Abstract
High-Performance Liquid Chromatography (HPLC) simulation software has long been recognized as an effective educational tool, yet we found that existing HPLC simulators are either too expensive, out-dated, or lack many important features we deemed necessary to make them widely useful for educational purposes. Here we describe a free, open-source HPLC simulator we developed that we believe meets this need. The web-based simulator is uniquely sophisticated, yet accessible to a diverse user group with varied expertise in HPLC. It features intuitive controls and indicators for a wide range of experimental conditions, and it displays a graphical chromatogram to provide immediate feedback when conditions are changed. The simulator can be found at hplcsimulator.org. At that website, we also provide a number of example problem sets that can be used by educators to more easily incorporate the simulator into their curriculum. Comments from students who used the simulator in an undergraduate Analytical Chemistry class were very positive.
Keywords: Analytical Chemistry, Chromatography, HPLC, Computer-Based Learning, Internet / Web-Based Learning, Upper-Division Undergraduate, Graduate Education
Introduction
High-Performance Liquid Chromatography (HPLC) is an important part of the undergraduate chemistry curriculum, but it can be a difficult technique to master. This difficulty can be attributed to the large number of often interrelated experimental variables that influence the outcome of each chromatographic analysis. For a student to gain an intuitive feel for how the important experimental parameters and their interplay affect HPLC separations, they would need to perform a large number of experiments. Given the time it takes to run individual separations and the constraints of a typical analytical chemistry laboratory session, it is unreasonable, if not impossible, for students to gain this type of experience.
Instead, computer-based HPLC simulators have long been used as a “lab” exercise that students can use to learn about and experience the fundamental principles of chromatography.1–5 HPLC simulators offer the advantages that they are easy to use, relatively inexpensive, there is no risk of damage to the instrumentation, no consumption of supplies, less supervision is required, and because simulators provide instant feedback, it can be more engaging than running real HPLC instruments. Of course, we are not suggesting that such simulators should be a replacement for using real HPLC instrumentation in the lab. Rather, they should be used as a supplementary tool to enhance the value of time spent on a real instrument. HPLC simulators allow students to explore the fundamental principles of HPLC more efficiently than they could in the lab.
For these reasons, we set out to incorporate an HPLC simulator into our own undergraduate and graduate course curricula. Several commercial HPLC simulators were available including Drylab (Molnar-Institute),6 LC & GC Simulator (Advanced Chemistry Development),2 and ChromSword (Iris Tech).7 While they are high-functioning, they were outside our budget. Even if we could have purchased them, we would have preferred to introduce students to a resource that they could continue to use after they graduate and no longer have ready access to our computer facilities.
Therefore, we set out to find a suitable HPLC simulator that was either free or low-cost. After an exhaustive internet and literature search, we found six HPLC simulators (Table 1). Two of those simulators stand out. HPLCSIM4 is an unusually sophisticated simulator. It handles both isocratic and gradient elution and gives control over a wide range of experimental parameters. HPLC for Windows5,8 is another notable HPLC simulator. It also handles a range of experimental conditions, but it puts a stronger emphasis on the practical considerations of running HPLC equipment (e.g. making sure to flip the power switch on first). Though potentially useful, both of these pieces of software run only on 32-bit Windows. As 64-bit computers continue to become the norm, the software is destined to become obsolete.
Table 1.
Free and Low-Cost HPLC Simulators
| Name | Web Address | Format |
|---|---|---|
| HPLCSIM | http://edu.chem.tue.nl/ce/simulations.htm | DOS application |
| HPLC for Windows | http://www.jce.divched.org/JCESoft/Programs/Collections/ACC/contents.html | Windows application |
| HPLC Simulation | http://www.colby.edu/chemistry/NMR/hplc.html | Web page (HTML) |
| HPLC Simulation | http://www.kabyn.com/hplc/index.php | Flash application |
| Chromulator | http://www.ohio.edu/people/gu/CHROM/ | Windows application |
| Chromatographic Separations | http://www.chem.uoa.gr/applets/AppletChrom/Appl_Chrom2.html | Java Applet |
Evidently there was a need in the educational community for a free HPLC simulator that is both sophisticated and able to run on modern operating systems, so we developed one ourselves. In this article, we describe a new, free, open-source HPLC simulator we created and made available at hplcsimulator.org,9 where we also provide several example problem sets to help instructors easily incorporate the simulator into their own curriculum. We discuss the key features of the HPLC simulator, other resources available on the website, an example of its use in an analytical chemistry class, and in the supporting information section we discuss the simulator's inner workings.
Calculations
The calculations performed by the HPLC simulator are given in the supporting information for interested readers and for reference, but it is unnecessary to understand them to be able to use the HPLC simulator; even someone new to HPLC should find it easy to use and intuitive.
In short, the HPLC simulator calculates gradient or isocratic elution chromatograms based on our experimental measurements of the retention behavior of 22 compounds on an Agilent Zorbax SB-C8 column.10 Each time any user-controlled experimental parameter is changed (e.g. temperature, flow rate, etc.), the HPLC simulator recalculates each of the simulated parameters (e.g. retention time, backpressure, etc.) and then redraws the chromatogram.
Experimental
Retention measurements
A Hewlett Packard (Palo Alto, CA) 1090 HPLC system and an Agilent (Santa Clara, CA) Zorbax SB-C8 column were used for all experiments. Mixtures of 3 or 4 solutes were generally injected at once. Solvents were mixed online.
In a typical experiment, k was measured for each compound at four eluent compositions (e.g. 20, 30, 40, and 50% organic modifier in water). From a linear fit to these measurements, we determined log kw and S in Eq. 8 at one temperature. Then the temperature was raised and the retention of each compound was measured at another four eluent compositions; the eluent compositions were slightly weaker to maintain useful k values (>1). A second set of log kw and S were calculated at the new temperature. Lastly, values of A’, B’, a’, and b’ were calculated for each compound from the two values of log kw and S (Eq. 9 and 10). These values are used by the HPLC simulator to calculate retention times under a range of temperatures and solvent compositions.
Software
The HPLC simulator was written in Java (Oracle Corporation, Redwood Shores, CA) for compatibility with the version 1.6.× compiler. Additional details on included libraries, how to compile the software, and a link to the source code can be found at hplcsimulator.org in the Development section.
Results and Discussion
Features
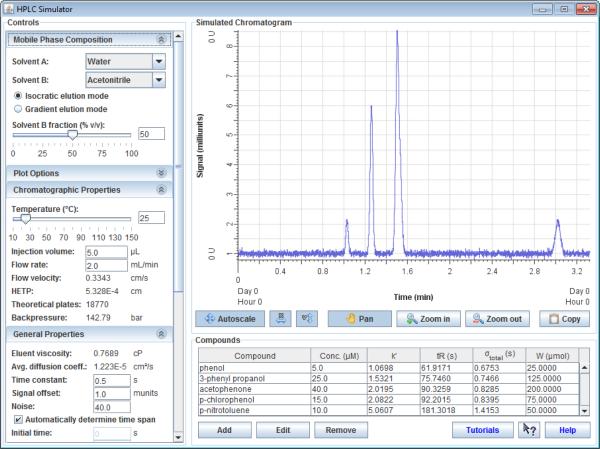
The new HPLC simulator (version 1.1.2 at this time) is located at hplcsimulator.org (Fig 1).9 It can be launched directly from an internet browser or offline, and runs on both Windows and Mac operating systems. The only notable system requirement is that the Java Runtime Environment (version 1.6.0 or above) must be installed. The vast majority of computers already have it installed (though it may need to be updated), but in the case that it is not installed or out of date, the website automatically directs the user to the website where the latest version can be downloaded.
Figure 1.
The HPLC simulator interface
The simulator features controls and indicators for a wide variety of experimental parameters and displays a graphical, simulated chromatogram. It provides immediate feedback any time experimental parameters are changed by updating all of the indicators and redrawing the chromatogram - in the case of the slider controls, the chromatogram and indicators update while the slider bar is being dragged. It includes controls for:
Organic modifier selection (currently acetonitrile or methanol)
Temperature
Injection volume
Flow rate
Detector time constant
Noise level
Column length and diameter
Particle size
Interparticle and intraparticle porosity
Reduced van Deemter terms
Sample composition (22 compounds to choose from)
% organic modifier in eluent (in isocratic mode)
Gradient program (may enter complex gradient programs with an unlimited number of steps)
Non-mixing dwell volume (in gradient mode)
Mixing dwell volume (in gradient mode)
and indicators for:
Flow velocity (open tube, interstitial, chromatographic, and reduced)
Total porosity
HETP
Reduced plate height
Number of theoretical plates
Backpressure
Eluent viscosity
Average analyte diffusion coefficient
Void volume
Void time
Retention factor of each solute
Retention time of each solute
Peak width of each solute
Since in gradient elution mode, some of those parameters change during the run, the simulator can also draw a second plot that shows any one of the following parameters as a function of time:
% organic modifier at the column inlet
Backpressure
Mobile phase viscosity
Retention factor of a selected compound
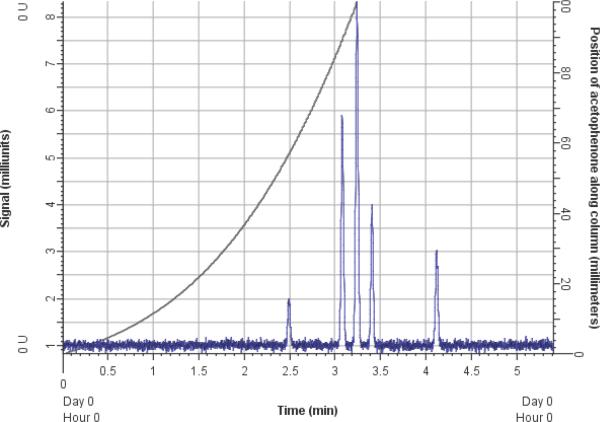
Position of a selected compound along the column (see Fig 2)
The simulator also features online help to explain the function of the controls and how values are calculated.
Figure 2.
A screenshot of the simulated chromatogram (blue line; left y-axis shows intensity) showing a plot of the position of a compound (the middle peak) along the column as a function of time (gray line; right y-axis shows position) during a 5 min gradient from ϕ = 0.05 to 0.95.
We must mention that our goal in developing the HPLC simulator was to design a tool that could be used to teach the fundamental principles of HPLC, not to teach the more practical aspects of operating a real HPLC instrument. For example, we omitted on/off switches, overpressure warnings, time delays (chromatograms are drawn instantly), etc. We also made no attempt to simulate any specific type of real-life detector. Instead, we simulate an ideal, concentration-sensitive detector that gives a response equal to the total concentration of compounds eluting from the column.
In addition to the simulator itself, the website also offers a number of other useful resources. Under the “Educational Resources” tab, we offer four problem sets that were designed to be used in undergraduate analytical chemistry courses. They cover a range of concepts and instructors are welcome to use them as they are or modify them however they wish. Under the “Forum” tab is a discussion forum where users can publicly ask questions, share ideas, or request additional features. Finally, the “Development” tab contains all of the files and information one would need to view or make modifications to the source code.
An example of a classroom exercise involving the HPLC simulator
One effective way to use the simulator is to introduce it in class before discussing solvent strength in reverse phase separations. (We have used the following exercise in both a Quantitative Analysis course comprised of junior and senior chemistry and biochemistry majors and in a senior level Instrumental Analysis course.) The students are first given the worksheet created by Mabbott (which can be downloaded under the Resources tab) that asks them to go to the HPLC simulator website and adjust the fraction of organic solvent, ϕ, to see what effect it has on a chromatogram containing five compounds. Students can easily find a value of ϕ that gives good resolution and they begin to see that small changes in ϕ can have a significant effect on retention and selectivity. They can also change particle size and see the effect on peak width and pressure. These are excellent lessons in themselves, but the simulator also provides an opportunity to generate data quickly, permitting one to build a predictive model. Such an exercise can be valuable practice in learning the process of doing science.
We then ask the students to find a mathematical relationship between the retention of a compound and the organic solvent fraction, ϕ. Is it linear? Is quadratic? What is it? Knowledge about how components in a mixture will behave could save us time finding the optimum separation conditions in the lab. The students gather data for one compound and see what type of equation fits. Six or seven data points between ϕ = 0.20 and 0.80 is enough to get a good idea. The students can easily evaluate the retention factor, k, enter it into a spreadsheet with the corresponding value of ϕ, and fit the data with an equation. This is a very good point to talk about fitting different models to data using the regression feature in the spreadsheet. What constitutes a good fit? Some may stop with a linear model, but a little discussion about residuals helps them see that the residuals follow a pattern with that choice. They are not random; the model is not very good. Soon someone discovers that k = ae-bϕ works best (it produces small random residuals).
We can take the discussion further. Let's assume that the retention of all compounds follow an exponential function of ϕ (of course, each has its own set of constants for a and b). How could we find the equations for the other four compounds in the mixture easily? Sometimes it helps to point out that if k = ae-bϕ, then ln(k) = ln(a) – bϕ. At minimum, all we need is data from two experiments in order to evaluate the parameters for each compound (of course, collecting more data points would improve the precision of the measured parameters). As a class we find the slope and intercept for each compound linking ln(k) and ϕ. Now we can make predictions!
Laub and Purnell11,12 demonstrated a strategy called “window analysis” that can be used here. It finds favorable conditions using selectivity factors for the pair of compounds in a mixture that are the most difficult to separate. With our parameters we can calculate ln(k) for each compound. We ask the students to generate a table of ln(k) at small increments in ϕ. (A partial table is shown in Table 2.) At each value of ϕ we find the most similar values of ln(k) by inspection, calculate the difference and then its absolute value. The result is ln(k2)-ln(k1 )= ln(α) where α is the selectivity factor. In other words, we have found the smallest value for the selectivity factor among these compounds at the specified value of ϕ. A plot of the minimum ln(α)versus ϕ produces triangular “windows.” The selectivity is the most favorable for separation under the conditions associated with the peak of the tallest window (in Table 2, the optimal condition is bolded and italicized). The students find that these conditions match those found by trial and error. It takes only 10-15 minutes to become comfortable with the simulator and nearly an hour to bring the class to the point of introducing window analysis. The actual construction of the window plot is assigned as homework following the session. Of course, this approach neglects any consideration of band spreading. However, that point makes an excellent place to launch into the investigation of parameters that influence bandwidth, something that can also be explored with the simulator (even before looking at the van Deemter equation).
Table 2.
Calculation of Minimum Selectivity at Various Solvent Compositions for Window Analysis
| Compound a | Compound b | Compound c | Compound d | Compound e | Minimum selectivity (α) | Closest pair | |
|---|---|---|---|---|---|---|---|
| ϕ | ln(ka) | ln(kb) | ln(kc) | ln(kd) | ln(ke) | ln(k2)-ln(k1) | |
| 0.100 | 3.662 | 4.707 | 4.920 | 5.403 | 4.644 | 0.063 | b and c |
| 0.120 | 3.534 | 4.556 | 4.772 | 5.243 | 4.512 | 0.044 | b and c |
| 0.140 | 3.407 | 4.405 | 4.624 | 5.083 | 4.380 | 0.025 | b and c |
| 0.160 | 3.280 | 4.254 | 4.476 | 4.923 | 4.249 | 0.006 | b and e |
| 0.180 | 3.153 | 4.103 | 4.329 | 4.764 | 4.117 | 0.014 | b and e |
| 0.200 | 3.025 | 3.952 | 4.181 | 4.604 | 3.985 | 0.033 | b and e |
| 0.220 | 2.898 | 3.801 | 4.033 | 4.444 | 3.853 | 0.052 | b and e |
| 0.240 | 2.771 | 3.651 | 3.885 | 4.284 | 3.722 | 0.071 | b and e |
| 0.260 | 2.643 | 3.500 | 3.737 | 4.124 | 3.590 | 0.090 | b and e |
| 0.280 | 2.516 | 3.349 | 3.590 | 3.965 | 3.458 | 0.109 | b and e |
| 0.300 | 2.389 | 3.198 | 3.442 | 3.805 | 3.326 | 0.116 | c and e |
| 0.320 | 2.262 | 3.047 | 3.294 | 3.645 | 3.195 | 0.099 | c and e |
| 0.340 | 2.134 | 2.896 | 3.146 | 3.485 | 3.063 | 0.083 | c and e |
| ⁞ | ⁞ | ⁞ | ⁞ | ⁞ | ⁞ | ⁞ | ⁞ |
| 0.800 | -0.793 | -0.576 | -0.253 | -0.190 | 0.032 | 0.063 | c and d |
A test of the pedagogical effectiveness of the HPLC simulator in an undergraduate Analytical Chemistry course
We performed a small-scale experiment with one of our undergraduate Analytical Chemistry classes to test the pedagogical effectiveness of using the simulator to teach some of the fundamental concepts of HPLC. All 28 students in the class were given a short homework assignment, but only half of them were given access to the HPLC simulator. Those students who were told about the simulator were selected randomly and given no training or special instructions in the operation of the software. The next day, they were all given a follow up quiz to assess their understanding of the material. The mean score of those students who used the simulator was 12.5/15 while those who did not use the simulator had a mean score of 11.7/15 (significant at the 67% confidence level). They were also asked to rate their confidence in their answers to the quiz questions on a scale of 1 to 10 (10 being the most confident). The mean confidence of those students who used the simulator was 7.9/10 while for those who did not it was 6.5/10 (significant at the 93% confidence level). These results were encouraging, but larger, more thorough studies would help to better understand more effective ways to incorporate the simulator into the classroom.
Comments written in by students who used the simulator were overwhelmingly positive. Not one student expressed difficulty in using the controls, making changes to the conditions, or understanding the information output by the simulator. The comments mainly focused on the HPLC simulator being easy to use and intuitive, and that they appreciated being able to see the effect of a change in conditions on the chromatogram. We continue to look for new and more effective ways to use the HPLC simulator in course curriculum and we welcome any suggestions.
Conclusions
The described HPLC simulator is a highly functional, easy-to-use tool that can be used for teaching a great number of important fundamental concepts in liquid chromatography. Other free and low-cost HPLC simulators exist, but they have significant shortcomings that limit their utility. We believe that the free, web-based software discussed in this manuscript overcomes those limitations and could find broad use in the classroom. Moreover, the downloadable problem sets lower the barrier for instructors to incorporate the simulator into their curriculum.
Students who used the HPLC simulator in a course had overwhelmingly positive comments and we expect the HPLC simulator will offer many benefits to both students and instructors. It provides a medium for instructors to easily demonstrate theoretical concepts, it quickly gives students an intuitive feel for HPLC before entering the lab, and it acquaints students with an online resource that could be a useful aid in their future work.
We will continue to improve the simulator and update it regularly. High priorities include adding more stationary phases (including normal phases), adding more compounds, and simulating peak tailing. We welcome any comments, suggestions, and feature requests on the hplcsimulator.org discussion forum. We have also made the source code publicly available on the website so that users may customize the software however they wish.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health [R01GM098290], the National Science Foundation [IOS-0923960 and MCB-0725149], the Minnesota Agricultural Experiment Station, and the Gordon and Margaret Bailey Endowment for Environmental Horticulture for financial support.
Footnotes
Supporting Information
Explanation of the chromatographic parameters in the HPLC simulator and their calculation.
Literature Cited
- 1.Rittenhouse RC. J. Chem. Educ. 1988;65:1050. [Google Scholar]
- 2.Stone DC. J. Chem. Educ. 2007;84:1488. [Google Scholar]
- 3.Bowater IC, McWilliam IG. J. Chem. Educ. 1994;71:674. [Google Scholar]
- 4.Reijenga JC. J. Chromatogr. A. 2000;903:41–48. doi: 10.1016/s0021-9673(00)00881-5. [DOI] [PubMed] [Google Scholar]
- 5.Shalliker RA, Kayillo S, Dennis GR. J. Chem. Educ. 2008;85:1265. [Google Scholar]
- 6.Imre M. J. Chromatogr. A. 2002;965:175–194. doi: 10.1016/s0021-9673(02)00731-8. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt EF, Lukulay P, Galushko S. J. Chromatogr. A. 2006;1107:79–87. doi: 10.1016/j.chroma.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Rittenhouse RC. J. Chem. Educ. 1995;72:1086. [Google Scholar]
- 9. [Oct 27, 2012];hplcsimulator.org - the free, open-source HPLC simulator. http://www.hplcsimulator.org.
- 10.Choo T. Thesis (Ph.D.) University of Minnesota; 1994. Lay Study of retention mechanism in reversed phase liquid chromatography. [Google Scholar]
- 11.Laub RJ, Purnell JH. Journal of Chromatography A. 1975;112:71–79. [Google Scholar]
- 12.Laub RJ, Purnell JH. Anal. Chem. 1976;48:799–803. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.