Abstract
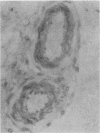
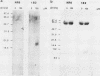
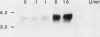
Analysis of the transforming growth factor alpha (TGF alpha) cDNA predicts that the mature TGF alpha polypeptide is cleaved from the extracellular domain of its precursor, which is an integral membrane protein. Furthermore, the cleavage sites for the release of this mitogen are compatible with the participation of an elastaselike protease. We have immunohistochemically localized TGF alpha to the vascular smooth muscle cells in the arterioles. To investigate whether polymorphonuclear (PMN) leukocytic elastase, a blood-borne protease, could process the cell surface TGF alpha, NR6 cells were transfected with the rat TGF alpha cDNA. The cDNA encoded the entire open reading frame, and its expression was under the control of the mouse metallothionein I promoter. A cloned transfectant, termed 1B2, synthesized the TGF alpha precursor in a zinc-inducible manner, and the precursor was localized to the cell surface. Western blot (immunoblot) analysis indicated that treatment of the zinc-induced 1B2 cells with either PMN leukocytic or pancreatic elastase resulted in the release of the mature TGF alpha polypeptide. The released TGF alpha was bioactive, as it was capable of both competing with epidermal growth factor for binding to its receptor and stimulating [3H]thymidine incorporation in the mitogenic assay. Formaldehyde fixation of the 1B2 cells eliminated basal release of TGF alpha but allowed normal processing by both PMN leukocytic and pancreatic elastase to occur. However, human cathepsin G, bovine pancreatic alpha 1-chymotrypsin, collagenase, trypsin, subtilisin, and plasmin failed to release any detectable fragments of the TGF alpha precursor from the fixed cells. The location of TGF alpha in the arterioles and ability of PMN leukocytic elastase to process the membrane-bound TGF alpha precursor suggests a novel role for this elastase at the wound site.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorge J. D., Paterson A. J., Kudlow J. E. Phorbol ester or epidermal growth factor (EGF) stimulates the concurrent accumulation of mRNA for the EGF receptor and its ligand transforming growth factor-alpha in a breast cancer cell line. J Biol Chem. 1989 Mar 5;264(7):4021–4027. [PubMed] [Google Scholar]
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989 Feb 24;56(4):691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Bringman T. S., Lindquist P. B., Derynck R. Different transforming growth factor-alpha species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987 Feb 13;48(3):429–440. doi: 10.1016/0092-8674(87)90194-2. [DOI] [PubMed] [Google Scholar]
- Brown C. F., Teng C. T., Pentecost B. T., DiAugustine R. P. Epidermal growth factor precursor in mouse lactating mammary gland alveolar cells. Mol Endocrinol. 1989 Jul;3(7):1077–1083. doi: 10.1210/mend-3-7-1077. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- De Cremoux H., Hornebeck W., Jaurand M. C., Bignon J., Robert L. Partial characterisation of an elastase-like enzyme secreted by human and monkey alveolar macrophages. J Pathol. 1978 Aug;125(4):171–177. doi: 10.1002/path.1711250402. [DOI] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P., Petrusz P., Bell G. I., Brown C. F., Korach K. S., McLachlan J. A., Teng C. T. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988 Jun;122(6):2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Twardzik D. R., Lim G. J., Ranchalis J. E., Lee D. C. Expression and characterization of transforming growth factor alpha precursor protein in transfected mammalian cells. Mol Cell Biol. 1987 May;7(5):1585–1591. doi: 10.1128/mcb.7.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh D. A., Amoss M. S., Hood D. M., Morgan S. J., Williams J. D. Epidermal growth factor-mediated effects on equine vascular smooth muscle cells. Am J Physiol. 1988 Oct;255(4 Pt 1):C447–C451. doi: 10.1152/ajpcell.1988.255.4.C447. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Kelly B., Davis R. J., Massagué J. Biologically active precursor for transforming growth factor type alpha, released by retrovirally transformed cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6307–6311. doi: 10.1073/pnas.83.17.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James H. L., Wachtfogel Y. T., James P. L., Zimmerman M., Colman R. W., Cohen A. B. A unique elastase in human blood platelets. J Clin Invest. 1985 Dec;76(6):2330–2337. doi: 10.1172/JCI112244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Kobrin M. S., Asa S. L., Samsoondar J., Kudlow J. E. Alpha-transforming growth factor in the bovine anterior pituitary gland: secretion by dispersed cells and immunohistochemical localization. Endocrinology. 1987 Oct;121(4):1412–1416. doi: 10.1210/endo-121-4-1412. [DOI] [PubMed] [Google Scholar]
- Kobrin M. S., Samsoondar J., Kudlow J. E. Alpha-transforming growth factor secreted by untransformed bovine anterior pituitary cells in culture. II. Identification using a sequence-specific monoclonal antibody. J Biol Chem. 1986 Nov 5;261(31):14414–14419. [PubMed] [Google Scholar]
- Kudlow J. E., Kobrin M. S., Purchio A. F., Twardzik D. R., Hernandez E. R., Asa S. L., Adashi E. Y. Ovarian transforming growth factor-alpha gene expression: immunohistochemical localization to the theca-interstitial cells. Endocrinology. 1987 Oct;121(4):1577–1579. doi: 10.1210/endo-121-4-1577. [DOI] [PubMed] [Google Scholar]
- Kudlow J. E., Kobrin M. S. Secretion of epidermal growth factor-like mitogens by cultured cells from bovine anterior pituitary glands. Endocrinology. 1984 Sep;115(3):911–917. doi: 10.1210/endo-115-3-911. [DOI] [PubMed] [Google Scholar]
- Kudlow J. E., Leung A. W., Kobrin M. S., Paterson A. J., Asa S. L. Transforming growth factor-alpha in the mammalian brain. Immunohistochemical detection in neurons and characterization of its mRNA. J Biol Chem. 1989 Mar 5;264(7):3880–3883. [PubMed] [Google Scholar]
- Lee D. C., Rose T. M., Webb N. R., Todaro G. J. Cloning and sequence analysis of a cDNA for rat transforming growth factor-alpha. Nature. 1985 Feb 7;313(6002):489–491. doi: 10.1038/313489a0. [DOI] [PubMed] [Google Scholar]
- Lobb D. K., Kobrin M. S., Kudlow J. E., Dorrington J. H. Transforming growth factor-alpha in the adult bovine ovary: identification in growing ovarian follicles. Biol Reprod. 1989 May;40(5):1087–1093. doi: 10.1095/biolreprod40.5.1087. [DOI] [PubMed] [Google Scholar]
- Mroczkowski B., Reich M., Chen K., Bell G. I., Cohen S. Recombinant human epidermal growth factor precursor is a glycosylated membrane protein with biological activity. Mol Cell Biol. 1989 Jul;9(7):2771–2778. doi: 10.1128/mcb.9.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. G., Kobrin M. S., Paterson A. J., Kudlow J. E. Transforming growth factor-alpha expression in the anterior pituitary gland: regulation by epidermal growth factor and phorbol ester in dispersed cells. Mol Endocrinol. 1989 Jun;3(6):976–983. doi: 10.1210/mend-3-6-976. [DOI] [PubMed] [Google Scholar]
- Pittelkow M. R., Lindquist P. B., Abraham R. T., Graves-Deal R., Derynck R., Coffey R. J., Jr Induction of transforming growth factor-alpha expression in human keratinocytes by phorbol esters. J Biol Chem. 1989 Mar 25;264(9):5164–5171. [PubMed] [Google Scholar]
- Rall L. B., Scott J., Bell G. I., Crawford R. J., Penschow J. D., Niall H. D., Coghlan J. P. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985 Jan 17;313(5999):228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- Samsoondar J., Kobrin M. S., Kudlow J. E. Alpha-transforming growth factor secreted by untransformed bovine anterior pituitary cells in culture. I. Purification from conditioned medium. J Biol Chem. 1986 Nov 5;261(31):14408–14413. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schneider C. A., Lim R. W., Terwilliger E., Herschman H. R. Epidermal growth factor-nonresponsive 3T3 variants do not contain epidermal growth factor receptor-related antigens or mRNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):333–336. doi: 10.1073/pnas.83.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Baur U., Bürgin M., Bühler F. R. Epidermal growth factor responsiveness in smooth muscle cells from hypertensive and normotensive rats. Hypertension. 1989 Apr;13(4):295–304. doi: 10.1161/01.hyp.13.4.295. [DOI] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Rice A. P., Gullick W. J., Cheng D. J., Kerr I. M., Waterfield M. D. Purification and characterization of vaccinia virus growth factor. Cell. 1985 Aug;42(1):383–393. doi: 10.1016/s0092-8674(85)80133-1. [DOI] [PubMed] [Google Scholar]
- Teixidó J., Gilmore R., Lee D. C., Massagué J. Integral membrane glycoprotein properties of the prohormone pro-transforming growth factor-alpha. 1987 Apr 30-May 6Nature. 326(6116):883–885. doi: 10.1038/326883a0. [DOI] [PubMed] [Google Scholar]
- Valentine R., Fisher G. L. Characteristics of bovine alveolar macrophage elastase. J Leukoc Biol. 1984 May;35(5):449–457. doi: 10.1002/jlb.35.5.449. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]