Abstract
Background:
Post-operative course after complex pediatric cardiac surgery is unpredictable. Although, change in arterial lactate levels has been used as a surrogate marker for many years, scientific evidence correlating the early perioperative lactate levels with outcome is still lacking.
Objective:
To evaluate the trends in lactate levels from intraoperative period to an extended post-operative period in pediatric intensive care unit (PICU) and to assess its usefulness as a prognostic marker.
Design:
Prospective observational study.
Setting:
Tertiary pediatric cardiac surgical unit.
Patients:
Thirty-five non-consecutive children aged 1-140 months who underwent surgery for congenital heart diseases (CHD) on cardiopulmonary bypass (CPB).
Intervention:
None.
Materials and Methods:
Arterial blood lactate levels were obtained at the following time points: After induction of anesthesia, 15 and 45 min after institution of CPB, at the start of rewarming, after sternotomy closure, then at 1, 6, 24, and 48 h in PICU. Other hemodynamic and clinical variables, CPB variables, blood gas values, and laboratory variables were also recorded.
Results:
Four patients died out of 35 patients (11.4%). Non-survivors showed significant persistent elevation in lactates (>4.0 mmol/l). Peak lactates correlate significantly with longer aortic cross clamp time, CPB duration, ventilation hours and PICU stay.
Conclusion:
Early point of care lactate can be a useful prognostic marker in post-cardiac surgery patients in adjunct with other parameters measured in PICU. This reiterates the importance of measuring lactates and timely recognition of at-risk patients, which on early intervention can help in reducing post-operative morbidity and mortality.
Keywords: Children, pediatric cardiac surgery, plasma lactate
Introduction
Predicting post-operative course after corrective surgery for complex congenital heart disease (CHD) is always challenging for surgeon and intensivists. If patients having potential to deteriorate can be identified early, overall patients’ outcome can be improved by timely intervention before the pathophysiological process becomes irreversible. For the hemodynamic assessment of post-cardiac surgery patients, various parameters have been studied including heart rate (HR), blood pressure (BP), urine output, peripheral perfusion, serum lactate, base excess, and central venous oxygen saturation but none of them was found to be an absolute predictor of adverse outcome.[1–2]
Children undergoing cardiac surgery may have diminished cardiac output despite stable hemodynamic indicators such as BP and HR.[3] Resultant systemic hypo-perfusion and tissue hypoxia lead to commencement of anaerobic respiration, reflected by elevated lactate levels with metabolic acidosis. Hence, plasma lactate levels are conventionally used as markers to assess the adequacy of tissue perfusion.
Association of elevated plasma lactates in children following cardiac repairs with adverse outcome is demonstrated by many authors but most of these studied the role of lactate levels on admission in to pediatric intensive care unit (PICU) or during cardiopulmonary bypass (CPB).[3–10] Studies demonstrating the role of lactate levels in the perioperative period are lacking, so we decided to look at the benefit of following trends from intraoperative period to an extended period in PICU.
The present study investigates the hypothesis that trends of lactate levels in the early perioperative period may be a useful marker to prognosticate the final outcome in pediatric cardiac surgery patients.
Materials and Methods
This prospective study was conducted in a tertiary pediatric cardiac surgical unit over a period of 6 months after obtaining clearance from the institutional ethical committee. Informed consent had been waived as this was an observational study utilizing variables that were obtained as a part of the standard clinical protocol of our institution.
Thirty-five non-consecutive patients who underwent cardiac surgery under CPB were enrolled in the study. Patients were selected without randomization or control of surgical procedure, anesthesia techniques, or CPB management. Patients aged more than 15 years, who required pre-operative ventilator or inotropic support, patients having acute or chronic liver failure, known case of inborn errors of metabolism or patients who died within 48 h of PICU stay were excluded from the study.
All the patients were premedicated with intramuscular morphine (0.2 mg/kg) and promethazine (0.5 mg/kg) about 30 to 45 min prior to induction of anesthesia. Anesthesia was induced with inhaled sevoflurane (2-8%) and rocuronium (0.1 mg/kg) was used as a muscle relaxant to accomplish endotracheal intubation. Anesthesia was maintained with inhaled sevoflurane (2-8%) with intravenous fentanyl (2-5 mcg/kg) and midazolam (0.05-0.1 mg/kg). All patients were mechanically ventilated and monitored as per the standard protocol, which included radial or femoral artery catheter for arterial BP monitoring and double or triple-lumen central venous catheter (femoral or right internal jugular) for intermittent blood sampling and medications, and esophageal and rectal temperature probes. Morphine (0.05 mg/kg) was given before incision. In all patients, methylprednisolone (30 mg/kg) was administered. Intravenous heparin (3 mg/kg) was used as anticoagulant before cardiac cannulation and a target-activated clotting time of more than 450 seconds was achieved and maintained. Appropriate age-specific aortic and venous cannulation was done to go on CPB. Bypass circuit was primed with a mixture of Ringer's lactate and one unit of blood. Additional plasma and blood was added to prime as and when required so as to maintain a circulating hematocrit of 25-30%. Standard bypass techniques with systemic hypothermia of 28-32°C were employed. Cardiac arrest was induced and maintained by using ante-grade intermittent cardioplegia. After weaning from CPB and removal of the cannula, heparin effect was reversed by using protamine sulphate at a 1:1 ratio.
About 1 ml of arterial blood was collected in heparinized syringe obtained through intra-arterial catheter inserted for arterial BP monitoring. Lactate levels and base excess were measured using point-of-care blood gas analyzer, ABL 800 FLEX (Radiometer America Inc.) which underwent daily calibration and quality control checks. Here, point-of-care testing can be defined as rapid availability of laboratory results at bedside to decrease the time to start intervention and to increase the therapeutic decision making.[11] Other parameters like blood gases, hematocrit, electrolytes, ionic calcium and glucose levels were also measured simultaneously. First blood sample was collected immediately after induction of anesthesia and subsequently at 15 min and 45 min after institution of CPB, at start of rewarming, after sternotomy closure and then 1, 6, 24, and 48 h after reaching PICU.
General patient data including age, sex, weight, diagnosis, and surgery performed were noted. Various hemodynamic and clinical parameters including HR, BP, oxygen saturation, and urine output were recorded at the same time points. Following perioperative and outcome variables were also analyzed and recorded: CPB duration, aortic cross clamp time, duration of mechanical ventilation, and vasopressor support, duration of PICU stay, complications, mortality and other laboratory variables. All data were analyzed using SPSS (version 16.0) software. Data are presented as median (range); Mean ± Standard deviation as appropriate. Paired variables were analyzed by Students’ or paired t-test while more than two groups were analyzed using repeated measures analysis of variance (ANOVA) test with appropriate post-tests whenever, required. P values < 0.05 were considered significant.
Results
A total of 35 patients (20 males and 15 females) were included in study, with a median age of 11 months (1 to 140 months), and median weight of 7.8 kg (range 2.6 to 25 kg). Among the 35 patients enrolled, four patients died during the hospital stay. Most of the patients included in study had ventricular septal defect (n = 9) followed by transposition of great arteries (n = 8), tetralogy of Fallots (n = 6), atrial septal defect (n = 3), two cases each of total anomalous pulmonary venous connection, tricuspid atresia and Ebstein's anomaly, and one each of double outlet right ventricle, sub-aortic membrane, and atrio-ventricular canal defect. Two patients died following arterial switch operation, whereas one each following repair of tricuspid atresia and Ebstein anomaly.
Mean lactate levels among survivors and non-survivors at different time points are shown in Table 1 and trends are depicted in Figure 1. Non-survivors exhibited a significantly higher mean lactate levels at all-time points than did survivors. A significant increase in mean lactate level was noted from baseline versus cooling (P < 0.0001), and cooling versus on-CPB (P = 0.0006). However, increase in lactates from CPB versus rewarming (P = 0.4744), rewarming versus the post-CPB period (P = 0.3606) was not significant. Similarly, a gradual but non-significant decline in lactate levels between the immediate post-CPB period versus PICU value (P = 0.3908) and between different lactate values in PICU was seen [Table 1].
Table 1.
Mean lactate levels among survivors and non-survivors
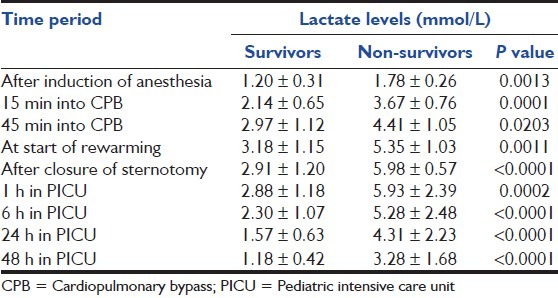
Figure 1.
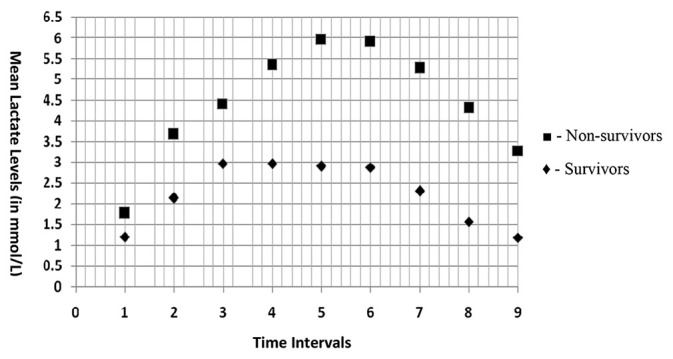
Trends of serum lactate levels in survivors and non-survivors at different time intervals (Time intervals: 1 -After induction of anaesthesia, 2 -15 min into CPB, 3 -45 min into CPB, 4 -At start of rewarming, 5 -After closure of sternotomy, 6 -1 hour in PICU, 7 -6 hours in PICU, 8 -24 hours in PICU, 9 -48 hours in PICU)
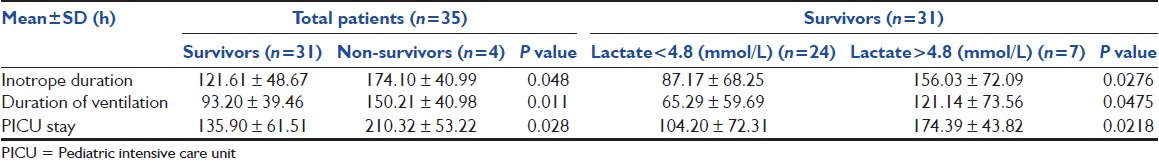
Among the survivors, 7 patients had peak lactate level more than 4.8 mmol/L (i.e., three times of the normal values), whereas in 24 patients, peak lactate level remained below 4.8 mmol/L. Mean durations of vasopressor therapy, mechanical ventilation and PICU stay were significantly higher in non-survivors than that of survivors. Similarly, these durations were significantly higher in patients with higher mean lactate levels (>4.8 mmol/L) than in patients who had mean lactate levels < 4.8 mmol/L [Table 2].
Table 2.
Duration of vasopressor therapy, mechanical ventilation and intensive care unit stay of the survivors and non-survivors
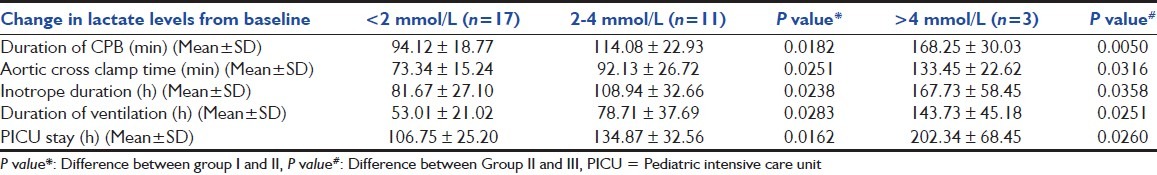
For the purpose of analysis, patients were further divided into three groups on the basis of change in lactate levels from baseline (i.e., peak - baseline lactate levels): Group I - <2 mmol/L, Group II - 2-4 mmol/L, Group III - >4 mmol/L. These groups included 17, 11, and 3 patients respectively. On comparison between these groups, a significant increase was seen from Group I to Group II, and from Group II to Group III in duration of CPB (P value - 0.0182, 0.0050), aortic cross clamp time (P value - 0.0251, 0.0316), vasopressor therapy (P value - 0.0238, 0.0358), mechanical ventilation (P value - 0.0283, 0.0251), and PICU stay (P value - 0.0162, 0.0260) [Table 3].
Table 3.
Relationship between changes in lactate levels and duration of cardiopulmonary bypass, vasopressor therapy, mechanical ventilation, and pediatric intensive care unit stays
No significant relationship was seen between change in lactate level and mean arterial BP, nadir temperature, lowest hematocrit value, blood gas parameters, and urine output during CPB and PICU stay.
Discussion
Hyperlactatemia is a complex condition characterized by increased blood lactate levels, which may result from several mechanisms. Normal blood lactate level is 1.0 ± 0.5 mmol/L in unstressed patients under normal conditions. Hyperlactatemia can be classified as mild (lactate level up to 2 mmol/L), moderate (lactate level 2-5 mmol/L, without metabolic acidosis) and severe (persistently increased lactate levels usually > 5 mmol/L, with metabolic acidosis).[11] Lactic acidosis has been classified into two categories: Type A (lactic acidosis along with clinical evidence of poor tissue perfusion) and Type B (lactic acidosis with no evidence of poor tissue perfusion).[12,13] Several studies have shown a strong positive correlation between elevated lactate levels and risk of morbidity and mortality in children undergoing surgery for CHDs. In contrast to previous reports demonstrating an association of hyperlactatemia on admission to PICU or during CPB[3–10] with morbidity and mortality, we demonstrated benefit of following the trend of lactate levels in extended perioperative period. In this study, significantly higher mean lactate levels were seen among non-survivors in comparison to survivors which started during CPB period and continued till post-operative period in PICU.
In our study, peak lactate levels were seen during rewarming phase in both survivors and non-survivors, which can be explained by the fact that due to increased oxygen demands, rewarming phase is at a higher risk for poor tissue perfusion. Munoz et al.[10] also reported that lactate levels peaked during rewarming phase, and increased lactate during CPB was associated with increased morbidity and mortality in post-cardiac surgery children. Toda et al.[14] studied the relation of lactate levels with prime solution and demonstrated that the poor physiological or prognostic implications of lactate levels in children who have a bloodless prime in comparison to blood prime. However, in our study, we used blood prime as a protocol in all the patients thus eliminating the confounding effect of prime solution.
In this study, higher lactates among non-survivors correlated significantly with adverse post-operative course as evidenced by increased duration of vasopressor support, duration of mechanical ventilation and PICU stay. Similarly, among survivors, seven patients with complicated course (i.e., increased duration of vasopressor support, mechanical ventilation and PICU stay) had higher mean lactates than 24 patients with uncomplicated course.
As no absolute lactate level or range predicting patient outcome has been defined, change in lactate over time is likely to be a better prognostic marker. Positive predictive value (PPV) of single lactate readings for mortality on PICU admission may not be high, as Hatherill et al.[4] reported that admission lactate value > 6 mmol/L had a low PPV for mortality in children undergoing cardiac surgery. However, Cheung et al.[9] demonstrated that accuracy of serum lactates to predict early survival was more than 80% and PPV of admission lactate < 7 mmol/L or peak lactate level at day one < 8 mmol/L for survival was 97% in comparison to only 43% PPV for non-survival when lactate levels exceeds these values. In addition, serial lactate levels identified survivors with neuro-developmental delay. Similarly, in a study by Siegel et al.[5] elevated lactates in post-cardiac surgery children had significant association with post-operative mortality and admission lactate levels > 4.2 mmol/L had almost 100% PPV.
In our study, change in lactates from baseline among survivors showed significant association with CPB duration and post-operative course including duration of vasopressor support, mechanical ventilation and PICU stay, as all these parameters increased significantly with increase in change in lactates.
Hatherill et al.[4] also demonstrated that children with complicated post-operative course had higher median serum lactates than in patients with an uncomplicated course. Duke[3] and Shemie et al.[7] have also been reported similar association between elevated lactate levels and complicated post-operative course. Charpie et al.[15] demonstrated the role of serial lactate levels as a predictor of mortality within 72 h of surgery or need for extracorporeal membrane oxygenation support in infants undergoing complex cardiac surgeries.
Few study limitations which should be mentioned are that the study would be more reproducible if data from similar kind of surgeries would be analyzed as inclusion of higher risk patients increases the chances of correlation of lactate levels with adverse outcome. In our study too, non-survivors had higher baseline lactates with a larger change during CPB than did survivors, which may reflect the diagnosis, pre-operative condition, and complexity of surgery with resultant longer CPB and circulatory arrest duration. Secondly, as a protocol, our patient received methylprednisolone on CPB, which may elevate lactate levels, as reported by Mayumi et al.[16] in their study. Similarly, high vasopressor support, especially adrenaline, has been associated with elevated lactate levels. However, as both the survivors and non-survivors have received methylprednisolone and vasopressors, these would have affected both the groups and did not appear to be significant confounding factors in this study. Nonetheless, this fact should also be taken into account in further studies. Lastly, because of small number of cases, the power of the study is not adequate.
Conclusion
Our study demonstrated that higher perioperative lactate levels in children undergoing cardiac repairs is an early indicator of adverse post-operative outcome and point-of-care lactate measurements helps in timely recognition of patients at increased risk, which may allow an early intervention so as to reduce post-operative morbidity and mortality. However, further randomized prospective studies and multivariate regression analysis are necessary giving due consideration to diagnosis, surgical complexity, and duration of CPB and circulatory arrest to determine whether changes in CPB management or other modifying factors will alter the final patient outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: Heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923–9. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bernardin G, Pradier C, Tiger F, Deloffre P, Mattei M. Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med. 1996;22:17–25. doi: 10.1007/BF01728326. [DOI] [PubMed] [Google Scholar]
- 3.Duke T, Butt W, South M, Karl TR. Early markers of major adverse events in children after cardiac operations. J Thorac Cardiovasc Surg. 1997;114:1042–52. doi: 10.1016/S0022-5223(97)70018-7. [DOI] [PubMed] [Google Scholar]
- 4.Hatherill M, Sajjanhar T, Tibby SM, Champion MP, Anderson D, Marsh MJ, et al. Serum lactate as a predictor of mortality after paediatric cardiac surgery. Arch Dis Child. 1997;77:235–8. doi: 10.1136/adc.77.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel LB, Hauser J, Hertzog JH, Hopkins RA, Hannah RL, Dalton HJ. Initial post-operative serum lactate predicts outcome in children after open heart surgery. Crit Care Med. 1995;23:A205. doi: 10.1007/BF01709563. [DOI] [PubMed] [Google Scholar]
- 6.Cheifetz IM, Kern FH, Schulman SR, Greeley WJ, Ungerleider RM, Meliones JN. Serum lactates correlate with mortality after operations for complex congenital heart disease. Ann Thorac Surg. 1997;64:735–8. doi: 10.1016/s0003-4975(97)00527-4. [DOI] [PubMed] [Google Scholar]
- 7.Shemie SD. Serum lactate predicts postoperative complications after pediatric cardiac surgery. Pediatr Res. 1996;39:54A. [Google Scholar]
- 8.Ranucci M, Isgrò G, Carlucci C, De La Torre T, Enginoli S, Frigiola A, et al. Central venous oxygen saturation and blood lactate levels during cardiopulmonary bypass are associated with outcome after pediatric cardiac surgery. Crit Care. 2010;14:R149. doi: 10.1186/cc9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung PY, Chui N, Joffe AR, Rebeyka IM, Robertson CM Western Canadian Complex Pediatric Therapies Project, Follow-up Group. Postoperative lactate concentrations predict the outcome of infants aged 6 weeks or less after intracardiac surgery: A cohort follow-up to 18 months. J Thorac Cardiovasc Surg. 2005;130:837–43. doi: 10.1016/j.jtcvs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Munoz R, Laussen PC, Palacio G, Zienko L, Piercey G, Wessel DL. Changes in whole blood lactate levels during cardiopulmonary bypass for surgery for congenital cardiac disease: An early indicator of morbidity and mortality. J Thorac Cardiovasc Surg. 2000;119:155–62. doi: 10.1016/s0022-5223(00)70231-5. [DOI] [PubMed] [Google Scholar]
- 11.Hannan RL, Ybarra MA, White JA, Ojito JW, Rossi AF, Burke RP. Patterns of lactate values after congenital heart surgery and timing of cardiopulmonary support. Ann Thorac Surg. 2005;80:1468–73. doi: 10.1016/j.athoracsur.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 12.Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20:80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Kruse JA, Carlson RW. Lactate metabolism. Crit Care Med. 1987;3:725–46. [PubMed] [Google Scholar]
- 14.Toda Y, Duke T, Shekerdemian LS. Influences on lactate levels in children early after cardiac surgery: Prime solution and age. Crit Care Resusc. 2005;7:87–91. [PubMed] [Google Scholar]
- 15.Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg. 2000;120:73–80. doi: 10.1067/mtc.2000.106838. [DOI] [PubMed] [Google Scholar]
- 16.Mayumi H, Zhang QW, Nakashima A, Masuda M, Kohno H, Kawachi Y, et al. Synergistic immunosuppression caused by high-dose methylprednisolone and cardiopulmonary bypass. Ann Thorac Surg. 1997;63:129–37. doi: 10.1016/s0003-4975(96)00682-0. [DOI] [PubMed] [Google Scholar]


