Abstract
Acute methotrexate toxicity rarely presents as medical emergency in form of multiorgan failure. Acute pneumonitis following low-dose methotrexate is rarely reported in literature. It is important to recognize this, as the drug must be discontinued immediately and rescue measures in form of folinic acid and hydration instituted promptly.
Keywords: Folinic acid, methotrexate, pneumonitis
Introduction
Methotrexate (MTX) is a dihydrofolate reductase (DHFR) inhibitor. High dose (HD) MTX (0.5 - 12 g/m2) has antiproliferative activity and is used in treatment of cancer while low dose (LD) MTX (10 - 40 mg/ m2) has anti-inflammatory and immunosuppressive properties.[1] Its long-term use is associated with hepatic and pulmonary toxicity.[2] Reports of acute MTX toxicity presenting as multiorgan failure and acute pneumonitis are rare (MTX-P).
Case Report
A 48-year-old, male with a 2-year history of plaque psoriasis affecting limbs and trunk was advised 5 mg of oral MTX thrice weekly. However, accidentally he ingested three tablets daily (5 mg each) for 4 days i.e., total dose of 60 mg. A week later, he presented to the emergency department with nausea, vomiting and painful skin erosions over the trunk and intertriginous areas. He developed yellowish discoloration of urine along with low urine output. Three days back he developed fever with cough and respiratory distress.
On examination was icteric, tachycardic (HR = 110/ min), arterial blood pressure BP = 136/54 mmHg, and tachypnoeic (RR = 38/min). He was conscious and oriented. At admission, laboratory investigations revealed pancytopenia, other investigation on day 1 are listed in Table 1. Bilateral diffuse infiltrates seen on chest X-ray [Figure 1], were believed to be due to pneumonia as his echocardiography and lower limb Doppler were all within normal limits.
Table 1.
Clinical and laboratory parameters
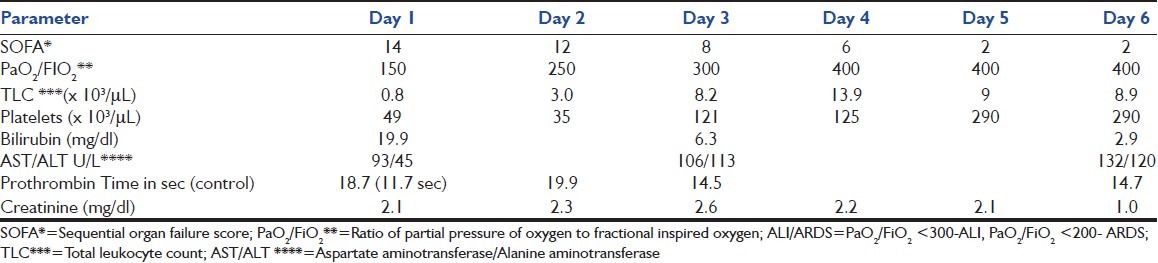
Figure 1.
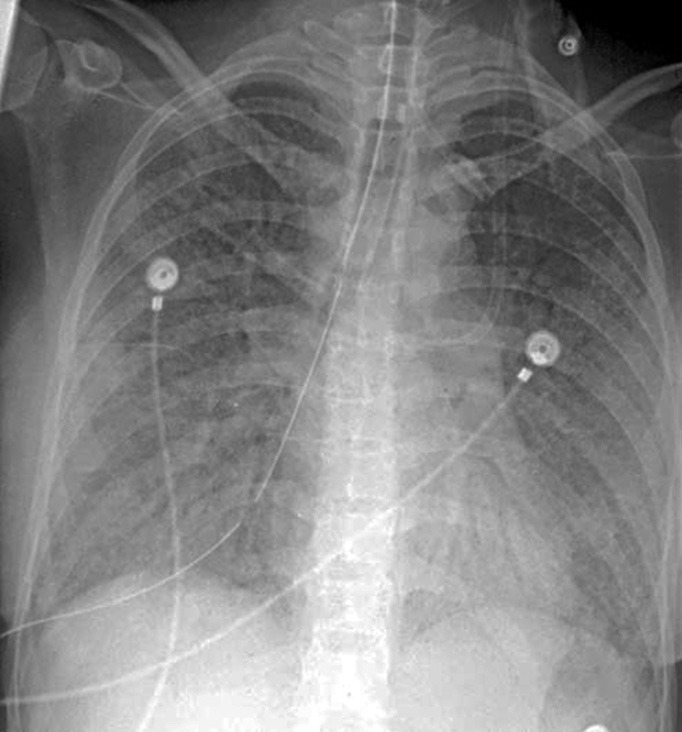
Day 1 chest X-ray suggestive of interstitial edema
Arterial blood gas analysis revealed hypoxemia with primary respiratory alkalosis. Diagnosis of acute MTX toxicity with multiorgan dysfunction was made. MTX was immediately discontinued. Intravenous folinic acid at dose of 15 mg was given 6 hourly (1 mg/kg). Subcutaneous granulocyte colony-stimulating factor (G-CSF) 350 μg was also given. In view of respiratory distress, patient was intubated and initiated on mechanical ventilation. As his urinary pH was 5.9, sodium bicarbonate 20 mEq/hr was added along with 1 l bolus followed by 100 ml/hr of normal saline to aid in alkalinization of urine (target urinary pH = 7.0).
After collecting cultures, patient was started on broad spectrum empiric antibiotics (Meropenem and Teicoplanin) and antifungals (Fluconazole) in view of neutropenia. Within 24 hours the patient's urine output improved and after 48 hours his platelet and leukocyte count also started increasing. Within 72 hours, he was extubated and was transferred to ward in another 2 days. As there was no evidence of infection (cultures sterile and normal procalcitonin) antibiotics were stopped after 5 days. Serial clinical and laboratory parameters are shown in [Table 1].
Discussion
Acute pneumonitis with LD- MTX (10 - 40 mg/m2) given over short span of time (4 days) has been rarely reported.[3,4] Patients with pre-existing lung disease, rheumatoid arthritis, old age and on previous use of disease-modifying anti-inflammatory rheumatic drugs (DMARDS) are at heightened risk of developing MTX pneumonia.[5]
Methotrexate pneumonitis occurs most frequently within the first year of treatment and its reported incidence varies from 0.86 to 6.9%.[6] We reviewed literature regarding development of acute pneumonitis within 1 week of LD-MTX exposure. Only three case reports, one by Ridley et al. about a middle-aged woman who developed acute pneumonitis 1 week after intake of 12.5 mg of MTX.[3] Similarly two cases of acute pneumonitis were reported in patients with ectopic pregnancy given MTX.[4]
In first case MTX pneumonitis was linked with rheumatoid arthritis, while other two cases were due to idiosyncratic reaction. MTX pneumonitis is of two types.
Type 1 occurs with low MTX exposure (<300 mg), with early (<6 months) onset, dominated by neutrophils, has lung fibrosis, and carries high mortality.
Type 2 occurs with high MTX exposure (>600 mg) with late (>6 months) onset, dominated by lymphocytes, has less lung fibrosis and carries low mortality.[7]
The mechanism of MTX-P is cell-mediated hypersensitivity reaction mediated by activated T-cells.[8] MTX stimulates type II alveolar cells to release cytokines which lead to inflammatory cells recruitment leading to alveolitis. Acute pulmonary toxicity however appears to be unrelated to either the dose or the duration of treatment. One important variable appears to be the frequency of drug administration; since patients receiving daily or even weekly doses have a higher risk of toxicity than those receiving the drug less frequently.[9] Although exact mechanism is still debatable. In our case it could be Type 1 Mtx pneumonitis (low dose and early).
Our patient had (1) acute onset of dyspnea, (2) fever >38°C, (3) tachypnoea >35/min, and dry cough, (4) radiological evidence of pulmonary interstitial infiltrates (5) WBC <1,000/cumm without eosinophilia, (6) Negative blood and sputum cultures, (7) PO2<60 mmHg on room air, which fulfilled seven of nine criteria for diagnosis of pneumonitis as described by Searles and McKendry.[10] Similarly according to 2008 diagnostic criteria for MTX pneumonitis, our patient fulfilled five out of eight criteria.[8] According to both these criteria our patient had definite MTX pneumonitis. High-resolution computerized tomography (HRCT) was contemplated but deferred since his clinical condition improved rapidly. Bronchoscopic alveolar lavage was not done initially due to thrombocytopenia.
MTX toxicity has its impact on skin, gastrointestinal mucosa, liver, kidneys, and bone marrow. Sites with rapid cell turnover like skin and gastrointestinal mucosa are the earliest to get involved. Ninety-eight percent of the oral MTX is excreted via the renal route. At low levels tubular reabsorption occurs, but at higher levels there is active tubular secretion. However, at extremely high tubular concentrations there is impairment of active tubular secretion which increases likelihood of toxicity. Nephrotoxicity is important in the pathogenesis of systemic toxicity. HD-MTX exposure patients with elevated baseline creatinine of ≥ 50%, have 55% incidence of myelosupression as against only 3% with normal renal function. Myelosupression occurs on day 8 - 10 and there is spontaneous recovery at three weeks.[11]
Immediate discontinuation of MTX is of utmost importance. Supportive measures include leucovorin (folinic acid), alkalinization of urine, intravenous hydration, G-CSF and blood products.[2] Other options include hemoperfusion and hemodialysis. Although corticosteroids are frequently administered, there are no large trials suggesting their efficacy as yet.[4] As steroids may increase the risk of superinfections we avoided it due to coexisting neutropenia in our case.
Our case had accidentally ingested large amount (60 mg) of LD-MTX, and developed immediate stomatitis, nausea and vomiting. After 96 hours, he developed cough with respiratory distress (acute pneumonitis), decreased urine output (AKI), and yellowish discoloration of urine, altered LFT (hepatitis). As his GFR diminished, there was accumulation of MTX leading to myelosupression and pancytopenia. This was noted after one week of ingestion. Thus the patient presented to us with multiorgan failure.
Conclusions
MTX-P though, related mostly to chronic LD-MTX but as in our case can also occur acutely if large doses of LD-MTX are ingested. It is a transient hypersensitivity reaction with good prognosis, if aggressive management is instituted promptly. This case report highlights the importance of early follow-up and regular laboratory investigations of patients on methotrexate therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Haematol. 2009;46:489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan KW, Tay YK. A case of acute methotrexate toxicity. Ann Acad Med Singapore. 2011;40:97–9. [PubMed] [Google Scholar]
- 3.Ridley MG, Wolfe CS, Mathews JA. Life threatening acute pneumonitis during low dose methotrexate treatment for rheumatoid arthritis: A case report and review of the literature. Ann Rheum Dis. 1988;47:784–8. doi: 10.1136/ard.47.9.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horrigan TJ, Fanning J, Marcotte MP. Methotrexate pneumonitis after systemic treatment of ectopic pregnancy. Am J Obstet Gynecol. 1997;176:714–5. doi: 10.1016/s0002-9378(97)70578-7. [DOI] [PubMed] [Google Scholar]
- 5.Saravanan V, Kelly CA. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology (Oxford) 2004;43:143–7. doi: 10.1093/rheumatology/keg466. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM, Alarcon GS, Weinblatt ME, Kaymakcian MV, Macaluso M, Cannon GW, et al. Clinical, laboratory, radiographic, and histopathologic features of methotrexate associated lung injury in patients with rheumatoid arthritis: A multicenter study with literature review. Arthritis Rheum. 1997;40:1829–37. doi: 10.1002/art.1780401016. [DOI] [PubMed] [Google Scholar]
- 7.Chikura B, Sathi N, Lane S, Dawson JK. Variation of immunological response in methotrexate-induced pneumonitis. Rheumatology (Oxford) 2008;47:1647–50. doi: 10.1093/rheumatology/ken356. [DOI] [PubMed] [Google Scholar]
- 8.Chikura B, Sathi N, Dawson JK. Methotrexate induced pneumonitis: A review article. Curr Respir Med Rev. 2009;5:12–20. [Google Scholar]
- 9.Nesbit M, Krivit W, Heyn R, Sharp H. Acute and chronic effects of methotrexate on hepatic, pulmonary and skeletal systems. Cancer. 1979;37:1048–54. doi: 10.1002/1097-0142(197602)37:2+<1048::aid-cncr2820370811>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: Potential risk factors. Four case reports and a review of the literature. J Rheumatol. 1987;14:1164–71. [PubMed] [Google Scholar]
- 11.Canestra J. Methotrexate poisoning. Emerg Med. 1996;8:89–92. [Google Scholar]


