Figure 2 shows the s-values for each of the samples. Importantly, the highest s-value was obtained for the 40% nano-BaSO4 sample which may be due to the high amount of nanoparticle surface area optimizing interactions with X-rays or the significant nanoparticle agglomeration which occurred during the extrusion process.
Figure 2.
Sample radiopacity s-values.
Note: Barium sulfate (BaSO4) 0% was used as a control, and the value of the control was subtracted from all the other experiential s-values.
AFM surface analysis
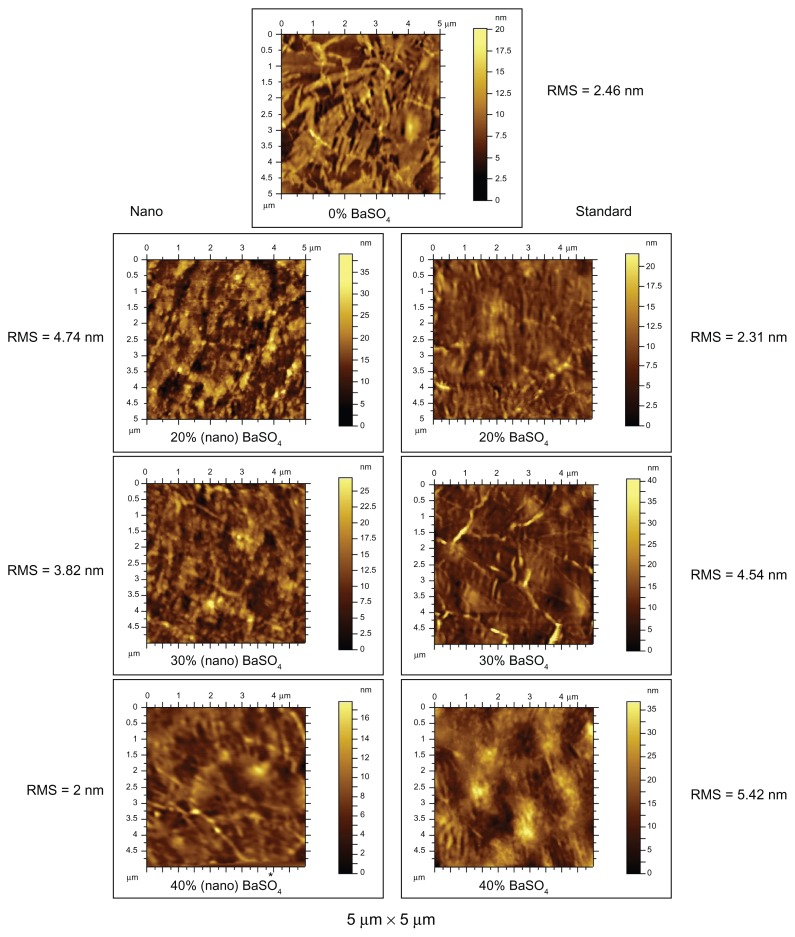
AFM results, as seen in Figure 3, showed that the samples with the nano-BaSO4 resulted in rougher surfaces with more nano-features which makes intuitive sense due to the presence of nanoparticles in the composites. However, the 40% nano-BaSO4 was extremely challenging to image due to the tip of the imaging cantilever repeatedly getting stuck on the sample. The RMS height values are a calculation of the standard deviation of the height distribution, or surface roughness. The RMS values from this study indicated that as the percentage of nanoparticles increased, the surface roughness actually decreased. This may be the result of the agglomeration of the nanoparticles into larger macroparticles as their percentage in the polymer increased. Figure 4 shows a compilation of all the numerical data collected from the AFM trials. Table 1 displays the descriptions of the height parameters as defined by ISO 25178 (adapted from the geometrical product specifications).15 According to the RMS and other quantified surface roughness parameters, the results indicated that the 40% nano-BaSO4 was the smoothest sample at the 5 μm by 5 μm AFM scan with the smallest height parameters in every category other than skewness. Although requiring further investigation, it is possible that the nanoparticles filled in portions of the surfaces to make a more smooth pellethane at this magnification. Thus, it makes intuitive sense that the 40% nano-BaSO4 sample was the smoothest at the micron scale, but all nanocomposites were more rough at the nanoscale.
Figure 3.
AFM 5 μm × 5 μm scan areas on polymer samples.
Note: *For 40% (nano) BaSO4 sample images of smaller areas was unattainable due to was repeated interaction between the sample surface and imaging tip.
Abbreviations: AFM, atomic force microscopy; BaSO4, barium sulfate; RMS, root mean square surface roughness.
Figure 4.
Compiled numerical measurements from the AFM analysis of polymer samples.
Abbreviations: AFM, atomic force microscopy; BaSO4, barium sulfate; Sq, root mean square height; Ssk, skewness; Sku, kurtosis; Sp, maximum peak height; Sv, maximum pit height; Sz, maximum height; Sa, arithmetical mean height.
Table 1.
Atomic force microscopy height parameters
| Roughness parameter abbreviation | Roughness parameter | Definition |
|---|---|---|
| Sq | Root mean square height | Standard deviation of the height distribution, or RMS surface roughness. Computes the standard deviation for the amplitudes of the surface (RMS). |
| Ssk | Skewness | Skewness of the height distribution. Third statistical moment, qualifying the symmetry of the height distribution. A negative Ssk indicates that the surface is composed with principally one plateau and deep and fine valleys. In this case, the distribution is sloping to the top. A positive Ssk indicates a surface with lots of peaks on a plane. |
| Sku | Kurtosis | Kurtosis of the height distribution. Fourth statistical moment, qualifying the flatness of the height distribution. |
| Sp | Maximum peak height | Height between the highest peak and the mean plane. |
| Sv | Maximum pit height | Depth between the mean plane and the deepest valley. |
| Sz | Maximum height | Height between the highest peak and the deepest valley. |
| Sa | Arithmetical mean height | Mean surface roughness. |
Adapted from the geometrical product specifications.16
Bacteria growth trials
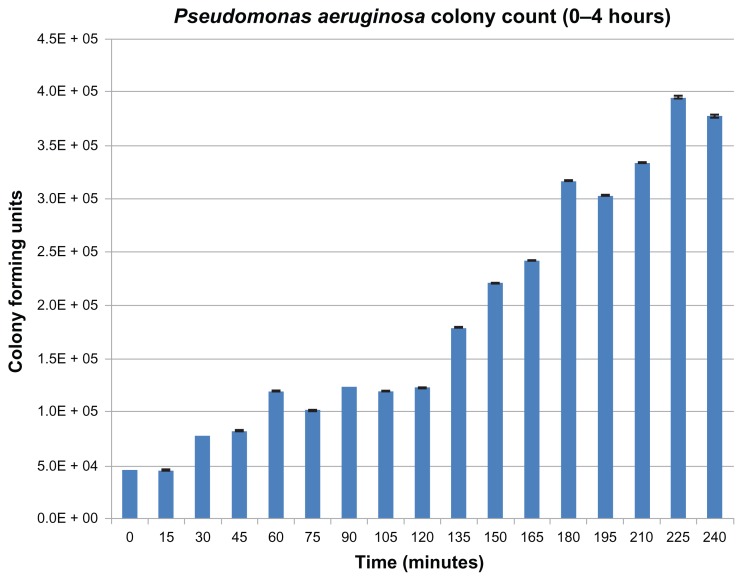
Results from the bacterial trials are presented in Figures 5–8. Figure 5 displays the growth curve of S. aureus at room temperature. The results shown in Figure 5 were used to create the red line in Figure 7. The data in Figure 4 were used to ensure that the results seen in Figure 6 were not affected by bacterial growth during the sample dilution phase of the experiment. Figures 6 and 8 are the results for P. aeruginosa. Most importantly, the bacteria results indicated a significant decrease in bacteria proliferation at certain concentrations of nano-BaSO4. In the case of S. aureus, significant decreases were observed on the 20% and 40% nano-BaSO4 polymers (Figure 7). In the case of P. aeruginosa, significant decreases were observed on 0% BaSO4 as well as the 30% and 40% nano-BaSO4 polymers (Figure 8). Additionally the 40% nano-BaSO4 led to a significant decrease in P. aeruginosa compared to the 40% (conventional or micron) BaSO4 polymer (Figure 8). Collectively, such results demonstrated that the 40% nano-BaSO4 pellethane decreased both bacteria growth the most and, thus, should be further studied for numerous medical device applications.
Figure 5.
Growth of Staphylococcus aureus at room temperature.
Note: Data = mean ± standard error of the mean.
Figure 8.
Pseudomonas aeruginosa colony count after 1.5 hours of contact with pellethane polymers.
Notes: Data = mean ± standard error of the mean. *A significant decrease when the marked sample is compared to the empty well control samples containing no polymer sample, as determined by P < 0.05; **a significant decrease when the marked sample is compared to the to the samples of 40% BaSO4, as determined by P < 0.05.
Abbreviation: BaSO4, barium sulfate.
Figure 7.
Staphylococcus aureus colony count after 1.5 hours of contact with pellethane polymers.
Notes: Data = mean ± standard error of the mean. *A significant decrease when the marked sample is compared to the samples of 0% BaSO4 as well as the empty well control samples containing no polymer sample, as determined by P < 0.05.
Abbreviation: BaSO4, barium sulfate.
Figure 6.
Growth of Pseudomonas aeruginosa at room temperature.
Note: Data = mean ± standard error of the mean.
Discussion
The results of these trials indicate, for the first time, that adding nano-BaSO4 to the extrusion process of pellethane was able to change the surface properties of the composites to reduce bacteria interactions. From the results, it appears that the 20% nano-BaSO4 and the 40% nano-BaSO4 blends yielded a marked reduction in S. aureus proliferation, while the 30% and 40% nano-BaSO4 blends showed marked decreases in P. aeruginosa proliferation. This indicates that compared to the collected AFM data, it is clear that the relative roughness of the 40% nano-BaSO4 polymer blend may have played a role in its ability to hinder bacterial proliferation. For P. aeruginosa, the bacteria proliferation results appeared to be more directly correlated to the surface roughness (RMS). Of course, longer antimicrobial trials should be conducted to investigate the potency and longevity of the antimicrobial effects seen here for the first time. This study also demonstrated the greatest radiopacity for the 40% nano-BaSO4 weight percentage which was the largest weight percentage of nanoparticles added (and which, as mentioned above, was the formulation that decreased the functions of both bacteria). It stands to reason that this composite would have the largest interaction between X-rays and nanoparticles of BaSO4 to lead to the greatest radiopacity; however, there has been no documented relationship between radiopacity and antibacterial properties; thus, future studies will have to elucidate what properties of this composite led to both the greatest radiopacity and antibacterial properties. Lastly, since it has been observed that nanoscale surface features can reduce the functions of other bacteria, we can hypothesize that the functions of other bacteria will be reduced on these same composites; however, clearly, such studies need to be conducted to confirm this.16
Of course, a paramount question for this study is why bacteria functions decreased on these nanocomposites compared to currently used micron-BaSO4 composites. While this question requires significantly more investigation, this study did provide some insights. Specifically, no change in contact angle was observed for any of the substrates made here. This suggests that all of the materials (even the composites) had a surface layer of pellethane without any exposed BaSO4, thus eliminating changes in surface chemistry and consequent surface energy as reasons why bacteria functions were reduced. However, this study did demonstrate interesting trends between surface roughness of the nanocomposites and bacteria responses; it is noteworthy that for the 0% BaSO4 and 40% BaSO4, P. aeruginosa density was similar as well as surface roughness. The composite that decreased the activity of both bacteria of interest to the present study was the 40% BaSO4, which had an RMS value of 2 nm on AFM 5 μm by 5 μm scans. Thus, we believe there is an optimal nanoscale surface roughness that needs to be determined as it relates to decreasing bacteria functions (Figure 9). Specifically, it has been previously proposed that certain nanoscale roughness values decreased bacteria adhesion due to their stiff membranes being unable to adjust to such surface features.15 Thus, it is clear that the nanocomposites formulated here did possess altered roughness at both the nanoscale and micron scale which needs to be further addressed in future studies to identify the exact roughness that inhibits bacteria adhesion and growth.
Figure 9.

Illustration comparing bacteria surface interactions with nanorough composites and conventional or nanosmooth composites.
Notes: Due to the high degree of roughness at the nanoscale on nanomaterial composites, rigid bacteria cell membranes cannot lay flush against the material surface. This may inhibit the preliminary steps leading to bacterial adhesion. As a result, bacterial activity on a nanocomposite surface is reduced. The exact nanoscale surface roughness that inhibits bacteria activity remains to be tested, but results from this study suggests that the 40% BaSO4 composite with an RMS roughness of 2 nm (when measured on 5 μm × 5 μm AFM scans) was the best at inhibiting both Pseudomonas aeruginosa and Staphylococcus aureus activity. Copyright © 2012, Dove Medical Press. Reproduced with permission from Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767–2781.16
Abbreviations: AFM, atomic force microscopy; BaSO4, barium sulfate; RMS, root mean square surface roughness.
It is also clear that composite processing parameters (such as extrusion viscosity, extrusion rate, nanoparticle dimension, weight percentage, nanoparticle morphology, nanoparticle dispersion, phase separation of the polymer and BaSO4, etc) need to be systematically changed and related to bacterial function. For example, increasing composite viscosity during the extrusion process would decrease the ability to disperse the nanoparticles which may create AFM RMS values higher than 2 nm (when measured on 5 μm by 5 μm AFM scans) leading to an increase in bacteria functions. Similarly, increasing the phase separation between the nanoparticles and polymers (created using high extrusion rates) may also lead to an increase in composite micron roughness leading to an increase in bacteria functions. All of these parameters need to be systematically studied in the future.
Conclusion
These trials indicated that, although the nano-BaSO4 did not change the hydrodynamic nature of the samples, there was a significant change in roughness when nano-BaSO4 was added to the polymer. Significantly, adding 40% BaSO4 to pellethane reduced bacteria proliferation. Further trials need to be completed to better correlate the surface properties of the composites to decreased bacteria functions and to extend such results into medical products.
Acknowledgments
Special thanks to Adriana Noemí Santiago and Gozde Durmus for their help in sample preparation and experimental procedure development. Additionally, the authors would like to thank Becky Sustak, Lindsay DuPont, and Advanced Radiology, RI, for help with the X-ray equipment. The authors would also like to thank Dattatri Nagesha for help with the AFM studies.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 3.Sheng WH, Wang JT, Lu DCT, Chie WC, Chen YC, Chang SC. Comparative impact of hospital-acquired infections on medical costs, length of hospital stay and outcome between community hospitals and medical centres. J Hosp Infect. 2005;59(3):205–214. doi: 10.1016/j.jhin.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 5.Simple intervention nearly eliminates catheter-related bloodstream infections [webpage on the Internet] Baltimore: Johns Hopkins Medicine Office of Corporate Communications;; 2004. [Accessed December 15, 2012]. Available from: http://www.hopkinsmedicine.org/Press_releases/2004/11_30_04.html. [Google Scholar]
- 6.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 7.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 8.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46(6):S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WW, Evans MJ, Slack MPE, Walmsley HL. The penetration of antibiotics into aggregates of mucoid and non-mucoid pseudomonas-aeruginosa. J Gen Microbiol. 1989;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- 10.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59(4):587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 11.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomedicine. 2011;6:1463–1473. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puckett SD, Lee PP, Ciombor DM, Aaron RK, Webster TJ. Nanotextured titanium surfaces for enhancing skin growth on transcutaneous osseointegrated devices. Acta Biomater. 2010;6(6):2352–2362. doi: 10.1016/j.actbio.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.S-Values – Digital radiology [webpage on the Internet] Palatine: American Chiropractic Registry of Radiologic Technologists; 2012. [Accessed December 15, 2012]. Available from: http://www.acrrt.com/index.php/11-articles/29-s-values-digital-radiology. [Google Scholar]
- 14.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 15.International Organization for Standardization. Geometrical product specifications (GPS)– Surface texture: Areal – Part 2: Terms, definitions and surface texture parameters. ISO 25178-25172:2012. [Google Scholar]
- 16.Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]