Abstract
In this work, we investigated the effects of lowered oxygen tension (20% and 5% O2) on the chondrogenesis and hypertrophy of articular chondrocytes (ACs), mesenchymal stem cells (MSCs) and their co-cultures with a 30:70 AC:MSC ratio. Cells were cultured for six weeks within porous scaffolds, and their cellularity, cartilaginous matrix production (collagen II/I expression ratio, hydroxyproline and GAG content) and hypertrophy markers (collagen X expression, ALP activity, calcium accumulation) were analyzed. After two weeks, hypoxic culture conditions had expedited chondrogenesis with all cell types by increasing collagen II/I expression ratio and matrix synthesis by ~2.5 – 11 and ~1.5 – 3.0 fold, respectively. At later times, hypoxia decreased cellularity but had little effect on matrix synthesis. ACs and co-cultures showed similarly high collagen II/I expression ratio and GAG rich matrix formation, whereas MSCs produced the least hyaline cartilage-like matrix and obtained a hypertrophic phenotype with eventual calcification. MSC hypertrophy was further emphasized in hypoxic conditions. We conclude that the most promising cell source for cartilage engineering was co-cultures, as they have a potential to decrease the need for primary chondrocyte harvest and expansion while obtaining a stable highly chondrogenic phenotype independent of the oxygen tension in the cultures.
Keywords: Cartilage tissue engineering, Chondrocyte, Co-culture, Gene expression, Mesenchymal stem cell
1. Introduction
Articular cartilage lesions and progressive cartilage loss caused by degenerative disease are major contributors to disability in the United States. Furthermore, cartilage related problems are expected to increase dramatically in the future due to the aging population and increasing incidence of obesity [1]. Cartilage is an avascular tissue with low metabolic activity and cell density, consisting mainly of collagen- and proteoglycan-rich extracellular matrix [2]. Poor innate access to reparative cell sources results in low regeneration capacity of damaged cartilage, and advanced treatment options relying on auto- and allografts have therefore been widely studied [3]. Current clinical therapies are unfortunately inadequate to regenerate the native hyaline cartilage structure in articulating joints, but instead produce mechanically inferior fibrocartilage highly increasing the risk of treatment failure in the long term. Therefore, novel cell sources and culture methods are needed before cell based therapies can reach their full potential [4].
The most common cell sources for cartilage engineering include articular chondrocytes (ACs) and mesenchymal stem cells (MSCs). Both of these cell types have inherent advantages and disadvantages. Autologous ACs have now been used for two decades in clinic with promising results [5]. However, the harvest of primary cells by cartilage biopsy is related with donor site morbidity [6] and the cultured cells lose their chondrocyte phenotype rapidly upon monolayer expansion [7]. MSC harvest, usually from bone marrow or adipose tissue, and subsequent expansion poses fewer complications, but effective chondrogenesis requires extensive differentiation in the presence of growth factors [8]. More problematically, the MSC differentiation does not arrest at the chondrocyte level, but can lead to hypertrophy and formation of calcified tissue replicating the process of endochondral ossification [9]. At present, there have been only few published investigations on the use of MSCs to treat cartilage defects in humans [10]. Co-cultures of ACs and MSCs have recently been proposed to mitigate problems associated with the corresponding monocultures. Based on the current in vitro evidence, such co-cultures are highly chondrogenic, demonstrate decreased hypertrophy and have a potential to minimize the need for chondrocyte harvest [11].
Due to the lack of vasculature, articular cartilage obtains nutrients and oxygen mainly by diffusion from synovial fluid. Oxygen tension within the tissue is estimated to range from approximately 7% on the joint surface to as low as 1% close to subchondral bone [12]. This range of hypoxic conditions is known to play crucial role in cartilage physiology and endochondral bone development in vivo, largely through the actions of HIF transcription factors [13]. Consequently, the effects of low oxygen tension have been investigated also on the maintenance and gaining of chondrogenic phenotype in vitro. Hypoxic cultures can promote the restoration of chondrogenic phenotype in passaged ACs [14] and increase the differentiation of MSCs toward chondrogenic lineage [15]. Changes on both the gene expression and protein levels can be seen already after one day of hypoxia [16, 17]. Still, hypoxic cultures have not been widely adopted in cartilage engineering applications so far.
Hypoxia and AC-MSC co-cultures have previously been studied separately as potential approaches to produce highly chondrogenic tissue engineering constructs in vitro, but to our knowledge there are no published records evaluating their combined effects on (re)differentiation and hypertrophy of chondrogenic 3D cultures. The aim of the current study was to investigate the effects of hypoxia on the chondrogenesis of AC-MSC co-cultures within porous polymer scaffolds. Furthermore, the roles of different cell sources and hypoxia in the stable maintenance of chondrogenic phenotype during long term 3D cultures were evaluated.
2. Materials and Methods
2.1. Scaffold preparation
Electrospun nonwoven poly(ε-caprolactone) (PCL) microfiber mats were fabricated with an average fiber diameter of approximately 10 μm as previously described [18]. The mats were inspected for consistent fiber morphology and diameter (9.5 ± 1.7 μm) using scanning electron microscopy and stored in a desiccator until use. Scaffolds were prepared by die-punching 8-mm-diameter discs from the electrospun mats using a dermal biopsy punch. The scaffolds (1.1 ± 0.1 mm thickness) were then sterilized by exposure to ethylene oxide (Andersen Sterilizers, Haw River, NC) for 14 h and aerated overnight to remove residual fumes. In order to eliminate air bubbles and to improve cell adhesion, scaffolds were prewetted by centrifugation through a graded series of ethanol (70 % – 20 %), followed by two rinses in phosphate buffered saline (PBS), and incubation in general culture medium (high-glucose DMEM, 10 % fetal bovine serum (BenchMark FBS; Gemini Bio-Products, West Sacramento, CA), penicillin/streptomycin/fungizone (PSF)) for four days.
2.2. Cell harvest and expansion
Bovine MSCs and ACs were harvested from 7-10 day old calves (Research 87, Boylston, MA), less than 24 h after slaughter using previously established protocols [19]. Briefly, marrow isolations from tibiae and femora were plated onto culture flasks and allowed to adhere for 48 h before washing with PBS to remove non-adherent cells and blood clots. Cultures were maintained in general medium until confluent and passaged for sub-cultures. After two passages, MSCs from a minimum of three animals were pooled, aliquoted, and cryopreserved in freezing medium (DMEM with 20 % FBS and 10 % dimethyl sulfoxide). Articular cartilage was collected from the femoral condyle area, minced to approximately 1×1×1 mm, washed with PBS and digested in chondrocyte culture medium (DMEM, 10% FBS, 1% non-essential amino acids, 50 μg / mL ascorbic acid, 46 μg / mL L-proline, 20 mM HEPES, PSF) containing 2 mg / mL collagenase type II (Worthington biochemical corporation, Lakewood, NJ). Digestions were incubated on a shaker table at 37 °C for 16 hours and passed through cell strainers. Primary ACs from a minimum of three animals were pooled, aliquoted, and cryopreserved in freezing medium.
2.3. Cell seeding and chondrogenic culture
The frozen AC and MSC aliquots were thawed and sub-cultured for one or two passages to establish seeding suspensions of 1.25×106 cells / mL. In preparation for cell seeding, prewetted scaffolds were press-fitted into custom made cylindrical polycarbonate cassettes (with the height, inner and outer diameters of 13 mm, 7.9 mm and 19 mm, respectively) designed to confine the seeding suspension. Cells (in ~200 μL of general medium) were then pipetted onto each scaffold to achieve a final seeding density of 4.5×106 cells / mL scaffold volume. Scaffolds were seeded with pure AC and MSC populations as well as their combination with a 30:70 AC:MSC ratio. The co-culture ratio was chosen based on our previous studies [19]. Cells were allowed to adhere for 4 h before culture medium was gently added to completely cover the cassettes. Scaffolds were removed from the cassettes after 24 h and placed in 12 well culture plates with 4 mL of serum free chondrogenic medium (high-glucose DMEM, 1 % ITS+ premix [BD Biosciences, San Jose, CA], 50 μg / mL ascorbic acid, 40 μg / mL L-proline, 10 mM Na-beta-glycerophosphate, 100 nM dexamethasone, 10 ng / mL TGF-β3 [PeproTech, Rocky Hill, NJ], PSF). Cultures were divided into normoxic (20 % O2) and hypoxic (5 % O2) conditions, and half of the medium was replenished two to three times a week. Four replicate scaffolds were harvested after 0, 14, 28 and 42 days of chondrogenic culture and washed with PBS.
2.4. Real-time reverse transcription polymerase chain reaction
Total RNA was isolated from pelleted cell seeding stocks and minced (approximately 1×1×1 mm pieces) 3D constructs cultured for 14 days, using RNeasy mini kit (Qiagen, Valencia, CA). Briefly, samples were immersed in lysis buffer and incubated at room temperature for 30 min with periodic vortexing. Cell lysate was then passed through a QIAshredder homogenization column and stored at −80 °C until further processing. An equal volume of 70 % ethanol was added to thawed lysates and RNA isolation was continued following the animal cell protocol provided by the manufacturer. Reverse transcription was then carried out to synthesize cDNA from purified RNA samples using Oligo(dT) primers (Promega, San Luis Obispo, CA) and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). Finally, cDNA was subjected to real-time PCR (Applied Biosystems 7300 Real-Time PCR System, Foster City, CA) using SYBR Green detection (PerfeCTa SYBR Green FastMix, ROX; Quanta Biosciences, Gaithersburg, MD) with custom designed primers (Integrated DNA Technologies, Coralville, IA).
Primer sequences are given in Table 1. Target gene expression was first normalized to the expression of the housekeeping gene GAPDH in the same sample (ΔCt), then to the average expression of that target gene measured in the AC group under normoxic conditions (ΔΔCt). Finally, the 2−ΔΔCt method was used to convert normalized gene expression levels to fold differences and statistics were calculated on these values [20]. Similarly, 2−ΔCt was used to calculate the ratios of collagen II / collagen I expression within individual samples.
Table 1.
Forward (F) and reverse (R) primers used for quantitative RT-PCR.
| Gene | Primer sequence | Product length | GenBank No. |
|---|---|---|---|
| Collagen type I (COL1A2) | F: 5′-CGGGTCTTGCTGGTCATCAT-3′ R: 5′-TGCACCAGGCTGTCCAATG-3′ |
125 | NM_174520.2 |
| Collagen type II (COL2A1) | F: 5′-AGTGGAAGAGCGGAGACTACTG-3′ R: 5′-GTTGGGAGCCAGGTTGTCAT-3′ |
233 | NM_001001135.2 |
| Collagen type X (COL10A1) | F: 5′-CTGAGCGATACCAAACACC-3′ R: 5′-CCTCTCAGTGATACACCTTTA-3′ |
106 | NM_174634.1 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
F: 5′-GAGTCCACTGGGGTCTTCACT-3′ R: 5′-GCGTGGACAGTGGTCATAAGTC-3′ |
251 | NM_001034034.1 |
2.5. Histology
A 3 mm biopsy punch was used to obtain individual samples from randomized scaffold location for each analysis. One biopsy sample from each scaffold was fixed for histology in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA), then immersed in 70% ethanol prior to embedding in HistoPrep freezing medium (Fisher Scientific). Frozen sections 5 μm thick were cut using a cryostat (Leica CM 1850 UV; Leica Biosystems Nussloch GmbH, Germany), mounted onto glass slides, and placed on a 42 °C slide warmer to facilitate adhesion. Sections were stained with Alcian Blue, Picrosirius Red and Fast Green to visualize the distribution of glycosaminoglycan (GAG), collagen and cells, respectively, in the 3D constructs. Images were obtained using a light microscope with a digital camera attachment (Axio Imager.Z2 equipped with AxioCam MRc5; Carl Zeiss MicroImaging GmbH, Germany).
2.6. Biochemical assays
Two biopsy samples were pooled together from each scaffold to be used for DNA, GAG and hydroxyproline (HYP) assays. The rest of the scaffold was minced (approximately 1×1×1 mm pieces) and used for alkaline phosphatase (ALP) and calcium assays. All samples were stored at −20 °C until further processing. Thawed biopsy samples were digested in proteinase K solution (1 mg / mL proteinase K, 0.01 mg / mL pepstatin A and 0.185 mg / mL iodoacetamide in a 50 mM tris(hydroxymethyl aminomethane) – 1 mM ethylenediaminetetraacetic acid buffer, pH 7.6) in a 56 °C water bath for 16 h, whereas minced samples were immersed in distilled water. Cell and extracellular matrix components were extracted via two additional freeze-thaw cycles followed by 10 min sonication in a water bath.
DNA content of the scaffolds was determined using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Eugene, OR). Briefly, cell lysate, assay buffer and dye solution were combined in duplicates, and allowed to incubate for 10 min at room temperature. Fluorescence was measured using excitation and emission wavelengths of 485 nm and 528 nm (FL x800 Fluorescence Microplate Reader; BioTek Instruments, Winooski, VT), respectively, and DNA concentrations were determined relative to a lambda DNA standard curve.
GAG content was determined using the colorimetric dimethylmethylene blue assay [21]. Briefly, cell lysate and color reagent were combined in duplicates, and allowed to incubate for 7 min at room temperature. Absorbance at 520 nm was measured (PowerWave x340 Microplate Reader; BioTek Instruments), and GAG concentrations were determined relative to a chondroitin sulfate standard curve. For glycosaminoglycan synthetic activity, the resulting GAG amounts were normalized to the amount of DNA for each sample.
HYP content, an indicator for total collagen, was determined in a colorimetric assay [22]. Briefly, an aliquot of cell lysate was combined with an equal volume of 4 N NaOH and hydrolyzed by autoclaving for 15 min, 121 °C (approximately 50 min total processing time). The solution was neutralized with HCl and acetic acid to pH 6.5 – 7.0 and divided into duplicate reactions. Chloramine-T and p-dimethylaminobenzaldehyde solutions were added sequentially, the absorbance at 570 nm was measured using a plate reader and HYP concentrations were determined relative to a trans-4-hydroxy-L-proline standard curve. For collagen synthetic activity, the resulting HYP amounts were normalized to the amount of DNA for each sample.
ALP enzymatic activity in the cell lysates was measured using alkaline buffer solution and phosphatase substrate tablets (Sigma-Aldrich, St. Louis, MO). Briefly, cell lysate and the reagents were combined in duplicates and incubated at 37 °C for 1 h. The reaction was stopped by addition of NaOH, the absorbance at 405 nm was measured using a plate reader and ALP activity was determined relative to a p-nitrophenol standard curve. Enzymatic activities were normalized to the amount of DNA for each sample.
After determining the ALP activity, acetic acid was added into the cell lysate in a final concentration of 0.5 M and the samples were incubated at room temperature for 16 h to dissolve calcium present in the minced scaffold. Calcium content was then determined in a colorimetric assay. Briefly, sample was combined with calcium arsenazo III reagent (Genzyme, Cambridge, MA) in duplicates, the absorbance at 650 nm was measured using a plate reader and Ca2+ concentrations were determined relative to a CaCl2 standard curve.
2.7. Statistics
Results are presented as means ± standard deviations. Statistical analysis was performed with an SPSS 16.0 software package (SPSS, Chicago, IL). Biochemical assay data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test, whereas RT-PCR data were analyzed using the Kruskal-Wallis test followed by the Mann-Whitney U test. Differences were considered significant at 95 % confidence level.
3. Results
3.1. Real-time reverse transcription polymerase chain reaction
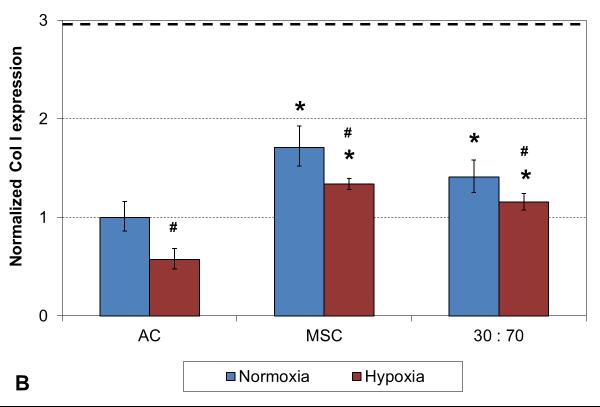
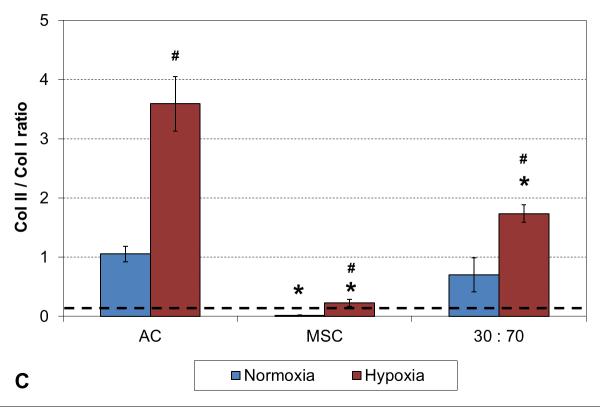
A two week culture experiment was performed to analyze early changes in chondrogenic gene expression in 3D cultures under different oxygen tensions. With all cell types expression of collagen type II was significantly higher and type I lower in hypoxia compared to normoxia, resulting in higher collagen type II-to-type I expression ratio in these conditions (Figure 1). In addition, comparison to the original monolayer expanded cell stocks used to seed the scaffolds indicated clear chondrogenic differentiation with TGF-β3 induction irrespective of the cell population used, although the AC and co-culture groups showed significantly stronger chondrogenesis than MSCs, as indicated by high collagen II expression. Furthermore, similar levels of collagen type X expression were observed with both cell stocks and all 3D constructs in normoxic conditions, whereas hypoxia increased this hypertrophy marker in the MSC and co-culture groups.
Figure 1.
RT-PCR analysis of chondrogenic and hypertrophic gene expression after 2 weeks of culture with various cell types and oxygen tensions. Collagen type II (A), type I (B), type II / I ratio (C) and collagen type X (D). Results are presented as mean ± SD with n = 4. The dashed line represents the AC stock used to seed the scaffolds. # and * denote statistically significant difference to the corresponding normoxic culture condition (same cell type) and AC group (same oxygen tension), respectively (p < 0.05).
3.2. Scaffold cellularity
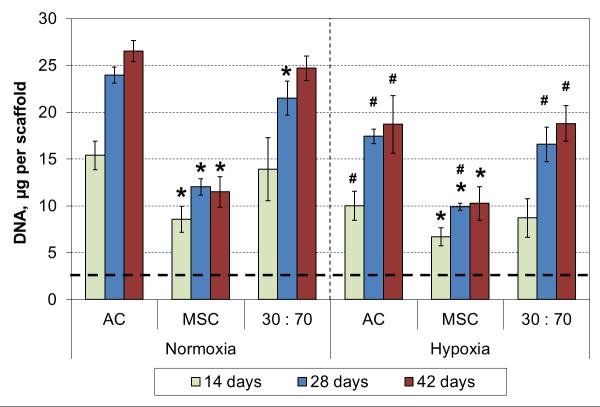
An independent six week culture was conducted to study long term effects of hypoxia in chondrogenic 3D cultures. Scaffold cellularity increased for 4 weeks, and at that point all cell types showed significantly decreased DNA contents in hypoxic conditions compared to normoxic conditions (Figure 2). Furthermore, the cellularities in the AC and co-culture groups were similar, and significantly higher than that in the MSC groups, irrespective of the oxygen tension.
Figure 2.
DNA content of cultured constructs with various cell types and oxygen tensions. Results are presented as mean ± SD with n = 4. The dashed line represents the cell stocks used to seed the scaffolds. # and * denote statistically significant difference to the corresponding normoxic culture condition (same cell type) and AC group (same oxygen tension), respectively (p < 0.05).
3.3. Collagen synthesis
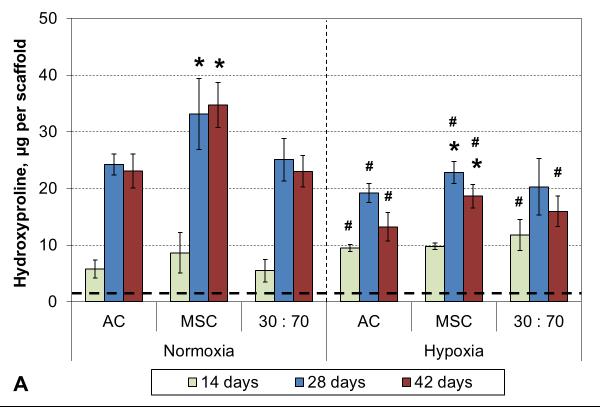
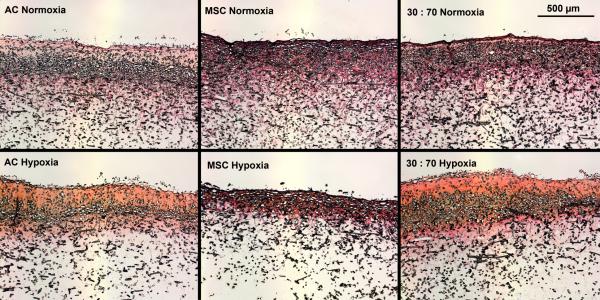
Hypoxia increased the total collagen, measured as HYP content and synthetic activity in early cultures (Figure 3). After 4 weeks, however, the situation was reversed and there was higher HYP content in normoxic than hypoxic conditions. In both conditions, MSCs showed higher levels of HYP synthesis than either ACs or co-cultures. These results were confirmed qualitatively in histological analysis using collagen-specific staining (Figures 4 and S1). Tissue formation occurred mostly within the topmost ~300 μm of and even over the porous scaffold.
Figure 3.
HYP content (A) and synthetic activity (B) with various cell types and oxygen tensions. Results are presented as mean ± SD with n = 4. The dashed line represents the cell stocks used to seed the scaffolds. # and * denote statistically significant difference to the corresponding normoxic culture condition (same cell type) and AC group (same oxygen tension), respectively (p < 0.05).
Figure 4.
Histological analysis of collagenous matrix production within porous scaffolds after 6 weeks of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Picrosirius Red and Fast Green staining.
3.4. GAG synthesis
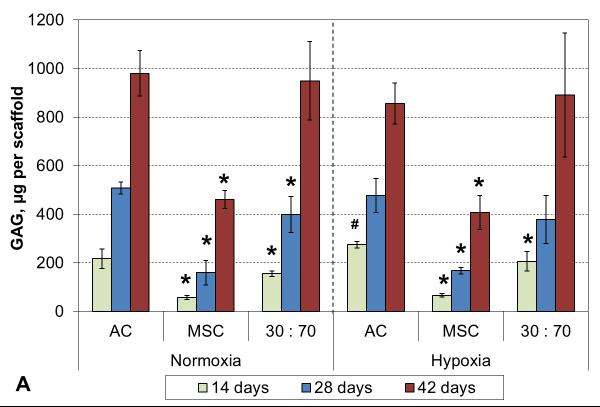
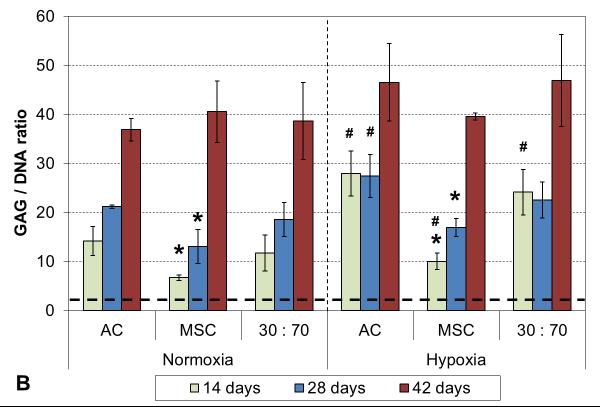
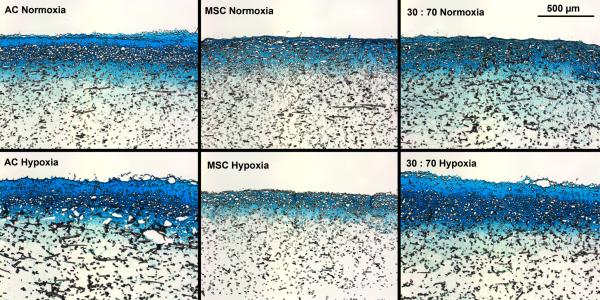
Oxygen tension had only minor effects on GAG deposition, and only in early cultures. GAG synthetic activity was significantly increased with all cell types after two weeks in hypoxia, but only the AC group showed corresponding increase also in GAG content (Figure 5). At later times, normoxic and hypoxic cultures demonstrated similar GAG contents and synthetic activities, and this was confirmed in histological evaluation (Figures 6 and S2). While GAG deposition increased gradually throughout the culture period with all cell types, MSCs always showed lower GAG contents than the corresponding AC and co-culture groups.
Figure 5.
GAG content (A) and synthetic activity (B) with various cell types and oxygen tensions. Results are presented as mean ± SD with n = 4. The dashed line represents the cell stocks used to seed the scaffolds. # and * denote statistically significant difference to the corresponding normoxic culture condition (same cell type) and AC group (same oxygen tension), respectively (p < 0.05).
Figure 6.
Histological analysis of GAG production within porous scaffolds after 6 weeks of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Alcian Blue staining.
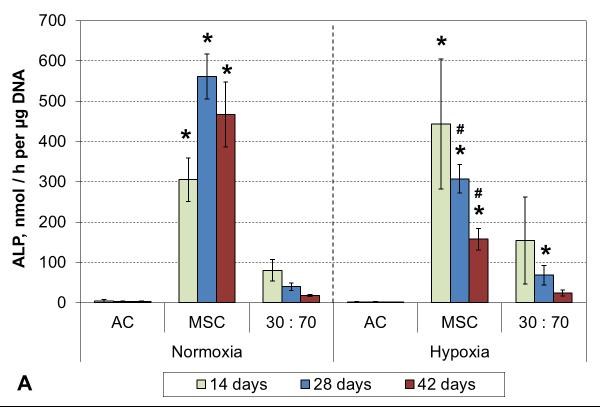
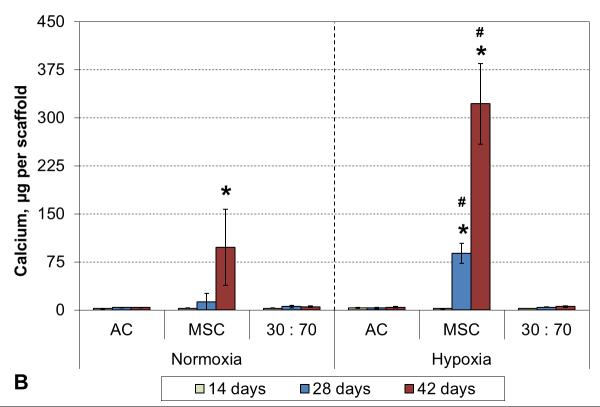
3.5 Hypertrophy markers
No ALP activity was seen in any AC culture, and while co-cultures exhibited some minor activity, that was not statistically different from the corresponding AC groups (Figure 7A). Similarly, none of the AC or the co-culture groups showed any signs of calcification. In contrast, MSC cultures showed strong induction of ALP activity already after 2 weeks of culture in both normoxic and hypoxic conditions. A decreasing trend was subsequently observed in hypoxia, whereas the activity continued to increase in normoxia and remained high even after 6 weeks. Furthermore, MSC cultures obtained a mineralizing phenotype, and this was expedited by hypoxic conditions. Calcium accumulation started by 4 and 6 weeks in hypoxia and normoxia, respectively (Figure 7B).
Figure 7.
ALP activity (A) and calcium content (B) with various cell types and oxygen tensions. Results are presented as mean ± SD with n = 4. # and * denote statistically significant difference to the corresponding normoxic culture condition (same cell type) and AC group (same oxygen tension), respectively (p < 0.05).
4. Discussion
Current articular cartilage repair strategies yield inconsistent treatment outcomes commonly resulting in the formation of fibrocartilaginous repair tissue and compromised long term functionality. Previous studies have demonstrated that hypoxic in vitro culture conditions and the use of heterotypic co-cultures can each increase the chondrogenic potential of tissue engineered 3D constructs, but there seem to be no studies investigating combined effects of these two approaches. Here, we hypothesized that AC-MSC co-cultures would show similarly increased chondrogenesis than corresponding monotypic cultures in hypoxia compared to normoxia. Furthermore, we hypothesized that the co-cultures would obtain stable chondrogenic phenotype without hypertrophy. To test these hypotheses, we conducted parallel chondrogenic 3D cultures of ACs, MSCs and their 30:70 mixture (AC:MSC) in normoxia and hypoxia. Cells were seeded on highly porous electrospun polymer scaffolds and their proliferation, cartilage-like matrix production and hypertrophy were followed for up to six weeks.
Low oxygen tension, mimicking the physiological conditions within cartilage tissue, has been proposed to increase chondrogenic potential of MSCs and chondrocytes. Hypoxia results in posttranslational stabilization of HIF transcription factors [23], and subsequent increase in chondrogenic gene expression [24, 25]. However, there is no established consensus for the optimal level or timing of the hypoxia for the purposes of cartilage engineering [26]. In recent literature, commonly used gas phase oxygen levels in hypoxic chondrogenic cultures seem to range from approximately 1% to 5% [27, 28]. It is noteworthy that there are always oxygen gradients in static cultures, and the oxygen tension within a metabolically active 3D construct is much lower than the equilibrium level near the air-liquid interface [29]. In addition, it is not clear whether hypoxic conditions have greater chondroinductive effect when applied in monolayer expansion phase or subsequent differentiating 3D cultures. With ACs, Egli et al. [27] showed enhanced cartilage formation with hypoxia expanded cells, but decreased chondrogenesis within hypoxic pellet cultures. In contrast, Ströbel et al. [30] and Schrobback et al. [14] did not see any benefits of hypoxic expansion, but reported positive effects of hypoxia in pellets. With MSCs, Adesida et al. [31] observed enhanced chondrogenesis in pellet cultures only when hypoxia was first applied in cell expansion, whereas Sheehy et al. [32] saw benefits of hypoxia only in 3D, and yet Müller et al.[33] reported best results with continuous exposure to hypoxic conditions. Furthermore, not only hypoxia but the change from a 2D to 3D culture itself is a strong chondrogenic inducer [34].
We did not compare different hypoxic regimens but chose only one set of conditions, i.e. 5% oxygen level applied in 3D cultures, and observed clear modulation of chondrogenic response in comparison to normoxic cultures. Our 3D constructs showed increased collagen type II-to-type I ratio and decreased cellularity in hypoxia with all tested cell populations. In addition, hypoxia increased matrix synthesis at early cultures but not at later times. Chondrogenic (re)differentiation upon prolonged exposure to exogenously added TGF-β3 might explain this, masking the effects of hypoxia in long term cultures [35]. Furthermore, diffusional constraints in static culture conditions commonly limit the cell growth onto the periphery of porous scaffolds and result in spontaneous development of hypoxia within the 3D constructs [36], irrespective of the surrounding atmosphere. Although medium changes were performed by replacing only half of the liquid volume at each time to retain some of the cell-produced growth factors and cytokines and to avoid major fluctuations in the pH and oxygen levels, better control of the culture environment would be desirable. Cartilage engineering might therefore benefit from bioreactor cultures to improve mass transfer while allowing precise regulation of culture pH and low oxygen conditions [37].
MSCs have garnered great attention as a potential cell source for cartilage engineering as they are relatively easy to harvest and expand, and their immunosuppressive properties might even allow the use of allogeneic cell sources [38]. Although MSCs can be readily differentiated toward chondrogenic lineage in vitro, their main mode of action in vivo might be as synthesizers of trophic factors rather than as direct producers of cartilaginous matrix and tissue [39]. Potential problems with in vitro differentiated MSCs include their unstable phenotype, often leading to the formation of fibrocartilage and even calcified tissue [40]. The propensity for terminal differentiation seems to be dependent on the MSC cell source [41], and using bone marrow derived cells this endochondral route has even been exploited in bone engineering [42]. These aspects were highlighted also in the current study, where MSC constructs had the lowest cellularity and GAG content, obtained high ALP activity, and produced collagen (type I) rich matrix with eventual mineralization. Various HIFs (1α and 2α) can have opposing effects on MSC chondrogenesis and hypertrophy [43], and their transcription and stability are differentially regulated in hypoxic conditions [44]. Current literature generally indicates that hypoxia should decrease the hypertrophic tendency of MSCs [24, 32, 45], although increased Col X and Runx2 expression has been occasionally reported [46]. In this study, MSC hypertrophy was increased in hypoxia diminishing the benefits of low oxygen conditions. This finding could be related to the evolving oxygen gradients within porous scaffolds and use of bovine cells, as MSC hypertrophy has not been previously studied in such a culture model.
Chondrogenic (re)differentiation of cultured cells was achieved in this study with a commonly used method of continuous application of dexamethasone and TGF-β in serum free culture medium. However, recent reports with both ACs [47] and MSCs [48] indicate that transient, two to three weeks, exposure might yield higher quality tissue engineered constructs with enhanced biochemical and biomechanical properties. The effects of such transient induction are not known in hypoxic conditions or in AC-MSC co-cultures and should warrant further investigations.
Co-cultures of MSCs and chondrocytes have recently emerged as a promising way to simultaneously increase the chondrogenic potential of tissue engineered constructs and to inhibit MSC hypertrophy [49, 50]. The major role of MSCs in such cultures seems to be the induction of chondrocytes to proliferate and produce cartilaginous matrix [51] while the chondrocytes can protect MSCs from hypertrophy [52]. In the current study, early induction of collagen X expression and minor ALP activity was detected with hypoxic co-cultures, but hypertrophy was soon arrested and the co-cultures never developed a calcifying phenotype. High proliferation rate of chondrocytes results in a gradual increase in the AC:MSC ratio [19, 53], likely increasing the hypertrophy inhibiting effect in long term cultures. The increased growth and redifferentiation of chondrocytes is important also because the strong correlation between monolayer expansion and loss of phenotype currently limits the applicability of autologous chondrocyte transplants. In addition, the microenvironment in injured cartilage tissue is often not only hypoxic but also inflammatory, and co-cultures have been shown to retain their chondrogenic capacity in such conditions [54]. Co-cultured 3D constructs need less chondrocytes at the seeding phase to produce similar end results than pure AC constructs, diminishing the requirements for the amount of harvested cartilage tissue and/or to the extent of monolayer expansion. Interestingly, the beneficial effects of co-cultures were not dependent on the oxygen tension, making hypoxic co-cultures an attractive culture modality for future studies.
5. Conclusions
In this work, we investigated the effects of hypoxic culture conditions on the chondrogenesis and hypertrophy of ACs, MSCs and their combinations as potential cell sources for cartilage engineering. All tested cell populations showed early enhancement of cartilaginous matrix production within porous scaffolds in hypoxia compared to normoxia, but this effect was diminished in prolonged cultures. The co-cultures were able to inhibit MSC hypertrophy in both conditions, whereas pure MSC cultures showed increased hypertrophy in hypoxia. The most promising cell source for cartilage engineering was co-cultures, as they have a potential to decrease the need for primary chondrocyte harvest and expansion while obtaining a stable highly chondrogenic phenotype independent of the oxygen tension in the cultures.
Supplementary Material
Figure S1. Histological analysis of collagenous matrix production within porous scaffolds after 2 weeks (A) and 4 weeks (B) of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Picrosirius Red and Fast Green staining.
Figure S2. Histological analysis of GAG production within porous scaffolds after 2 weeks (A) and 4 weeks (B) of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Alcian Blue staining.
Acknowledgements
This study was supported by the National Institutes of Health grant R01 AR057083.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: New insights - part 1: The disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- [2].Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28:203–15. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- [3].Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration evolution and overview. Clin Orthop. 2011;469:2696–705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012;347:613–27. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation - a review of recent developments. J Bone Joint Surg Am. 2003;85A:109–15. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- [6].Matricali GA, Dereymaeker GPE, Luyten FP. Donor site morbidity after articular cartilage repair procedures : A review. Acta Orthop Belg. 2010;76:669–74. [PubMed] [Google Scholar]
- [7].Dell’Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16–27. doi: 10.1016/s0014-4827(03)00036-3. [DOI] [PubMed] [Google Scholar]
- [8].Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: The indisputable role of growth factors. Injury-Int J Care Inj. 2012;43:259–65. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- [9].Dickhut A, Pelttari K, Janicki P, Wagner W, Eckstein V, Egermann M, et al. Calcification or dedifferentiation: Requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–26. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- [10].Tang QO, Carasco CF, Gamie Z, Korres N, Mantalaris A, Tsiridis E. Preclinical and clinical data for the use of mesenchymal stem cells in articular cartilage tissue engineering. Expert Opin Biol Ther. 2012;12:1361–82. doi: 10.1517/14712598.2012.707182. [DOI] [PubMed] [Google Scholar]
- [11].Leijten JCH, Georgi N, Wu L, Van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: Shifting from monocultures to cocultures. Tissue Eng Part B: Reviews. 2013;19:31–40. doi: 10.1089/ten.TEB.2012.0273. [DOI] [PubMed] [Google Scholar]
- [12].Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg B. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. [DOI] [PubMed] [Google Scholar]
- [13].Araldi E, Schipani E. Hypoxia, hifs and bone development. Bone. 2010;47:190–6. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schrobback K, Klein TJ, Crawford R, Upton Z, Malda J, Leavesley DI. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012;347:649–63. doi: 10.1007/s00441-011-1193-7. [DOI] [PubMed] [Google Scholar]
- [15].Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- [16].Coyle CH, Izzo NJ, Chu CR. Sustained hypoxia enhances chondrocyte matrix synthesis. J Orthop Res. 2009;27:793–9. doi: 10.1002/jor.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of sox9. Bone. 2005;37:313–22. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- [18].Dahlin RL, Meretoja VV, Ni M, Kasper FK, Mikos AG. Hypoxia and flow perfusion modulate proliferation and gene expression of articular chondrocytes on porous scaffolds. AIChE J. 2013 Available from URL: http://onlinelibrary.wiley.com/doi/10.1002/aic.13958/pdf (doi:10.1002/aic.13958)
- [19].Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33:6362–9. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2^(-delta delta ct) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [21].Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- [22].Stegeman H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- [23].Wei W, Yu XD. Hypoxia-inducible factors: Crosstalk between their protein stability and protein degradation. Cancer Lett. 2007;257:145–56. doi: 10.1016/j.canlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [24].Duval E, Bauge C, Andriamanalijaona R, Benateau H, Leclercq S, Dutoit S, et al. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012;33:6042–51. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- [25].Lafont JE, Talma S, Hopfgarten C, Murphy CL. Hypoxia promotes the differentiated human articular chondrocyte phenotype through sox9-dependent and -independent pathways. J Biol Chem. 2008;283:4778–86. doi: 10.1074/jbc.M707729200. [DOI] [PubMed] [Google Scholar]
- [26].Malda J, Martens DE, Tramper J, van Blitterswijk CA, Riesle J. Cartilage tissue engineering: Controversy in the effect of oxygen. Crit Rev Biotechnol. 2003;23:175–94. [PubMed] [Google Scholar]
- [27].Egli RJ, Bastian JD, Ganz R, Hofstetter W, Leunig M. Hypoxic expansion promotes the chondrogenic potential of articular chondrocytes. J Orthop Res. 2008;26:977–85. doi: 10.1002/jor.20603. [DOI] [PubMed] [Google Scholar]
- [28].Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Soballe K, Bunger C, et al. Combined 3d and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011;82:234–40. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, Schulz MB, et al. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- [30].Ströbel S, Loparic M, Wendt D, Schenk AD, Candrian C, Lindberg RLP, et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther. 2010;12:R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3:9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and-endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305–10. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- [33].Müller J, Benz K, Ahlers M, Gaissmaier C, Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011;20:1589–602. doi: 10.3727/096368910X564094. [DOI] [PubMed] [Google Scholar]
- [34].Caron MMJ, Emans PJ, Coolsen MME, Voss L, Surtel DAM, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2d and 3d cultures. Osteoarthr Cartilage. 2012;20:1170–8. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- [35].Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived mscs and articular chondrocytes. Osteoarthr Cartilage. 2010;18:1345–54. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [36].Lewis MC, MacArthur BD, Malda J, Pettet G, Please CP. Heterogeneous proliferation within engineered cartilaginous tissue: The role of oxygen tension. Biotechnol Bioeng. 2005;91:607–15. doi: 10.1002/bit.20508. [DOI] [PubMed] [Google Scholar]
- [37].Concaro S, Gustavson F, Gatenholm P. Kasper C, VanGriensven M, Portner R, editors. Bioreactors for tissue engineering of cartilage. Bioreactor systems for tissue engineering. Advances in biochemical engineering-biotechnology. 2009:125–43. doi: 10.1007/978-3-540-69357-4_6. [DOI] [PubMed] [Google Scholar]
- [38].Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- [39].Caplan A. Why are mscs therapeutic? New data: New insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury-Int J Care Inj. 2008;39:S58–S65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- [41].Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A. 2012;18:1161–70. doi: 10.1089/ten.tea.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–6. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by hif-2 alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–83. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- [44].Lin Q, Cong XY, Yun Z. Differential hypoxic regulation of hypoxia-inducible factors 1 alpha and 2 alpha. Mol Cancer Res. 2011;9:757–65. doi: 10.1158/1541-7786.MCR-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gawlitta D, van Rijen MHP, Schrijver EJM, Alblas J, Dhert WJA. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng Part A. 2012;18:1957–66. doi: 10.1089/ten.TEA.2011.0657. [DOI] [PubMed] [Google Scholar]
- [46].Ghone NV, Grayson WL. Recapitulation of mesenchymal condensation enhances in vitro chondrogenesis of human mesenchymal stem cells. J Cell Physiol. 2012;227:3701–8. doi: 10.1002/jcp.24078. [DOI] [PubMed] [Google Scholar]
- [47].Ng KW, O’Conor CJ, Kugler LE, Cook JL, Ateshian GA, Hung CT. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491–500. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63:148–58. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–45. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18:1542–51. doi: 10.1089/ten.TEA.2011.0715. [DOI] [PubMed] [Google Scholar]
- [52].Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- [53].Wu L, Leijten JCH, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17:1425–36. doi: 10.1089/ten.TEA.2010.0517. [DOI] [PubMed] [Google Scholar]
- [54].Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, et al. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr Cartilage. 2011;19:1210–8. doi: 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Histological analysis of collagenous matrix production within porous scaffolds after 2 weeks (A) and 4 weeks (B) of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Picrosirius Red and Fast Green staining.
Figure S2. Histological analysis of GAG production within porous scaffolds after 2 weeks (A) and 4 weeks (B) of culture with various cell types and oxygen tensions. Scale bar represents 500 μm. Alcian Blue staining.