Synopsis
Aging is associated with an increased prevalence of cardiac arrhythmias, which contribute to higher morbidity and mortality in the elderly. The frequency of cardiac arrhythmias, particularly atrial fibrillation and ventricular tachyarrhythmia, is projected to increase as the population ages, greatly impacting health care resource utilization. Several clinical factors associated with the risk of arrhythmias have been identified in the population, yet the molecular bases for the increased predisposition to arrhythmogenesis in the elderly are not fully understood. Therefore, only limited therapeutic strategies directed at pathophysiological processes that enhance cardiac vulnerability to arrhythmias are available. This is further compounded by the paucity of outcome studies providing evidence on which optimal management guidelines can be formulated for the very elderly. This review highlights the epidemiology of cardiac dysrhythmias, changes incardiac structure and function associated with aging, and the basis for arrhythmogenesis in the elderly, the understanding of which is critical to formulate preventive strategies.
Keywords: aging, arrhythmias, mechanisms, elderly, atrial fibrillation, ventricular fibrillation, sudden cardiac death
Introduction
Aging is associated with an increased prevalence of cardiac arrhythmias, which contribute to higher morbidity and mortality in the elderly.1–4 With the aging of the population, the frequency of cardiac arrhythmias, particularly atrial fibrillation (AF) and ventricular tachyarrhythmia, is projected to increase and thereby greatly impact health care resource utilization.4–7 Several clinical factors associated with the risk of arrhythmias have been identified in the population,8,9 yet the molecular bases for the increased predisposition to arrhythmogenesis in the elderly are not fully understood. Therefore, only limited therapeutic strategies directed at pathophysiological processes that enhance cardiac vulnerability to arrhythmias are available. This is further compounded by the paucity of outcome studies10,11 that provide evidence on which optimal management guidelines can be formulated for the very elderly.2,10,12–14 In this review, we highlight the epidemiology of cardiac dysrhythmias, changes in cardiac structure and function associated with aging and the basis for arrhythmogenesis in the elderly, the understanding of which is critical to formulate preventive strategies.
Epidemiology
The incidence of cardiac dysrhythmias, both brady- and tachyarrhythmias, increases with advancing age.12,15,16 The median age of pacemaker recipients for bradyarrhythmias in the United States is 75 years with more than 80% of pacemakers implanted in those 65 years or older.16 The major indication for pacemaker implantation in the elderly is to relieve symptoms due to bradycardia and/or chronotropic incompetency from sinus node dysfunction or His-Purkinje disease as a result of aging-related degenerative changes in the atrial pacemaker complex and conduction system that usually manifest in the seventh or eighth decade of life.16–19
Among tachyarrhythmias, AF is the most common arrhythmia encountered in clinical practice with a 100-fold higher prevalence in octogenarians (8–10%) compared to those younger than 55 years of age (<0.1%; Fig 1).2,12,20–22 With a 1-in-4 lifetime risk of development,23 AF contributes to increased morbidity in the elderly, not only by adversely affecting quality of life but also by deterioration in myocardial function, which increases susceptibility to heart failure, stroke, hospitalization and mortality. AF carries an annual healthcare cost exceeding $15 billion.5,8,9,20,23–27 The median age of patients with AF is around 75 years with 84% of the patients older than 65. With the rapid increase in the number of elderly7 and concomitant cardiovascular comorbidities,24,28 a sixfold increase in the prevalence of AF (to 15.9 million) is projected,20 highlighting the magnitude of the epidemic of AF and its far reaching implications on the health and economics of the country.20,24,29
Fig. 1.
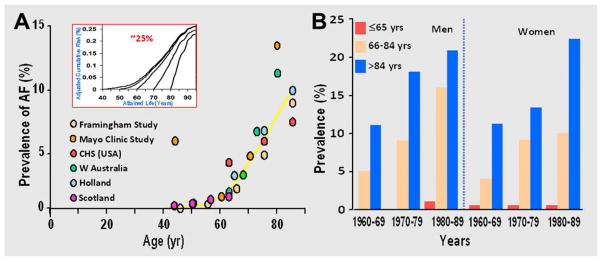
A: Prevalence of atrial fibrillation (AF) in older adults doubles with each decade. Prevalence increases 80–100-fold in the very elderly (0.1% at 40 years to ~8–10% in ≥ 80 years).
(Adapted from Feinberg et al. Prevalence, age distribution, and gender of patients with atrial fibrillation analysis and implications. Arch Int Med 1995;155:469–73, with permission from American Medical Association.)
Inset: Cumulative lifetime risk for the development of AF (~25%) in the adult population.
(Adapted from Lloyd-Jones et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 2004;110:1042–6, with permission from Wolters Kluwer Health.)
B: Secular trends in prevalence of AF in Olmsted County, MN: 1960–1989.
(Adapted from Tseng et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol 2003;42:93–100, with permission from Elsevier.)
Aging is also associated with a progressive increase in the incidence of ventricular dysrhythmias, both benign and malignant, with or without structural heart disease. Unexpected death from cardiovascular causes occurs in 250,000 to 300,000 individuals annually with up to 75–80% resulting from ventricular fibrillation or tachycardia, more so in elderly patients with coronary artery disease, and may be the presenting event in 50% of patients.2,3,30,31 In 15–20% of sudden cardiac death (SCD) victims, advanced atrioventricular block or asystole is documented.32,33 However, an increase in pulseless electrical activity with a proportionate decrease in ventricular fibrillation as the presenting arrhythmia has been reported in more recent studies.34,35 The true incidence of bradyarrhythmias causing sudden death in the elderly is not known because by the time the first rhythm is recorded, an arrhythmia beginning as ventricular tachyarrhythmia may degenerate into or appear as asystole. SCD accounts for 13% of all natural deaths and 50% of all deaths from cardiovascular causes.15,36 Despite advancement in the management of cardiovascular disease, the incidence of SCD in the general population (0.1% to 0.2% per year) has decreased only marginally and is expected to grow with the aging of the population.11,15,30,31,34 The persistent dismal survival rate of 4–5% after an out-of-hospital cardiac arrest37 calls for improvement in risk stratification and means to prevent SCD15 by better defining mechanisms underlying aging-associated increase in the susceptibility to arrhythmogenesis and gathering evidence from clinical trials to develop cost-effective strategies to reduce the burden of arrhythmias in the elderly.13
Structural and functional changes in the aging heart
Structural and functional alterations in cardiac mechanical and electrical system, as well as energetics and metabolism associated with the aging process increase predisposition to cardiac arrhythmias.1,38–40 Bradyarrhythmias, due to reduced normal automaticity and delayed conduction are common in the very elderly, even in the absence of apparent heart disease, and are further exacerbated by comorbidities or use of medications resulting in symptoms that require pacemaker implantation.16,17,19,41,42 The intrinsic heart rate and heart rate reserve, as determined following cardiac parasympathetic and sympathetic blockade, decreases with aging.43 This results from aging-associated replacement of pacemaker cells within the sinoatrial node and atrioventricular conduction fibers with an extracellular matrix composed of collagen and elastin fibers44 and impairment of receptor and post-receptor signaling via beta-adrenergic receptors contributing to the diminished heart rate response and heart rate variability, with resultant reduction in aerobic work capacity in the elderly.16,44–46 In addition, amyloid, lipid and lipofuscin deposition within the myocardium, particularly around the atrial pacemaker tissue, further contributes to bradyarrhythmias in the aging heart.1,12,47,48 By age 75, the number of functional pacemaker cells decreases to less than 10% of those in young adults, which, along with the reduction in the expression of ion channels, promotes reduced automaticity.49 Senescence-induced degenerative changes in the cardiac skeleton, particularly in areas close to the AV node, His-Purkinje tissue and bundle branches, delay conduction and predispose elderly patients to dysrhythmias.47,50 Electrical and structural remodeling with action potential duration prolongation and connexin remodeling increases the refractoriness of cardiac tissue and slows conduction.51–53 These changes, along with a blunted response to neurohumoral activation, promote age-related increase in the propensity for chronotropic and dromotropic impairment and for the development of brady- and tachyarrhythmias.1
Susceptibility to both supraventricular and ventricular arrhythmogenesis is enhanced in the senescent heart even in the absence of apparent structural abnormalities and is further exaggerated with comorbidities accompanying the aging process.12,15 This results from both structural and functional alterations – including cell loss, myocyte hypertrophy and interstitial fibrosis – resulting in altered cellular coupling and exaggerated directional differences in conduction (anisotropy), increasing heterogeneity in impulse propagation properties and refractoriness of the myocardium. This creates zones of functional slowing or conduction block that stabilize reentry, enhancing susceptibility to arrhythmogenesis.46,54–56 Changes in expression, distribution and regulation of ion channels alter action potential waveforms and propagation, further enhancing vulnerability to dysrhythmias.49,57 In the senescent heart, the action potential duration and repolarization are prolonged,58–60 in part due to downregulation of potassium currents, including the Ca2+-activated potassium, transient outward (Ito), and ATP-sensitive potassium channels and partly due to delay in the inactivation of the calcium current (ICaL).58,61,62 Along with an increase in sodium-calcium exchanger, this delay increases predilection to Ca2+-overload-mediated triggered activity and reentrant arrhythmias.59,62–67 A reduction in the expression of the sarcoplasmic reticulum Ca2+-ATPase (SERCA-2)68,69 and post-translational modifications in the function of SERCA-2, phospholamban and the sarcoplasmic reticulum Ca2+-release channel (RYR2) further alters calcium homeostasis and susceptibility of the aging heart towards arrhythmogenesis.60,70–74
The heart, an aerobic organ with high energy demand, is dependent on adequate energy supply from mitochondrial oxidative phosphorylation, which provides more than 70% of ATP.75 A decline in mitochondrial function including oxidative phosphorylation,37,76–79 with reduction in the activity of respiratory chain components, adenine nucleotide translocase activity, changes in mitochondrial matrix and membrane lipid pattern, occurs with the aging process and contributes to an enhanced susceptibility to myocardial dysfunction under conditions of increased energy demand, such as during tachyarrhythmias.39,59,72,76,80–89 The contribution of age-related changes in cardiac microstructure, including sarcolemma, cytoskeleton, intercellular gap junctions, cellular geometry and interstitium as well as mitochondria7,90 and other intracellular organelles, on the regulation of cardiac excitability or arrhythmogenesis are not well defined and warrant further studies.
Electrophysiological substrates for AF in the elderly
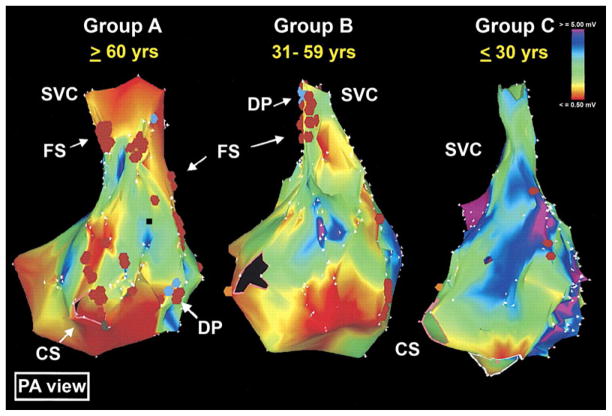
AF is a heterogeneous disorder with variable etiology, clinical profile and natural history.77 In the majority of elderly patients, AF occurs in the setting of structural heart disease, with only a small percentage exhibiting AF as a primarily electrical disorder.14,91–95 Aging is associated with changes in expression, distribution and/or function of ion channels that alter action potential waveforms, propagation and calcium handling, increasing vulnerability to AF.46,78,79,96,97 In addition, aging-associated loss of atrial cardiomyocytes and increased interstitial fibrosis occurs even in the absence of structural heart disease and promote the substrate for arrhythmogenesis. In humans, this can be demonstrated as fractionated electrocardiograms and low-voltage zones that increase with advancing age (Fig 2).38 Changes in hemodynamic, mechanical, neurohumoral, metabolic and inflammatory factors that accompany aging or aging-associated diseases, such as heart failure, valvular heart disease, hypertension, myocardial infarction and diabetes contribute to the development of AF, yet the common mechanistic link between these factors and the development of the substrate for AF or its progression in the elderly is not fully understood.
Fig. 2.
Age-related changes in human left atrial voltage loss determined by three-dimensional electroanatomic bipolar voltage mapping. Group A includes those ≥60 years of age; Group B 31–59 years; and Group C ≤30 years. Color annotation: Bipolar voltages from 0.5 mV (in red) to voltages 5 mV (in purple). CS = coronary sinus; DP = double potentials; FS = fractionated signals; PA = posteroanterior; SVC = superior vena cava.
(Reproduced from Kistler et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 2004;44:109–16, with permission from Elsevier.)
The electrophysiologic basis for the initiation and/or maintenance of AF varies depending on the patient’s age, underlying heart disease or other electrophysiological modulating factors.8,9,98 Both enhanced impulse generation due to increased automaticity or triggered activity within the atria or pulmonary veins and conduction slowing is responsible for the initiation and maintenance of AF in the elderly. A range of adaptive and maladaptive processes in response to day-to-day stressors, such as volume or pressure overload, stretch, ischemia or rapid rates, contribute to triggers that further alter cellular excitability, cell-to-cell coupling and anisotropy. This results in directional slowing of impulse propagation54 and a source/sink mismatch,53 creating a milieu that increases predisposition to AF in the senescent atria.38,52–55,63,73,74,78,99–101
AF may exist in paroxysmal (spontaneously terminates within 7 days), persistent (requires intervention for termination) or permanent forms, with one-fourth of patients with paroxysmal AF progressing to the permanent form,9,14 which becomes resistant to pharmacological and nonpharmacological therapies.102 Extended follow-up of a unique population of young patients (mean age 44 ± 11 years) from Olmsted County, Minnesota, with lone AF (in the absence of structural heart disease or hypertension)103 allowed assessment of the natural history of AF and determination of the effect of aging and aging-associated comorbidities on the progression of AF and subsequent risk of stroke, heart failure and mortality.14 The 30-year cumulative probability of development of permanent AF was 29% (95% confidence interval 16–42%), with minimum risk of heart failure, stroke or mortality. Older age at diagnosis, or presence of QRS abnormalities on electrocardiography were predictors of progression to permanent AF, whereas the presence of premature supraventricular complexes/tachycardia was protective and associated with decreased risk of permanent AF. These findings suggest that young patients with premature complexes and supraventricular arrhythmias in the absence of structural heart disease had a different electrophysiological substrate, with a primary electrical disorder compared to older patients or those with electrocardiographic or overt structural abnormalities104 or with the development of hypertension, heart failure, diastolic dysfunction or ischemic heart disease with advancing age.9,14,105 resulting in different progression rates to permanent AF.12, 14
Aging and its associated comorbidities have been known to increase the risk of progression of AF and its complications, however, the common link connecting these risk factors to the development or progression of AF is not fully understood. Myocardial fibrosis is a common factor associated with aging and co-morbidities such as heart failure, ischemic or valvular heart disease or other forms of cardiomyopathy and could contribute to aging associated progression of atrial fibrillation. Further research in defining the role of myocardial cell loss and replacement fibrosis is warranted. In animal models, the facilitative role of rapid atrial rate in the initiation and maintenance of sustained AF has been demonstrated.78,79,106 A progressive decrease in atrial refractoriness, increased heterogeneity and loss of the normal rate-dependent adaptation of refractoriness occurs in this animal model.78 Slowing of the conduction velocity occurs within the atrium, although late. These changes in electrophysiological properties, termed “electrical remodeling,” of the atrium likely result from reduction in the L-type Ca2+ current and transient outward potassium current, a progressive late reduction in the density of voltage-gated Na+ channels, and gap junction redistribution that contributes to the slowing of conduction, thus increasing vulnerability of the atria to reentry and creating a condition in which AF perpetuates AF.79,102,106,107 These electrophysiological changes are different from structural changes that are observed in patients with heart failure or in the senescent atria with gradual loss of myofibrils, myocyte hypertrophy, fragmentation of sarcoplasmic reticulum and fibrosis with changes in the structure and shape of mitochondria.1,46,62,108–117 Changes in protein expression, similar to a de-differentiation process toward a partially fetal phenotype or that seen in hibernating myocardium, also take place with chronic AF.108,110 The structural substrate with interstitial fibrosis and atrial enlargement appears to be more important in the pathogenesis of AF and its progression in humans than the electrical remodeling, as suggested by animal models in which AF was induced by heart failure or mitral regurgitation,78,111–113 and by its high prevalence in the elderly.9,114 This is further supported by observations that despite reversibility of atrial refractoriness with restoration of sinus rhythm, AF inducibility or recurrences continued to be high,115,116 indicating that strategy of prompt termination of AF to avoid adverse electrical remodeling is of little clinical benefit in the elderly.
The precise mechanisms underlying atrial structural remodeling in humans are not fully understood, but impaired intracellular calcium handling, oxidative stress and altered energetics appear to play an important role,107 and mechanisms coupling calcium loading to structural remodeling need to be further defined. Additional unknown factors are operative102,112,117,118 and need to be elucidated for better understanding of the pathogenesis of AF in the elderly so effective strategies can be instituted for primary and secondary prevention of AF and thereby limit its burden on health care resources.
Electrophysiological substrates for ventricular arrhythmias/SCD in the elderly
The substrate for ventricular arrhythmias in the elderly varies depending on the presence of structural heart disease. The effect of aging on cardiac hemodynamics, structure and function is complex and challenging to study in humans because of difficulty in isolating the effect of aging from diseases associated with the aging process. Ventricular dysfunction and fibrosis after myocardial infarction or with nonischemic cardiomyopathy predispose to ventricular arrhythmogenesis, which contributes to the majority of SCD in the elderly.119–123 The risk factors for arrhythmias leading to SCD have been well described; however, precise mechanisms underlying the initiation and maintenance or prediction of timing for the development of dysrhythmias causing SCD in the elderly are not completely understood. This is due to interactions between dynamic transient factors (ischemia, hypoxia, catecholamines, pH and electrolyte changes, stretch or inflammation) on the underlying myocardial substrate that precipitate arrhythmias.8,78,100 Ventricular tachyarrhythmias may be initiated by one mechanism, perpetuated by another and then degenerate into a different mechanism. This is mainly due to the complex interactions between myocardial cellular and extracellular substrates and triggers that define the overall risk of arrhythmia susceptibility.78,99,100,101
Acute ischemia triggering lethal ventricular tachyarrhythmias contributes to the majority of SCD in the elderly.124 In 50% of patients with coronary artery disease, sudden death is the initial manifestation.37,125–127 Acute changes in coronary plaque morphology such as disruption or thrombus were found in more than 50% of the victims of sudden death.128–130 Cardiac events due to inherited arrhythmia syndromes such as congenital long QT syndrome, short QT syndrome, Brugada syndrome or catecholaminergic polymorphic ventricular tachycardia account for only a small percentage of sudden deaths in the elderly.131,132 Familial clustering of cardiac events, however, does suggest a role of genetic factors in predisposition to sudden death,133–135 which, in the elderly, appears to be due to influences that increase the risk of a coronary event.37,125,136–138 Patients with long QT syndrome maintain a high risk for life-threatening cardiac events even in later years, although the risk of aborted cardiac arrest or death conferred by long QT syndrome is attenuated, most likely due to the higher prevalence of comorbidities associated with senescence.139–143
Ventricular arrhythmias are common in the elderly, affecting more than 70% of individuals age 60 years and older. With advancing age and presence of structural heart disease, not only do the prevalence and complexity of ventricular arrhythmias worsen but so does their prognostic significance.15,144–147 Even in asymptomatic elderly the prevalence of ventricular arrhythmias on ambulatory monitoring is as high as 60–90%. Complex premature beats, such as pairs and triplets, occur in up to 10% of such individuals and may be present in up to 60% of the older elderly during exercise. In the absence of heart disease, asymptomatic premature ventricular complexes (PVC) presenting at rest are benign, but when elicited during exercise148 or post-exercise recovery period,149 carry adverse prognosis and increased risk of cardiovascular death. In patients with reduced ventricular function, frequent PVCs have poor prognostic significance.147,150–156 The mechanisms underlying ventricular arrhythmogenesis are variable and depend on the underlying myocardial substrate. During ischemia or the acute phase of myocardial infarction, functional reentry can precipitate ventricular fibrillation, whereas reentry circuits around the scar tissue late after acute myocardial infarction result in ventricular tachycardia that degenerates into fibrillation. The vulnerability of the aging heart to arrhythmogenesis is increased during the peri-infarct period, with a higher likelihood of inhospital cardiac arrest in those ≥75 years and older compared with younger patients.157 Ventricular tachyarrhythmias occurring within 48 hours of acute ischemic event are associated with an increase in hospital death, however, long-term mortality is not affected unless significant ventricular dysfunction persists.158 The incidence of scar-related reentrant ventricular arrhythmias increases exponentially, with reduction in left ventricular ejection fraction to below 30%.125,159
Evaluation and management of elderly patients with cardiac arrhythmias
Several noninvasive and invasive risk stratification protocols for patients at risk for cardiac arrhythmias who may benefit from interventions to reduce complications and risk of life-threatening events have been developed.15,16,77 A simple 12-lead electrocardiogram allows identification of the underlying structural or functional substrate, such as conduction system abnormalities, prior infarction, ventricular hypertrophy, arrhythmogenic right ventricular cardiomyopathy or primary electrical disorders, such as long or short QT syndrome and Brugada syndrome. A prolonged QTc interval in the elderly and a QRS duration longer than 150 ms in patients with severely depressed ventricular function predicts a higher risk of SCD.3,16,160 A prolonged PR interval or delayed conduction in the atria increases the risk of atrial fibrillation.77 On signal-averaged electrocardiogram (SAECG), absence of late potentials has a high negative predictive value in excluding wide complex tachycardia as a cause of unexplained syncope in the elderly patient with coronary artery disease.161,162 Appearance of exercise-induced complex ventricular ectopy or ventricular tachycardia in the elderly during exercise testing may predict an increased risk of mortality compared to patients with simple ectopy observed at rest only.148,149,163 Microvolt fluctuation in the amplitude or morphology of T waves during rest,164 exercise testing or atrial pacing may identify high-risk postinfarction or cardiomyopathy patients.165,166 Assessment of atrial and ventricular dimensions and contractile function with imaging techniques such as echocardiogram are an essential part of cardiac evaluation of patients at risk for arrhythmias.16,77,167 In patients suspected of ventricular arrhythmias triggered by ischemia,168 exercise or pharmacological testing to detect ischemia can be performed with imaging using echocardiogram, magnetic resonance imaging or nuclear perfusion scans.169,170 In patients with ventricular arrhythmias or aborted sudden death, coronary angiography is useful to assess for coronary artery disease. Invasive electrophysiology testing is useful for risk stratification for SCD in elderly patients with ischemic heart disease and moderate left ventricular dysfunction or syncope, but is of limited utility for patients with dilated cardiomyopathy or inherited arrhythmia syndromes.171–179 Use of multiple risk markers in combination may better predict arrhythmogenic events than a single parameter given the complexity and variability of the underlying substrates predisposing to arrhythmogenesis and SCD.31
Discussion of the management of cardiac dysrhythmias are beyond the scope of this article but can be obtained from recent guidelines.15,16,31,77,180–186 However, little information is available from clinical trials focusing on the efficacy of various therapeutic modalities in the older-elderly such as antiarrhythmic agents, ablation procedures for atrial and ventricular tachyarrhythmias or implantable cardioverter-defibrillators.13,98 Information about the efficacy of therapies for cardiac arrhythmias in the very elderly with limited life expectancy is difficult to assess, especially in view of the fact that only a select few individuals more than 80 years old were included in randomized clinical trials and nonrandomized data suffers from selection bias.129 A diminished benefit of therapies such as implantable cardioverter-defibrillator has been demonstrated with pooled analysis of SCD prevention trials due to an increased number of nonarrhythmic cardiac and noncardiac deaths in the very elderly with multiple comorbidities.130
Summary
Cardiac dysrhythmias are common in the elderly and their overall prevalence and impact on health care costs is expected to increase with the changing population demographics. Despite rise in the number of elderly with atrial and ventricular arrhythmias, only limited insight into mechanisms underlying aging-associated increase in the susceptibility of the heart to arrhythmogenesis is available. In addition, evidence from well-designed clinical trials in the very elderly supporting safer and effective management decisions is lacking, which limits specific practice guidelines. Ongoing research to fulfill this unmet need may provide novel insights into the pathogenesis of cardiac arrhythmias and improvement in preventive and therapeutic strategies.13
Key Points.
Aging is associated with an increased prevalence of cardiac arrhythmias, which contribute to higher morbidity and mortality in the elderly. The frequency of cardiac arrhythmias, particularly atrial fibrillation and ventricular tachyarrhythmia, is projected to increase as the population ages, greatly impacting health care resource utilization.
Several clinical factors associated with the risk of arrhythmias have been identified in the population, yet the molecular bases for the increased predisposition to arrhythmogenesis in the elderly are not fully understood. Therefore, only limited therapeutic strategies directed at pathophysiological processes that enhance cardiac vulnerability to arrhythmias are available.
This is further compounded by the paucity of outcome studies providing evidence on which optimal management guidelines can be formulated for the very elderly.
Acknowledgments
Funding Sources: Drs. Jahangir and Mirza’s research effort was in part supported by the National Heart, Lung, and Blood Institute grants RO1 HL101240-03 and R01 HL089542-04.
The authors gratefully acknowledge Joe Grundle and Katie Klein for the editorial preparation of the manuscript.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mahek Mirza, Email: mahek.mirza@aurora.org.
Anton Strunets, Email: anton.strunets@aurora.org.
References
- 1.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–64. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go ASHE, Phillips KA, Chang Y, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 6.Jahangir A, Shen WK. Pacing in elderly patients. Am Heart J. 2003;146:750–3. doi: 10.1016/S0002-8703(03)00454-X. [DOI] [PubMed] [Google Scholar]
- 7.Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol. 2007;103:2120–8. doi: 10.1152/japplphysiol.00647.2007. [DOI] [PubMed] [Google Scholar]
- 8.Jahangir A, Munger T, Packer D, Crijns H. Atrial fibrillation. In: Potrid PJ, Kowey PR, editors. Cardiac Arrhythmia: Mechanisms, Diagnosis, and Management. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 457–99. [Google Scholar]
- 9.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 10.Aronow WS. Heart disease and aging. Med Clin North Am. 2006;90:849–62. doi: 10.1016/j.mcna.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–25. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 accf/aha/hrs focused updates incorporated into the acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:e269–36713. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 13.Liu XK, Jahangir A, Shen WK. Cardiac arrhythmias. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard’s Geriatric Medicine and Gerontology. 6. New York: McGraw Hill; 2009. pp. 951–65. [Google Scholar]
- 14.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 15.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Rosenqvist M, Obel IW. Atrial pacing and the risk for AV block: is there a time for change in attitude? Pacing Clin Electrophysiol. 1989;12(1 Pt 1):97–101. doi: 10.1111/pace.1989.12.p1.97. [DOI] [PubMed] [Google Scholar]
- 18.Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854–62. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- 19.Vlietstra RE, Jahangir A, Shen WK. Choice of pacemakers in patients aged 75 years and older: ventricular pacing mode vs. dual-chamber pacing mode. Am J Geriatr Cardiol. 2005;14:35–8. doi: 10.1111/j.1076-7460.2005.03329.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–7. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 25.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980–1998. Am J Epidemiol. 2002;155:819–26. doi: 10.1093/aje/155.9.819. [DOI] [PubMed] [Google Scholar]
- 26.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–6. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 27.Ozcan C, Jahangir A, Friedman PA, et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med. 2001;344:1043–51. doi: 10.1056/NEJM200104053441403. [DOI] [PubMed] [Google Scholar]
- 28.Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 29.Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and co-morbidities of atrial fibrillation in a privately insured population. Curr Med Res Opin. 2005;21:1693–9. doi: 10.1185/030079905X65475. [DOI] [PubMed] [Google Scholar]
- 30.Myerburg RJ, Hendel RC. Expanding risk-profiling strategies for prediction and prevention of sudden cardiac death. J Am Coll Cardiol. 2010;56:215–7. doi: 10.1016/j.jacc.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luu M, Stevenson WG, Stevenson LW, et al. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675–80. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- 33.Bayés de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–9. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 34.Cobb LA, Fahrenbruch CE, Olsufka M, et al. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–13. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 35.Herlitz J, Andersson E, Bang A, et al. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Göteborg. Eur Heart J. 2000;21:1251–8. doi: 10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- 36.Gillum RF. Geographic variation in sudden coronary death. Am Heart J. 1990;119:380–9. doi: 10.1016/s0002-8703(05)80031-6. [DOI] [PubMed] [Google Scholar]
- 37.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–16. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 39.Preston CC, Oberlin AS, Holmuhamedov EL, et al. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008;129:304–12. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts-Thomson KC, Kistler PM, Sanders P, et al. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage, and conduction velocity. Heart Rhythm. 2009;6:587–91. doi: 10.1016/j.hrthm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez RD, Schocken DD. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics. 1990;45:26–30. 33–6. [PubMed] [Google Scholar]
- 42.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–32. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 43.Jose AD. Effect of combined sympathetic and parasympathetic blockade on heart rate and cardiac function in man. Am J Cardiol. 1966;18:476–8. doi: 10.1016/0002-9149(66)90073-7. [DOI] [PubMed] [Google Scholar]
- 44.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–67. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 45.Fleg JL, O’Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 46.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lie JT, Hammond PI. Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin Proc. 1988;63:552–64. doi: 10.1016/s0025-6196(12)64885-x. [DOI] [PubMed] [Google Scholar]
- 48.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 49.Tellez JO, McZewski M, Yanni J, et al. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp Physiol. 2011;96:1163–78. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 50.Cheitlin MD. Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol. 2003;12:9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 51.Huang C, Ding W, Li L, et al. Differences in the aging-associated trends of the monophasic action potential duration and effective refractory period of the right and left atria of the rat. Circ J. 2006;70:352–7. doi: 10.1253/circj.70.352. [DOI] [PubMed] [Google Scholar]
- 52.Liu XK, Jahangir A, Terzic A, et al. Age- and sex-related atrial electrophysiologic and structural changes. Am J Cardiol. 2004;94:373–5. doi: 10.1016/j.amjcard.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 53.Spach MS, Heidlage JF, Dolber PC, et al. Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study. Heart Rhythm. 2007;4:175–85. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koura T, Hara M, Takeuchi S, et al. Anisotropic conduction properties in canine atria analyzed by high-resolution optical mapping: Preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation. 2002;105:2092–8. doi: 10.1161/01.cir.0000015506.36371.0d. [DOI] [PubMed] [Google Scholar]
- 55.de Bakker JM, van Rijen HM. Continuous and discontinuous propagation in heart muscle. J Cardiovasc Electrophysiol. 2006;17:567–73. doi: 10.1111/j.1540-8167.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 56.Hao X, Zhang Y, Zhang X, et al. TGF-beta1-mediated fibrosis and ion channel remodeling are key mechanisms in producing the sinus node dysfunction associated with SCN5A deficiency and aging. Circ Arrhythm Electrophysiol. 2011;4:397–406. doi: 10.1161/CIRCEP.110.960807. [DOI] [PubMed] [Google Scholar]
- 57.Nerbonne JM, Kass RS. Physiology and molecular biology of ion channels contributing to ventricular repolarization. In: Gussak I, Antzelevitch C, editors. Contemporary cardiology: cardiac repolarization: bridging basic and clinical science. Totowa, NJ: Humana Press; 2003. pp. 25–62. [Google Scholar]
- 58.Josephson IR, Guia A, Stern MD, et al. Alterations in properties of L-type Ca channels in aging rat heart. J Mol Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- 59.Jahangir A, Holmuhamedov EL, Cabrera Aguilera CC, et al. Molecular basis for the increased vulnerability of the aging heart to injury (abstract) Eur Heart J. 2006;27(Suppl 1):5101. [Google Scholar]
- 60.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circulation Res. 2010;106:659–73. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janczewski AM, Spurgeon HA, Lakatta EG. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol. 2002;34:641–8. doi: 10.1006/jmcc.2002.2004. [DOI] [PubMed] [Google Scholar]
- 62.Walker KE, Lakatta EG, Houser SR. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc Res. 1993;27:1968–77. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- 63.Jahangir A, Terzic A. KATP channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toro L, Marijic J, Nishimaru K, et al. Aging, ion channel expression, and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhou YY, Lakatta EG, Xiao RP. Age-associated alterations in calcium current and its modulation in cardiac myocytes. Drugs Aging. 1998;13:159–71. doi: 10.2165/00002512-199813020-00007. [DOI] [PubMed] [Google Scholar]
- 66.Xiao RP, Zhu W, Zheng M, et al. Subtype-specific [alpha]1- and [beta]-adrenoceptor signaling in the heart. Trends in Pharmacological Sciences China. 2006;27:330–7. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Morita N, Lee JH, Bapat A, et al. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am J Physiol Heart Circ Physiol. 2011;301:H180–91. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lompré AM, Lambert F, Lakatta EG, et al. Expression of sarcoplasmic reticulum Ca2-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ Res. 1991;69:1380–8. doi: 10.1161/01.res.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 69.Taffet GE, Tate CA. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Am J Physiol. 1993;264:H1609–14. doi: 10.1152/ajpheart.1993.264.5.H1609. [DOI] [PubMed] [Google Scholar]
- 70.Koban MU, Moorman AF, Holtz J, et al. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37:405–23. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 71.Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;275:H2087–94. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- 72.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 73.Anyukhovsky EP, Sosunov EA, Chandra P, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 74.Kawamura S, Takahashi M, Ishihara T, et al. Incidence and distribution of isolated atrial amyloid: histologic and immunohistochemical studies of 100 aging hearts. Pathol Int. 1995;45:335–42. doi: 10.1111/j.1440-1827.1995.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 75.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–93. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 76.Loeb LA, Wallace DC, Martin GM. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci U S A. 2005;102:18769–70. doi: 10.1073/pnas.0509776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Nattel S, Shiroshita-Takeshita A, Brundel BJ, et al. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Finet JE, Rosenbaum DS, Donahue JK. Information learned from animal models of atrial fibrillation. Cardiol Clin. 2009;27:45–54. viii. doi: 10.1016/j.ccl.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Lisa F, Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009;46:775–80. doi: 10.1016/j.yjmcc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seppet E, Eimre M, Peet N, et al. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Mol Cell Biochem. 2005;270:49–61. doi: 10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 83.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 84.Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal. 2003;5:503–6. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- 85.Bak MI, Wei JY, Ingwall JS. Interaction of hypoxia and aging in the heart: analysis of high energy phosphate content. J Mol Cell Cardiol. 1998;30:661–72. doi: 10.1006/jmcc.1997.0633. [DOI] [PubMed] [Google Scholar]
- 86.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 87.Lesnefsky EJ, Gudz TI, Migita CT, et al. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–28. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 88.Pepe S. Effect of dietary polyunsaturated fatty acids on age-related changes in cardiac mitochondrial membranes. Exp Gerontol. 2005;40:751–8. doi: 10.1016/j.exger.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 89.Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–15. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 90.Jahangir A, Ozcan C, Holmuhamedov EL, et al. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–86. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 91.Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–92. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 92.Olson TM, Alekseev AE, Liu XK, et al. Kv1. 5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–91. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 93.Olson TM, Alekseev AE, Moreau C, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–6. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellinor PT, Yoerger DM, Ruskin JN, et al. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–84. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 95.Andalib A, Brugada R, Nattel S. Atrial fibrillation: evidence for genetically determined disease. Curr Opin Cardiol. 2008;23:176–83. doi: 10.1097/HCO.0b013e3282fa7142. [DOI] [PubMed] [Google Scholar]
- 96.Jahangir A, Sattiaraju S, Shen WK. Senescence and arrhythmogenesis. In: Gussak I, Antzelevitch C, editors. Electrical Disease of the Heart: Genetics, Mechanisms, Treatment, and Prevention. London: Springer; 2008. pp. 247–60. [Google Scholar]
- 97.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 98.Mirza M, Shen WK, Jahangir A. Senescence and arrhythmogenesis. In: Gussak I, Antzelevitch C, editors. Electrical disease of the heart: Genetics, mechanisms, treatment, and prevention. London: Springer; 2012. (in press) [Google Scholar]
- 99.Bonnema DD, Webb CS, Pennington WR, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J Card Fail. 2007;13:530–40. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adamson PB, Barr RC, Callans DJ, et al. The perplexing complexity of cardiac arrhythmias: beyond electrical remodeling. Heart Rhythm. 2005;2:650–9. doi: 10.1016/j.hrthm.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Polyakova V, Miyagawa S, Szalay Z, et al. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allessie M. The “second factor”: a first step toward diagnosing the substrate of atrial fibrillation? J Am Coll Cardiol. 2009;53:1192–3. doi: 10.1016/j.jacc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 103.Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–74. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 104.Osranek M, Bursi F, Bailey KR, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26:2556–61. doi: 10.1093/eurheartj/ehi483. [DOI] [PubMed] [Google Scholar]
- 105.Nieuwlaat R, Eurlings LW, Cleland JG, et al. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2009;53:1690–8. doi: 10.1016/j.jacc.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 106.Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 107.Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–56. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 108.Ausma J, Coumans WA, Duimel H, et al. Atrial high energy phosphate content and mitochondrial enzyme activity during chronic atrial fibrillation. Cardiovasc Res. 2000;47:788–96. doi: 10.1016/s0008-6363(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 109.Ausma J, Dispersyn GD, Duimel H, et al. Changes in ultrastructural calcium distribution in goat atria during atrial fibrillation. J Mol Cell Cardiol. 2000;32:355–64. doi: 10.1006/jmcc.1999.1090. [DOI] [PubMed] [Google Scholar]
- 110.Dispersyn GD, Ausma J, Thone F, et al. Cardiomyocyte remodelling during myocardial hibernation and atrial fibrillation: prelude to apoptosis. Cardiovasc Res. 1999;43:947–57. doi: 10.1016/s0008-6363(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 111.Knackstedt C, Gramley F, Schimpf T, et al. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovasc Pathol. 2008;17:318–24. doi: 10.1016/j.carpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Corradi D, Callegari S, Maestri R, et al. Structural remodeling in atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:782–96. doi: 10.1038/ncpcardio1370. [DOI] [PubMed] [Google Scholar]
- 113.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 114.Xu J, Cui G, Esmailian F, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–8. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 115.Fynn SP, Garratt CJ. The effectiveness of serial cardioversion therapy for recurrence of atrial fibrillation. Eur Heart J. 2002;23:1487–9. doi: 10.1053/euhj.2002.3251. [DOI] [PubMed] [Google Scholar]
- 116.Everett TH, 4th, Li H, Mangrum JM, et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102:1454–60. doi: 10.1161/01.cir.102.12.1454. [DOI] [PubMed] [Google Scholar]
- 117.Lin CS, Pan CH. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol Life Sci. 2008;65:1489–508. doi: 10.1007/s00018-008-7408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin PH, Lee SH, Su CP, et al. Oxidative damage to mitochondrial DNA in atrial muscle of patients with atrial fibrillation. Free Radic Biol Med. 2003;35:1310–8. doi: 10.1016/j.freeradbiomed.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Myerburg RJ. Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol. 2001;12:369–81. doi: 10.1046/j.1540-8167.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 120.Geelen P, Lorga Filho A, Primo J, et al. Experience with implantable cardioverter defibrillator therapy in elderly patients. Eur Heart J. 1997;18:1339–42. doi: 10.1093/oxfordjournals.eurheartj.a015447. [DOI] [PubMed] [Google Scholar]
- 121.Panotopoulos M, Panagiotis T, Axtell K, et al. Efficacy of the implantable cardioverter-defibrillator in the elderly. J Am Coll Cardiol. 1997;29:556–60. doi: 10.1016/s0735-1097(96)00527-x. [DOI] [PubMed] [Google Scholar]
- 122.Saksena S, Mathew P, Giorgberidze I, et al. Implantable defibrillator therapy for the elderly. Am J Geriatr Cardiol. 1998;7:11–13. [PubMed] [Google Scholar]
- 123.Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing-or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 124.Penn J, Goldenberg I, Moss AJ, et al. Improved outcome with preventive cardiac resynchronization therapy in the elderly: a MADIT-CRT substudy. J Cardiovasc Electrophysiol. 2011;22:892–7. doi: 10.1111/j.1540-8167.2011.02011.x. [DOI] [PubMed] [Google Scholar]
- 125.Myerburg RJ, Castellanos A. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart Rhythm. 2006;3:235–9. doi: 10.1016/j.hrthm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 126.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 127.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 128.van Rees JB, Borleffs CJ, Thijssen J, et al. Prophylactic implantable cardioverter-defibrillator treatment in the elderly: therapy, adverse events, and survival gain. Europace. 2012;14:66–73. doi: 10.1093/europace/eur255. [DOI] [PubMed] [Google Scholar]
- 129.Jahangir A, Shen WK, Neubauer SA, et al. Relation between mode of pacing and long-term survival in the very elderly. J Am Coll Cardiol. 1999;33:1208–16. doi: 10.1016/s0735-1097(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 130.Krahn AD, Connolly SJ, Roberts RS, et al. Diminishing proportional risk of sudden death with advancing age: implications for prevention of sudden death. Am Heart J. 2004;147:837–40. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 131.Grimm W. Outcomes of elderly heart failure recipients of ICD and CRT. Int J Cardiol. 2008;125:154–160. doi: 10.1016/j.ijcard.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 132.Kocovic DZ. Cardiac resynchronization therapy and other new approaches for the treatment of heart failure in the elderly. Am J Geriatr Cardiol. 2006;15:108–13. doi: 10.1111/j.1076-7460.2006.05465.x. [DOI] [PubMed] [Google Scholar]
- 133.Foley PW, Chalil S, Khadjooi K, et al. Long-term effects of cardiac resynchronization therapy in octogenarians: a comparative study with a younger population. Europace. 2008;10:1302–7. doi: 10.1093/europace/eun263. [DOI] [PubMed] [Google Scholar]
- 134.Silva RM, Mont L, Nava S, et al. Radiofrequency catheter ablation for arrhythmic storm in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2004;27:971–5. doi: 10.1111/j.1540-8159.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 135.Scheinman MM. NASPE Survey on Catheter Ablation. Pacing Clin Electrophysiol. 1995;18:1474–8. doi: 10.1111/j.1540-8159.1995.tb06733.x. [DOI] [PubMed] [Google Scholar]
- 136.Tchou P, Jazayeri M, Denker S, et al. Transcatheter electrical ablation of right bundle branch. A method of treating macroreentrant ventricular tachycardia attributed to bundle branch reentry. Circulation. 1988;78:246–57. doi: 10.1161/01.cir.78.2.246. [DOI] [PubMed] [Google Scholar]
- 137.Stevenson WG, Khan H, Sager P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–70. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 138.Gurevitz OT, Glikson M, Asirvatham S, et al. Use of advanced mapping systems to guide ablation in complex cases: experience with noncontact mapping and electroanatomic mapping systems. Pacing Clin Electrophysiol. 2005;28:316–23. doi: 10.1111/j.1540-8159.2005.09477.x. [DOI] [PubMed] [Google Scholar]
- 139.Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol. 2005;16:S52–S58. doi: 10.1111/j.1540-8167.2005.50163.x. [DOI] [PubMed] [Google Scholar]
- 140.Pedrinazzi C, Durin O, Agricola P, et al. Efficacy and safety of radiofrequency catheter ablation in the elderly. J Interv Card Electrophysiol. 2007;19:179–85. doi: 10.1007/s10840-007-9153-6. [DOI] [PubMed] [Google Scholar]
- 141.Inada K, Roberts-Thomson KC, Seiler J, et al. Mortality and safety of catheter ablation for antiarrhythmic drug-refractory ventricular tachycardia in elderly patients with coronary artery disease. Heart Rhythm. 2010;7:740–4. doi: 10.1016/j.hrthm.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 142.Kelly P, Ruskin JN, Vlahakes GJ, et al. Surgical coronary revascularization in survivors of prehospital cardiac arrest: Its effect on inducible ventricular arrhythmias and long-term survival. J Am Coll Cardiol. 1990;15:267–73. doi: 10.1016/s0735-1097(10)80046-4. [DOI] [PubMed] [Google Scholar]
- 143.Brugada J, Aguinaga L, Mont L, et al. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol. 2001;37:529–33. doi: 10.1016/s0735-1097(00)01133-5. [DOI] [PubMed] [Google Scholar]
- 144.Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: detection by 24-hour ambulatory electrocardiography. Chest. 1982;81:302–7. doi: 10.1378/chest.81.3.302. [DOI] [PubMed] [Google Scholar]
- 145.Kennedy HL, Whitlock JA, Sprague MK, et al. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–7. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 146.Hinkle LE, Jr, Carver ST, Stevens M. The frequency of asymptomatic disturbances of cardiac rhythm and conduction in middle-aged men. Am J Cardiol. 1969;24:629–50. doi: 10.1016/0002-9149(69)90451-2. [DOI] [PubMed] [Google Scholar]
- 147.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: The Framingham Study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 148.Jouven X, Zureik M, Desnos M, et al. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med. 2000;343:826–33. doi: 10.1056/NEJM200009213431201. [DOI] [PubMed] [Google Scholar]
- 149.Frolkis JP, Pothier CE, Blackstone EH, et al. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–90. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 150.Messerli FH, Ventura HO, Elizardi DJ, et al. Hypertension and sudden death: Increased ventricular ectopic activity in left ventricular hypertrophy. Am J Med. 1984;77:18–22. doi: 10.1016/0002-9343(84)90430-3. [DOI] [PubMed] [Google Scholar]
- 151.Bigger JT, Jr, Fleiss JL, Kleiger R, et al. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–8. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 152.Huikuri HV, Mäkikallio TH, Raatikainen MJ, et al. Prediction of sudden cardiac death: appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation. 2003;108:110–5. doi: 10.1161/01.CIR.0000077519.18416.43. [DOI] [PubMed] [Google Scholar]
- 153.Stevenson WG, Stevenson LW, Middlekauff HR, et al. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993;88:2953–61. doi: 10.1161/01.cir.88.6.2953. [DOI] [PubMed] [Google Scholar]
- 154.Aronow WS, Ahn C, Mercando AD, et al. Prevalence and association of ventricular tachycardia and complex ventricular arrhythmias with new coronary events in older men and women with and without cardiovascular disease. J Gerontol A Biol Sci Med Sci. 2002;57:M178–80. doi: 10.1093/gerona/57.3.m178. [DOI] [PubMed] [Google Scholar]
- 155.Volpi A, Cavalli A, Turato R, et al. Incidence and short-term prognosis of late sustained ventricular tachycardia after myocardial infarction: Results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-3) Data Base. Am Heart J. 2001;142:87–92. doi: 10.1067/mhj.2001.115791. [DOI] [PubMed] [Google Scholar]
- 156.Trusty JM, Beinborn DS, Jahangir A. Dysrhythmias and the athlete. AACN Clin Issues. 2004;15:432–48. doi: 10.1097/00044067-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 157.Ornato JP, Peberdy MA, Tadler SC, et al. Factors associated with the occurrence of cardiac arrest during hospitalization for acute myocardial infarction in the second national registry of myocardial infarction in the US. Resuscitation. 2001;48:117–23. doi: 10.1016/s0300-9572(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 158.Behar S, Goldbourt U, Reicher-Reiss H, et al. Prognosis of acute myocardial infarction complicated by primary ventricular fibrillation. Am J Cardiol. 1990;66:1208–11. doi: 10.1016/0002-9149(90)91101-b. [DOI] [PubMed] [Google Scholar]
- 159.Huikuri HV, Castellanos A, Myerburg RJ. Medical progress: Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 160.Schouten EG, Dekker JM, Meppelink P, et al. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–23. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 161.Steinberg JS, Berbari EJ. The signal-averaged electrocardiogram: update on clinical applications. J Cardiovasc Electrophysiol. 1996;7:972–88. doi: 10.1111/j.1540-8167.1996.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 162.Cook JR, Flack JE, Gregory CA, et al. Influence of the preoperative signal-averaged electrocardiogram on left ventricular function after coronary artery bypass graft surgery in patients with left ventricular dysfunction. The CABG Patch Trial. Am J Cardiol. 1998;82:285–9. doi: 10.1016/s0002-9149(98)00335-x. [DOI] [PubMed] [Google Scholar]
- 163.Podrid PJ, Graboys TB. Exercise stress testing in the management of cardiac rhythm disorders. Med Clin North Am. 1984;68:1139–52. doi: 10.1016/s0025-7125(16)31089-6. [DOI] [PubMed] [Google Scholar]
- 164.Couderc JP, Zareba W, McNitt S, et al. Repolarization variability in the risk stratification of MADIT II patients. Europace. 2007;9:717–23. doi: 10.1093/europace/eum131. [DOI] [PubMed] [Google Scholar]
- 165.Bloomfield DM, Bigger JT, Steinman RC, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 166.Chow T, Kereiakes DJ, Bartone C, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820–7. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 167.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American college of cardiology/American heart association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography) J Am Coll Cardiol. 2003;42:954–70. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 168.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC Guidelines for the Clinical Use of Cardiac Radionuclide Imaging--Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42:1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 169.Azaouagh A, Churzidse S, Konorza T, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a review and update. Clin Res Cardiol. 2011;100:383–94. doi: 10.1007/s00392-011-0295-2. [DOI] [PubMed] [Google Scholar]
- 170.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wilber DJ, Garan H, Finkelstein D, et al. Out-of-hospital cardiac arrest. Use of electrophysiologic testing in the prediction of long-term outcome. N Engl J Med. 1988;318:19–24. doi: 10.1056/NEJM198801073180105. [DOI] [PubMed] [Google Scholar]
- 172.Chen LY, Jahangir A, Decker WW, et al. Score indices for predicting electrophysiologic outcomes in patients with unexplained syncope. J Interv Card Electrophysiol. 2005;14:99–105. doi: 10.1007/s10840-005-4593-3. [DOI] [PubMed] [Google Scholar]
- 173.Bachinsky WB, Linzer M, Weld L, et al. Usefulness of clinical characteristics in predicting the outcome of electrophysiologic studies in unexplained syncope. Am J Cardiol. 1992;69:1044–9. doi: 10.1016/0002-9149(92)90861-r. [DOI] [PubMed] [Google Scholar]
- 174.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 175.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009;30:2631–71. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation. 2002;105:1342–7. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 177.Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of brugada syndrome and no previous cardiac arrest. Circulation. 2003;108:3092–6. doi: 10.1161/01.CIR.0000104568.13957.4F. [DOI] [PubMed] [Google Scholar]
- 178.Nienaber CA, Hiller S, Spielmann RP, et al. Syncope in hypertrophic cardiomyopathy: multivariate analysis of prognostic determinants. J Am Coll Cardiol. 1990;15:948–55. doi: 10.1016/0735-1097(90)90222-b. [DOI] [PubMed] [Google Scholar]
- 179.Buxton AE, Lee KL, Hafley GE, et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease: An analysis of patients enrolled in the multicenter unsustained tachycardia trial. Circulation. 2002;106:2466–72. doi: 10.1161/01.cir.0000037224.15873.83. [DOI] [PubMed] [Google Scholar]
- 180.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. doi: 10.1007/s10840-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 181.Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59:1995–2027. doi: 10.1016/j.jacc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 182.Skanes AC, Healey JS, Cairns JA, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–36. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 183.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 184.Dubner S, Auricchio A, Steinberg JS, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs) Europace. 2012;14:278–93. doi: 10.1093/europace/eur303. [DOI] [PubMed] [Google Scholar]
- 185.Curtis AB. Update on the clinical management of atrial fibrillation: guidelines and beyond. Postgrad Med. 2011;123:7–20. doi: 10.3810/pgm.2011.11.2491. [DOI] [PubMed] [Google Scholar]
- 186.Savelieva I, Kakouros N, Kourliouros A, et al. Upstream therapies for management of atrial fibrillation: Review of clinical evidence and implications for european society of cardiology guidelines. Part I: Primary prevention. Europace. 2011;13:308–28. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]