Abstract
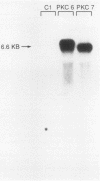
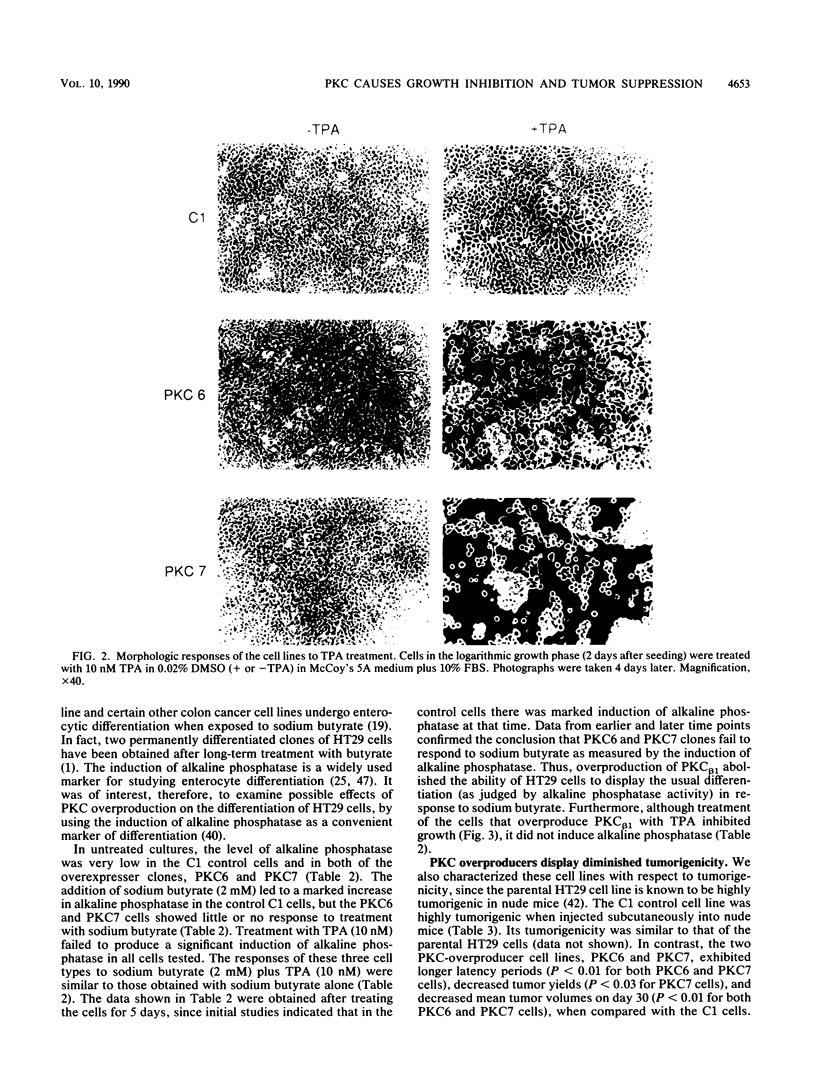
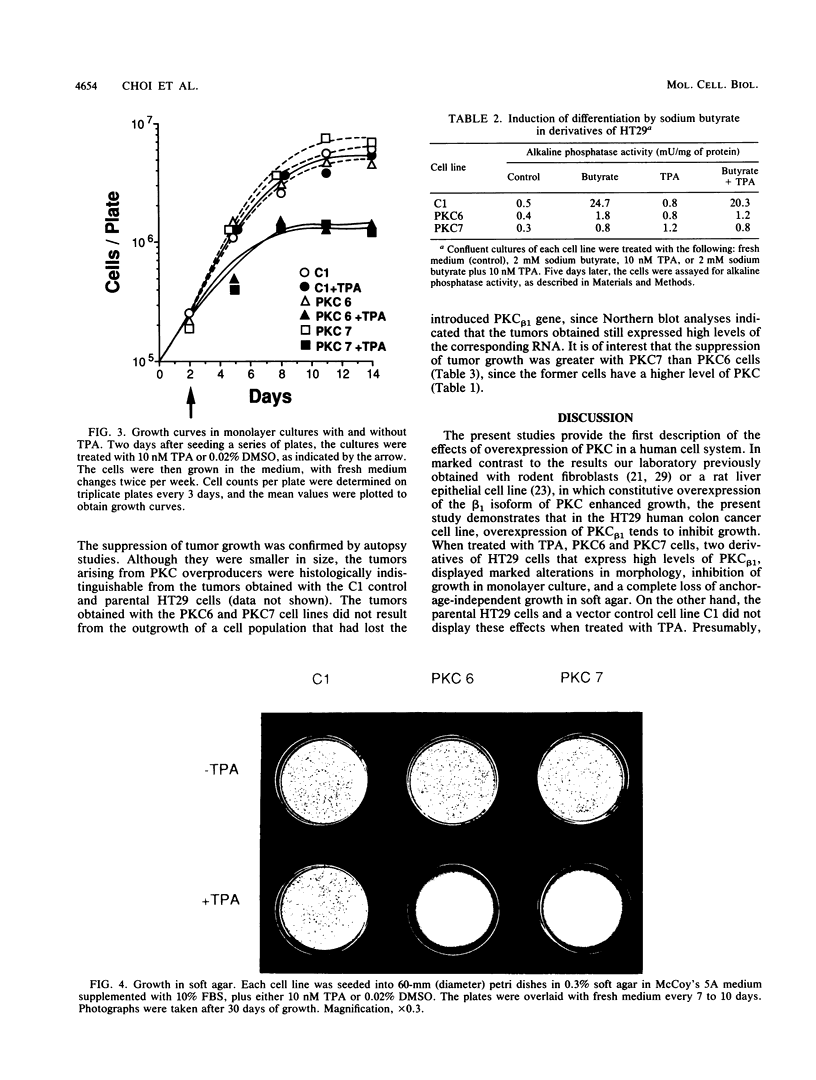
By using a retrovirus-derived vector system, we generated derivatives of the human colon cancer cell line HT29 that stably overexpress a full-length cDNA encoding the beta 1 isoform of rat protein kinase C (PKC). Two of these cell lines, PKC6 and PKC7, displayed an 11- to 15-fold increase in PKC activity when compared with the C1 control cell line that carries the vector lacking the PKC cDNA insert. Both of the overexpresser cell lines exhibited striking alterations in morphology when exposed to the tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate (TPA). Following exposure to TPA, PKC6 and PKC7 cells displayed increased doubling time, decreased saturation density, and loss of anchorage-independent growth in soft agar; but these effects were not seen with the C1 cells. Also, in contrast to the control cells, the PKC-overproducing cells failed to display evidence of differentiation, as measured by alkaline phosphatase activity, when exposed to sodium butyrate. In addition, the PKC-overexpresser cells displayed decreased tumorigenicity in nude mice, even in the absence of treatment with TPA. These results provide the first direct evidence that PKC can inhibit tumor cell growth. Thus, in some tumors, PKC might act as a growth-suppressor gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augeron C., Laboisse C. L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984 Sep;44(9):3961–3969. [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987 Feb;79(2):532–541. doi: 10.1172/JCI112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer C. J., O'Brian C. A., Guillem J. G., Weinstein I. B. The regulation of protein kinase C by chenodeoxycholate, deoxycholate and several structurally related bile acids. Carcinogenesis. 1987 Feb;8(2):217–220. doi: 10.1093/carcin/8.2.217. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Friedman E. A. Differential response of premalignant epithelial cell classes to phorbol ester tumor promoters and to deoxycholic acid. Cancer Res. 1981 Nov;41(11 Pt 1):4588–4599. [PubMed] [Google Scholar]
- Friedman E., Gillin S., Lipkin M. 12-O-Tetradecanoylphorbol-13-acetate stimulation of DNA synthesis in cultured preneoplastic familial polyposis colonic epithelial cells but not in normal colonic epithelial cells. Cancer Res. 1984 Sep;44(9):4078–4086. [PubMed] [Google Scholar]
- Friedman E., Isaksson P., Rafter J., Marian B., Winawer S., Newmark H. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res. 1989 Feb 1;49(3):544–548. [PubMed] [Google Scholar]
- Friedman E., Urmacher C., Winawer S. A model for human colon carcinoma evolution based on the differential response of cultured preneoplastic, premalignant, and malignant cells to 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Apr;44(4):1568–1578. [PubMed] [Google Scholar]
- Gescher A. Antiproliferative properties of phorbol ester tumour promoters. Biochem Pharmacol. 1985 Aug 1;34(15):2587–2592. doi: 10.1016/0006-2952(85)90552-0. [DOI] [PubMed] [Google Scholar]
- Guillem J. G., Hsieh L. L., O'Toole K. M., Forde K. A., LoGerfo P., Weinstein I. B. Changes in expression of oncogenes and endogenous retroviral-like sequences during colon carcinogenesis. Cancer Res. 1988 Jul 15;48(14):3964–3971. [PubMed] [Google Scholar]
- Gum J. R., Kam W. K., Byrd J. C., Hicks J. W., Sleisenger M. H., Kim Y. S. Effects of sodium butyrate on human colonic adenocarcinoma cells. Induction of placental-like alkaline phosphatase. J Biol Chem. 1987 Jan 25;262(3):1092–1097. [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Housey G. M., O'Brian C. A., Johnson M. D., Kirschmeier P., Weinstein I. B. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1065–1069. doi: 10.1073/pnas.84.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Tsao D., Siddiqui B., Whitehead J. S., Arnstein P., Bennett J., Hicks J. Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer. 1980 Mar 15;45(5 Suppl):1185–1192. doi: 10.1002/1097-0142(19800315)45:5+<1185::aid-cncr2820451324>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kirschmeier P. T., Housey G. M., Johnson M. D., Perkins A. S., Weinstein I. B. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988 Apr;7(3):219–225. doi: 10.1089/dna.1988.7.219. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Krauss R. S., Housey G. M., Johnson M. D., Weinstein I. B. Disturbances in growth control and gene expression in a C3H/10T1/2 cell line that stably overproduces protein kinase C. Oncogene. 1989 Aug;4(8):991–998. [PubMed] [Google Scholar]
- Lipkin M., Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985 Nov 28;313(22):1381–1384. doi: 10.1056/NEJM198511283132203. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McKay I., Collins M., Taylor-Papadimitriou J., Rozengurt E. An inhibitory effect of tumour promoters on human epithelial cell growth can be dissociated from an effect on junctional communication. Exp Cell Res. 1983 May;145(2):245–254. doi: 10.1016/0014-4827(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Morita A., Tsao D., Kim Y. S. Effect of sodium butyrate on alkaline phosphatase in HRT-18, a human rectal cancer cell line. Cancer Res. 1982 Nov;42(11):4540–4545. [PubMed] [Google Scholar]
- Nishizuka Y. Perspectives on the role of protein kinase C in stimulus-response coupling. J Natl Cancer Inst. 1986 Mar;76(3):363–370. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Ovadia H., Hanna N., Nelken D. Effect of Normal Immunosuppressive Protein (NIP) on tumor growth in mice. Eur J Cancer. 1975 Jun;11(6):413–417. doi: 10.1016/0014-2964(75)90072-9. [DOI] [PubMed] [Google Scholar]
- Pakkanen R., Vaheri A. Cytovillin and other microvillar proteins of human choriocarcinoma cells. J Cell Biochem. 1989 Sep;41(1):1–12. doi: 10.1002/jcb.240410102. [DOI] [PubMed] [Google Scholar]
- Persons D. A., Wilkison W. O., Bell R. M., Finn O. J. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988 Feb 12;52(3):447–458. doi: 10.1016/s0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Pettit G. R. Bryostatins: potent, new mitogens that mimic phorbol ester tumor promoters. Biochem Biophys Res Commun. 1985 Nov 15;132(3):939–945. doi: 10.1016/0006-291x(85)91898-4. [DOI] [PubMed] [Google Scholar]
- Trainer D. L., Kline T., McCabe F. L., Faucette L. F., Feild J., Chaikin M., Anzano M., Rieman D., Hoffstein S., Li D. J. Biological characterization and oncogene expression in human colorectal carcinoma cell lines. Int J Cancer. 1988 Feb 15;41(2):287–296. doi: 10.1002/ijc.2910410221. [DOI] [PubMed] [Google Scholar]
- Vandenbark G. R., Niedel J. E. Phorbol diesters and cellular differentiation. J Natl Cancer Inst. 1984 Nov;73(5):1013–1019. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Positive and negative controls on cell growth. Biochemistry. 1989 Oct 17;28(21):8263–8269. doi: 10.1021/bi00447a001. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Wice B. M., Trugnan G., Pinto M., Rousset M., Chevalier G., Dussaulx E., Lacroix B., Zweibaum A. The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J Biol Chem. 1985 Jan 10;260(1):139–146. [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Hennings H., Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982 Jun;42(6):2344–2349. [PubMed] [Google Scholar]